Abstract
Cardiovascular disease (CVD) is a well-described complication of schizophrenia, however, mechanisms connecting CVD with other facets of psychotic disorders, such as neurocognition, are not understood. The current study examined folate metabolism as a potential mechanism of CVD and neurocognitive deficits by: 1) using endothelial dysfunction as a biomarker of CVD, and 2) comparing enzymes associated with neurocognition, CVD, and critical to folate metabolism, methylenetetrahydrofolate reductase (MTHFR) and catechol-o-methyl transferase (COMT). Endothelial function was assessed in 147 participants with schizophrenia, schizoaffective disorder, and psychotic disorder not otherwise specified grouped by MTHFR and COMT allele status. Regression models were used to compare neurocognitive performance based on the Brief Assessment of Cognition in Schizophrenia (BACS). Overall, endothelial function predicted BACS composite z-scores after controlling for age, race, level of education, serum folate levels, and MTHFR/COMT risk allele status. Participants with at least one or more MTHFR and/or COMT risk alleles had lower BACS Composite and BACS Symbol Coding adjusted mean z-scores than those with both MTHFR CC and COMT Met/Met genotypes. Thus, endothelial dysfunction may contribute to the neurocognitive deficits seen in psychotic disorders. CVD interventions may not only reduce CVD-related morbidity, but also lessen progressive neurocognitive deficits reported in psychotic disorders.
Keywords: schizophrenia, neurocognition, folate, pharmacogenomics, endothelial function, cardiovascular disease
1.1. Introduction
Psychotic disorders such as schizophrenia are often associated with cardiovascular diseases (CVD). Previous reports show that two-thirds of people with schizophrenia are diagnosed with CVD, compared to one-half of the general population, and this high rate of CVD morbidity is directly related to decreased life expectancy in schizophrenia (Fan et al., 2013; Hennekens et al., 2005).
One potential mechanism for the development of CVD may be found within enzymes responsible for the metabolism of folate, methylenetetrahydrofolate reductase (MTHFR) C677T and catechol-O-methyltransferase (COMT) Val158Met, as variant or risk alleles (MTHFR T and COMT Val alleles) are linked to CVD risk in schizophrenia ( Burghardt and Ellingrod, 2013; Ellingrod et al., 2011; Kullo and Malik, 2007).
Additionally, MTHFR and COMT risk alleles are associated with reduced prefrontal cortex functioning and neurocognitive deficits in schizophrenia (Roffman et al., 2011a; Roffman et al., 2008a; Roffman et al., 2011b; Roffman et al., 2007; Roffman et al., 2008b). Together, these findings suggest abnormal folate metabolism may be related to the development of both the CVD and neurocognitive deficits often seen in schizophrenia.
MTHFR and COMT enzymes are each involved in the AldoMet cycle, a key pathway in folate metabolism. Within this cycle, MTHFR C677T catalyzes the formation of methyltetrahydrofolate (5-methyl THF) from dietary folate. Abnormal folate metabolism may be a consequence of the MTHFR 677T allele, as single T allele carriers show a 35% reduction in activity and TT homozygotes show a 70% reduction. The presence of the MTHFR T allele results in reduced folate metabolism that can lead to elevated levels of homocysteine or hyperhomocysteinemia, which has been associated with CVD in the general population (Klerk et al., 2002).
In addition to MTHFR, the COMT variant (158Val/Met) is another crucial component within the AldoMet cycle, as the COMT Val/Val genotype exhibits 30–50% greater activity than the Met/Met genotype (Chen et al., 2004), which may also lead to hyperhomocysteinemia. Moreover, the effects of the COMT 158 variant may be exaggerated in individuals who also have an MTHFR T allele (Tunbridge et al., 2008).
While both COMT and MTHFR genotypes have been individually and additively associated with neurocognitive deficits in schizophrenia (Ceaser et al., 2013; Kontis et al., 2013; Roffman et al., 2011b; Roffman et al., 2008b), the relationship between folate metabolism, neurocognition, and CVD in schizophrenia has not been examined. Accordingly, an important biomarker for CVD is dysfunction of the endothelium (Kullo & Malik, 2007; Haynes, 2003; Ross, 1999; Rubinshtein et al., 2010). Briefly, the endothelium is a vital organ lining all blood vessels of the body that regulates inflammation and is also related to neurocognitive deficits in clinical populations, e.g., Alzheimer’s disease (Dede et al., 2007), and depression (Smith et al., 2007). Hence, understanding the relationship between endothelial function, pharmacogenetically regulated folate metabolism through MTHFR and COMT enzymes, and neurocognition in schizophrenia may help to identify those at greatest risk for significant impairments. Since deficits in endothelial functioning are potentially reversible, determining the role of CVD in the development of neurocognitive deficits in schizophrenia would be invaluable, as targeted interventions could potentially help to reverse negative outcomes.
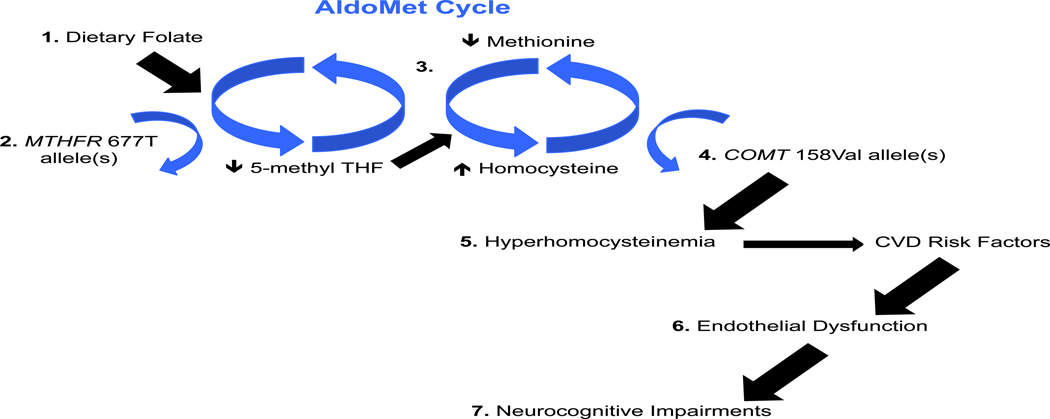
Thus, the aim of the current study was to determine the impact of MTHFR 677C/T, COMT 158 Val/Met, and endothelial functioning on neurocognition in psychotic disorders. We hypothesized that presence of MTHFR and/or COMT risk alleles and the occurrence of a specific CVD risk factor (endothelial dysfunction), would be associated with impaired neurocognition. Figure 1 provides a detailed description of this hypothesis.
Figure 1.
The AldoMet cycle begins with: 1) dietary folate, which is converted to 5-methyl THF by the MTHFR enzyme. 2) Presence of the MTHFR 677T allele(s) is associated with a 35 to 70% reduction in the metabolism of folate. 3) In the next phase of the Aldo Met cycle, 5-methyl THF is needed to form methionine, which is then converted to homocysteine by the COMT enzyme. 4) Presence of the COMT 158Val allele(s) is associated with a more efficient conversion of methionine to homocysteine, resulting in an elevation of homocysteine and possible hyperhomocysteinemia. 5) Previous reports have linked hyperhomocysteinemia with CVD risk factors such as metabolic syndrome (e.g., elevated BMI) 6) Additionally, endothelial dysfunction may be a marker of these CVD risk factors, 7) and endothelial dysfunction is associated with neurocognitive impairments. THF = tetrahydrofolate; MTHFR = methylenetetrahydrofolate reductase; COMT = catechol-o-methyl transferase; CVD = cardiovascular disease; BMI = body mass index.
2.1. Methods and Materials
2.1.1. Participants
A total of 147 participants with a DSM-IV Axis I primary diagnosis of schizophrenia (n = 61), schizoaffective disorder (n = 71), or psychotic disorder not otherwise specified (n = 15) were included in this analysis. Participants were part of a larger study examining the metabolic side effects of antipsychotic medications and were included if they: 1) had a DSM-IV (American Psychiatric Association, 2000) diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychotic disorder not otherwise specified, 2) were between the ages of 18–90, and 3) had received at least 6 months of antipsychotic medication treatment. Participants were excluded if they: 1) were unwilling to participate, 2) lacked the ability to give informed consent 3) were diagnosed with type II diabetes prior to treatment with antipsychotic medications, or 4) had an active substance abuse diagnosis. The University of Michigan Medical School Institutional Review Board (IRBMED), Washtenaw County Health Organization (WCHO), Ann Arbor Veterans Affairs Medical Center, and Detroit-Wayne County Community Mental Health Agency (DWCCMHA) approved the study protocol. Each participant was assessed at the Michigan Clinical Research Unit (MCRU) at the University of Michigan Medical Center.
2.1.2. Diagnostic, clinical, and demographic assessment
Participants underwent an informed consent process followed by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID; First et al., 1997). Participants also completed the Beck Depression Inventory (BDI; Beck et al., 1996). Level of education was determined by the eight classifications provided by the SCID (1 = completed grade 6 or less, 2 = completed grade 7 to 12 without graduating high school, 3 = graduated high school or high school equivalent, 4 = completed some college courses without graduating, 5 = graduated from a 2 year college, 6 = graduated from a 4 year college, 7 = completed some graduate/professional courses without graduating, and 8 = completed graduate/professional school). Participants were grouped by low and high education, with levels 1 to 2 categorized as low education (no high school diploma) and levels 3 to 8 categorized as high education (high school diploma, GED, or higher).
2.1.3. Metabolic assessment
Participants completed a fasting blood draw (12 hours) that included assays for both serum folate and homocysteine serum levels, which were collected to determine the relationship between MTHFR/COMT risk alleles and neurocognition independent of serum folate and homocysteine levels.
2.1.4. Neurocognitive battery
The Brief Assessment of Cognition in Schizophrenia (BACS; Keefe, 1999; Keefe et al., 2004) measures four domains: verbal memory (List learning), working memory (Digit sequencing), processing speed (Token Motor Task, Verbal Fluency: category instances and letter fluency, and Symbol Coding), and executive function (Tower of London; Keefe et al., 2008). BACS z-scores were based on a group of 63 healthy controls from our database.
2.1.5. Endothelial function
Endothelial functioning was assessed with the EndoPAT 2000 (Itamar Medical Inc, Caesarea, Israel), which has been validated and described in previous studies as a non-invasive method using peripheral arterial tonometry (Bonetti, 2002; Bonetti et al., 2004; Goor et al., 2004; Halligan et al., 2004; Lavie et al., 2000; Rubinshtein et al., 2010; Tziomalos et al., 2010). The Reactive Hyperemia Index (RHI) is calculated using the EndoPAT 2000 software. RHI scores ≤ 1.67 meet the endothelial dysfunction criterion (Bonetti et al., 2004). See Ellingrod et al. (2011) for a detailed account of the procedure. In the current study, only clinical participants were assessed for endothelial function.
2.1.6. Genotyping
Genotyping was done with Pyrosequencing™ Technology. Participants were genotyped for MTHFR 677C/T (rs1801133) and COMT Val158Met (rs4680) variants. Details are available in previous reports (Ellingrod et al., 2008; Ellingrod et al., 2012).
2.1.7. Risk allele status
Participants with both MTHFR CC and COMT Met/Met genotypes were included in the low-risk allele group. Participants with one or more MTHFR T alleles and/or COMT Val alleles were included in the risk allele group.
2.1.8. Statistical Method
Statistical analyses were performed using JMP Pro 11 statistical software (SAS Institute Inc.). Demographic differences between risk allele groups were examined with a one-way ANOVA and standard t-test for continuous variables, and a chi-square for nominal variables. Linear regression models were used for the main analyses. For the linear regression models, as recommended by Field (2009), known predictors (age and education), were followed by new predictors (serum folate levels, risk allele status, and endothelial functioning score). Known predictors were determined by similar reports that controlled for age and education (cf. LeBlanc et al., 2012; Meyer et al., 2005). Education was based on the previously described continuous SCID levels of education (1–8) and categorized as low or high. Based on previous reports of racial differences in genotypes (Roffman et al., 2011a; Roffman et al., 2007), race was also entered as a predictor (i.e., Caucasian or non-Caucasian). Adjusted means for BACS Composite and subscale z-scores are reported, along with standardized betas. A p-value less than 0.05 was considered significant. For analysis of BACS subscales, a Bonferroni correction was used (p value of 0.05 / six subscales = 0.0083).
3.1. Results
3.1.1. Demographics and Genotypes
Table 1 provides demographic information for all participants. MTHFR and COMT genotypes were both in Hardy Weinberg equilibrium (p > 0.05). There were no significant demographic differences between risk allele groups, with 17 participants in the low-risk allele group and 130 in the risk allele group. Additionally, within the risk allele group, 49 participants had at least one MTHFR T allele (38%) and 121 participants had at least one COMT Val allele (93%). A total of 40 participants had at least one MTHFR T allele and COMT Val allele (31%).
Table 1.
Demographic, clinical, and metabolic characteristics for all participants and risk allele groups. Level of education is defined by rankings that range from 1–8 (1 = completed grade 6 or less, 2 = completed grade 7 to 12 without graduating high school, 3 = graduated high school or high school equivalent, 4 = completed some college courses without graduating, 5 = graduated from a 2 year college, 6 = graduated from a 4 year college, 7 = completed some graduate/professional courses without graduating, and 8 = completed graduate/professional school). Race is a categorical variable comprised of Caucasian and non-Caucasian participants. Serum homocysteine and folate levels are displayed in micromole/liter and nanogram/milliliter, respectively. Participants taking statin medications: atorvastatin, cerivastatin, fluvastatin, lovastatin, mevastatin, pitavastatin, pravastatin, rosuvastatin, or simvastatin are listed according to group. Beck Depression Inventory scores were not available for all clinical participants (n = 103), risk allele group (n = 98), low-risk allele group (n = 6). Diagnosis was compared among the three psychotic disorders according to allele group membership.
| All clinical participants (n = 147) |
Risk allele group (n = 130) |
Low-risk allele group (n = 17) |
Healthy Controls (HC; n = 63) |
F statistic, t-test, Chi- square or FET p value* |
Post-hoc results | |
|---|---|---|---|---|---|---|
| Age in years (M ± SD) | 45.4 ± 10.4 | 45.5 ± 10.7 | 45.1 ± 8.5 | 43.5 ± 11.3 | 0.48 | -- |
| Gender (% Male) | 89 (61%) | 78 (60%) | 11 (65%) | 41 (65%) | 0.77 | -- |
| Level of education (1–8; M ± SD) | 4.1 ± 1.5 | 4.1 ± 1.5 | 3.9 ± 1.6 | 6.2 ± 1.7 | <0.001 | HC > both allele groups |
| Race (% Caucasian) | 80 (54%) | 68 (52%) | 12 (71%) | 46 (70%) | 0.01 | HC > risk allele group |
| Diagnosis | -- | -- | -- | -- | 0.21 | -- |
| SZ (%) | 61 (41%) | 51 (39%) | 10 (59%) | -- | -- | -- |
| SA (%) | 71 (48%) | 64 (49%) | 7 (41%) | -- | -- | -- |
| P-NOS (%) | 15 (10%) | 15 (12%) | 0 (0%) | -- | -- | -- |
| Comorbid with depressive disorder | 4 (3%) | 4 (3%) | 0 (0%) | -- | -- | -- |
| Serum homocysteine levels (umol/L; M ± SD) | 10.1 ± 3.1 | 10.2 ± 3.1 | 8.8 ± 2.5 | -- | 0.08 | -- |
| Serum folate levels (ng/ml; M ± SD) | 17.7 ± 6.3 | 17.8 ± 6.2 | 17.0 ± 6.7 | -- | 0.63 | -- |
| Statins (%) | 31 (21%) | 26 (20%) | 5 (29%) | -- | 0.39 | -- |
| Current smoker (%) | 71 (48%) | 60 (46%) | 11 (65%) | -- | 0.15 | -- |
| History of smoking (%) | 63 (43%) | 53 (41%) | 10 (59%) | -- | 0.17 | -- |
| Metabolic syndrome diagnosis (%) | 66 (45%) | 58 (45%) | 8 (47%) | -- | 0.87 | -- |
| Body Mass Index (M ± SD) | 32.7 ± 8.1 | 32.5 ± 8.0 | 34.4 ± 8.9 | -- | 0.35 | -- |
| Duration of illness in years (M ± SD) | 19.7 ± 11.4 | 19.9 ± 11.6 | 18.7 ± 10.6 | -- | 0.70 | -- |
| Atypical antipsychotics (%) | 121 (82%) | 107 (82%) | 14 (82%) | -- | 1.00 | -- |
| CPZ equivalents (M ± SD) | 678.2 ± 695.6 | 682.7 ± 708.9 | 644.0 ± 603.1 | -- | 0.83 | -- |
| Beck Depression Inventory (M ± SD) | 13.8 ± 10.4 | 13.7 ± 10.4 | 16.7 ± 11.1 | -- | 0.49 | -- |
One-way ANOVA and Chi-square were used to compare risk allele groups with healthy controls; t-tests and Chi-square were used to compare: 1) all clinical participants with healthy controls, and 2) the risk allele group with the low-risk allele group. Tukey’s HSD and Chi-square were used for post-hoc analyses. M = Mean; SD = Standard deviation; CPZ = chlorpromazine; FET = Fisher’s Exact Test; SZ = schizophrenia; SA = schizoaffective disorder; P-NOS = psychotic disorder not otherwise specified.
3.1.2. Endothelial function
The mean RHI was 1.89 ± 0.59 for all participants and 44.2% (n = 65) of participants met criteria for endothelial dysfunction (RHI ≤ 1.67). A linear regression model showed RHI was not related to current smoking, history of smoking, cigarettes smoked per year among all participants (p > 0.05 for all variables). However, RHI scores were predictive of Body Mass Index (β = −0.17, ρ = 0.039) and participants meeting criteria for metabolic syndrome had significantly lower RHI scores (M = 1.77) than those participants who did not meet metabolic syndrome criteria (M = 1.97), t(144) = 4.45, p = 0.03. Furthermore, a linear regression model showed RHI was not related to BDI scores among all participants (p > 0.05).
3.1.3. Prediction of BACS Composite z-scores
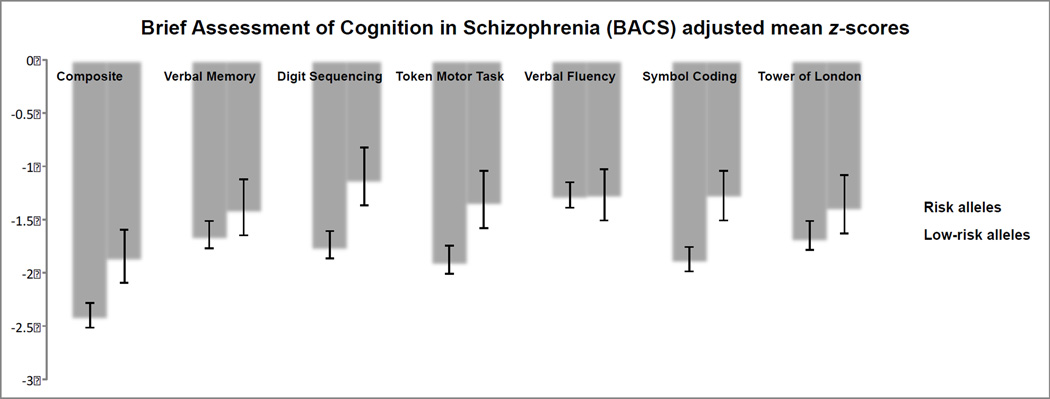
Means and adjusted means for BACS Composite z-scores are listed in Table 2 and Figure 2, respectively. RHI scores also predicted BACS Composite z-scores in a linear regression model with the additional covariates of age, race, education, serum folate levels, and risk allele status (β = 0.19, ρ = 0.009; See Table 3). Within this model, there were differences in neurocognition according to risk allele status, as participants in the low-risk allele group had significantly higher BACS Composite z-scores, adjusted M = −1.85, than participants in the risk allele group, adjusted M = −2.40; β = 0.16, ρ = 0.032. In looking at participants with risk alleles using a similar linear regression model with almost identical covariates (age, race, education, and serum folate levels), RHI scores significantly predicted BACS Composite z-scores, β = 0.20, ρ = 0.008. However, for participants with no risk alleles, RHI scores did not predict BACS Composite z-scores, β = 0.03, ρ = 0.923.
Table 2.
Means and standard errors for BACS Composite and subscale z-scores for 147 participants with psychotic disorders (schizophrenia, schizoaffective disorder, and psychotic disorder not otherwise specified).
| Brief Assessment of Cognition in Schizophrenia (BACS) |
All clinical participants (n = 147) |
|---|---|
| M (SE) | |
| Composite | −1.88 (0.09) |
| Verbal Memory | −1.28 (0.10) |
| Digit Sequencing | −1.31 (0.10) |
| Token Motor Task | −1.60 (0.09) |
| Verbal Fluency | −1.02 (0.08) |
| Symbol Coding | −1.52 (0.08) |
| Tower of London | −1.18 (0.11) |
Figure 2.
Table 3.
Demographic, risk allele, and endothelial contributors to Brief Assessment of Cognition in Schizophrenia (BACS) Composite z-scores for 147 participants with psychotic disorders (schizophrenia, schizoaffective disorder, and psychotic disorder not otherwise specified). Race is a categorical variable comprised of Caucasian and non-Caucasian participants. Reactive Hyperemia Index (RHI) measures endothelial function and higher scores indicate endothelial dysfunction. Education is a categorical variable comprised of lower (less than high school education) and higher (high school diploma/equivalent or higher) classification groups. Risk allele group is a categorical variable compromised of participants with risk alleles (one or more of the MTHFR 667T and/or COMT 158Val variants) and participants without risk alleles (MTHFR CC and COMT Met/Met genotypes). MTHFR = methylenetetrahydrofolate reductase; COMT = catechol-o-methyl transferase.
| Predictor variable | Variable statistics | Model statistics | ||||
|---|---|---|---|---|---|---|
| β | t | p | R2 | F | p | |
| Model 1: All participants (n=147) | -- | -- | -- | 0.32 | 10.75 | <0.001 |
| Age | −0.25 | −3.56 | <0.001 | |||
| Race category | 0.22 | 2.92 | 0.004 | |||
| Education group | 0.35 | 4.89 | <0.001 | |||
| Serum folate levels | 0.12 | 1.57 | 0.118 | |||
| RHI | 0.19 | 2.65 | 0.009 | |||
| Risk allele group | 0.16 | 2.17 | 0.032 | |||
| Model 2A: No risk alleles (n=17) | -- | -- | -- | 0.25 | 0.75 | 0.603 |
| Age | −0.39 | −1.36 | 0.297 | |||
| Race category | 0.30 | 0.95 | 0.315 | |||
| Education group | 0.11 | 0.35 | 0.869 | |||
| Serum folate levels | 0.31 | 1.11 | 0.300 | |||
| RHI | 0.03 | 0.10 | 0.827 | |||
| Model 2B: Risk alleles (n=130) | -- | -- | -- | 0.33 | 12.05 | <0.001 |
| Age | −0.24 | −3.26 | 0.001 | |||
| Race category | 0.23 | 2.91 | 0.004 | |||
| Education group | 0.38 | 5.04 | <0.001 | |||
| Serum folate levels | 0.10 | 1.23 | 0.223 | |||
| RHI | 0.20 | 2.68 | 0.008 | |||
3.1.4. Prediction of BACS subscale z-scores
Means and adjusted means for BACS subscale z-scores are listed in Table 2 and Figure 2, respectively. After correcting for multiple comparisons, RHI scores also predicted BACS Tower of London z-scores in a linear regression model with the additional covariates of age, race, education, serum folate levels, and risk allele status (β = 0.23, ρ = 0.002; See Table 3). As observed in the BACS Composite z-scores, within this model the low-risk allele group had significantly higher BACS Symbol Coding z-scores, adjusted M = −1.26, than participants in the risk allele group, adjusted M = −1.87; β = 0.21, ρ = 0.007. In looking at participants with risk alleles, RHI scores significantly predicted BACS Symbol Coding z-scores, β = 0.17, ρ = 0.034. However, for participants with no risk alleles, RHI scores did not predict BACS Composite z-scores, β = −0.14, ρ = 0.648.
4.1. Discussion
The current study explored the relationship between pharmacogenomic risk alleles associated with folic acid metabolism, endothelial functioning, and neurocognition in psychotic disorders such as schizophrenia, schizoaffective disorder, and psychotic disorder not otherwise specified. It was found that endothelial functioning (a biomarker for general CVD) is positively related to overall neurocognitive performance, even after controlling for age, race, education, serum folate levels, and MTHFR/COMT risk allele status. In particular, the relationship between endothelial function and neurocognition appeared within carriers of MTHFR T and/or COMT Val alleles while those with both MTHFR CC and COMT Met/Met genotypes did not show this relationship. Additionally, a positive relationship between RHI and executive function was observed, suggesting that endothelial dysfunction may affect executive function more than any other neurocognitive domain. Moreover, a difference in neurocognition according to the presence of MTHFR/COMT risk alleles was also observed. Carriers of at least one MTHFR T and/or COMT Val allele demonstrated lower overall neurocognitive performance and processing speed performance compared to those with both MTHFR CC and COMT Met/Met genotypes. Thus, abnormal folate metabolism associated with MTHFR and COMT risk alleles and endothelial dysfunction may adversely affect neurocognition in psychotic disorders.
To our knowledge, this is the first study to examine the relationship between endothelial dysfunction and neurocognition in psychotic disorders, although previous work has examined other markers of CVD and neurocognition. Friedman et al. (2010) found a relationship between CVD risk factors (e.g., metabolic syndrome criteria), such as hypertension and elevated Body Mass Index (BMI), and neurocognition in schizophrenia. Similarly, Abdul Rashid et al. (2013) examined the role of BMI on neurocognition in schizophrenia and reported an indirect effect of BMI on neurocognition. The relationship between CVD risk factors and neurocognitive deficits may be explained by atherosclerosis, as both hypertension and BMI are established atherosclerosis risk factors, and atherosclerosis impairs neurocognition (Friedman et al., 2010). Endothelial dysfunction is also associated with the development of atherosclerosis (Munzel et al., 2008; Ross, 1993), which could affect cerebral perfusion, causing deterioration and loss of function in neuronal cells, and lead to neurocognitive deficits (Reijmer et al., 2011). Additionally, both endothelial dysfunction and atherosclerosis impact nitric oxide (NO) levels (Matthys and Bult, 1997), and infusion of sodium nitroprusside, an NO donor, has been shown to alleviate neurocognitive deficits in schizophrenia (Maia-de-Oliveira et al., 2015). Together, the current and previous findings serve to highlight the potentially deleterious impact of CVD risk factors on neurocognition in psychotic disorders.
In light of the current findings, lifestyle changes consisting of increased physical activity and a healthier diet could potentially prevent CVD (Pearson, 2002) and endothelial function (Joris et al., 2014), along with neurocognitive deficits, in psychotic disorders. Additionally, therapeutic interventions such as antioxidants (e.g., Vitamin C) and angiotensin-converting enzyme (ACE) inhibitors have been shown to improve endothelial function in those with heart failure (Yang et al., 2015) and may attenuate endothelial dysfunction and neurocognitive deficits in psychotic disorders. However, there is controversy as to whether or not neurocognitive deficits are stable or continuous in schizophrenia. It has been postulated that neurocognitive deficits begin before the onset of schizophrenia manifest (or around the same time) and Harvey et al. (1999) reported neurocognitive deterioration over an average 2.5 year period in an older cohort of schizophrenia (M = 77.8, 44% male) also treated for diabetes mellitus (4%) and cardiac-related illness (28%) at follow-up. It should be noted that these participants may have also experienced additional contributors to endothelial dysfunction (e.g., hypertension). Thus, preventing significant CVD may also help to avert progressive neurocognitive deficits seen in some samples of schizophrenia.
The current study supports previous findings regarding the effects of MTHFR and COMT on neurocognition in schizophrenia, as evidenced by impaired performance on BACS Composite and Symbol Coding according to MTHFR T allele and COMT Val allele status. Similarly, Roffman and colleagues reported deficits in working memory and executive function were related to decreased dorsolateral prefrontal cortex (DLPFC) activity and were moderated by an MTHFR T and COMT Val allele interaction (Roffman et al., 2008a; Roffman et al., 2008b). While BACS Symbol Coding is considered a processing speed task, all three constructs are closely related, as increased processing speed allows for decreased working memory load, and similar DLPFC activation has been observed for all three constructs (Barbey et al., 2013). A possible mechanism for the combined effects of MTHFR and COMT on DLPFC activation in schizophrenia begins with the MTHFR T allele, which is associated with reduced methylation of genomic DNA, leading to reduced COMT promoter methylation in the DLPFC. Supporting this hypothesis, reduced COMT promoter methylation in the DLPFC has been reported in schizophrenia (Abdolmaleky et al., 2006). The MTHFR T allele may also cause an increase in COMT expression, resulting in the attenuation of dopamine signaling in the DLPFC. Additionally, reduced COMT promoter methylation and COMT expression in DLPFC caused by the MTHFR T allele may be exacerbated by the hyperactive COMT Val allele, leading to some of the observed neurocognitive impairments in schizophrenia (Roffman et al., 2008a; Roffman et al., 2008b).
4.1.1. Limitations
The low-risk allele group was small (n = 17) and increases the risk of Type II error. However, this small group size is expected, as rates of homozygosity for the MTHFR C and COMT Met alleles range from 10 to 15% of patients (Kontis et al., 2013; Roffman et al., 2008b). Additional CVD risk factors were not included in the regression models due to multicollinearity, as BMI and metabolic syndrome diagnosis were both strongly associated with endothelial function. Future studies should include larger sample sizes to examine the independent effect of endothelial function in comparison to other CVD risk factors. Additionally, while depression has been linked to endothelial dysfunction and cognitive impairments (Smith et al., 2007), within the current study Beck Depression Inventory scores were not related to endothelial dysfunction and only a small number of participants had a comorbid psychotic disorder and major depression (n = 4). Future studies should also include both clinical and non-clinical populations to examine the differences between endothelial dysfunction and cognitive impairments according to diagnosis (e.g., psychotic disorders compared to depression and healthy controls).
5.1. Conclusions
The results of the current study show a relationship between risk for cardiovascular disease and impaired neurocognition in psychotic disorders such as schizophrenia, schizoaffective disorder, and psychotic disorder not otherwise specified. Enzymes crucial to the metabolism of folate, MTHFR C677T and COMT Val158Met, along with endothelial functioning, are associated with neurocognitive performance in psychotic disorders, even after controlling for age, education, race, and serum folate levels. Patients with risk alleles may not only be the most vulnerable for the development of CVD, but also poorer neurocognition, and as such, specific interventions related to cardiovascular health need to be developed along with increased monitoring for the development of CVD. The assessment of endothelial function could provide a means for monitoring overall CVD risk and identifying those who may benefit from early intervention. Consequently, implementation of potential early interventions may not only improve cardiovascular health and reduce the risk of sudden cardiac death, but also ameliorate neurocognitive impairment, which itself is an overall risk for poorer outcomes in schizophrenia (Green, 1996; Green et al., 2004; Green, 2000).
Acknowledgements
The authors thank the staff of the Michigan Clinical Research Unit, Washtenaw County Health Organization, Ann Arbor Veterans Affairs Medical Center, and Detroit-Wayne County Community Mental Health Agency for their assistance in data collection. This study was supported by grants from The National Institute for Mental Health under Award Number R01MH082784, the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2UL1TR000433-06, and the Chemistry Core of the Michigan Diabetes Research and Training Center funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of Funding Source
The NIH provided financial support and had no further role in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Vicki Ellingrod designed the study and wrote the study protocol. Tyler Grove managed the literature searches and analyses. Tyler Grove and Dr. Vicki Ellingrod undertook the statistical analysis, and Tyler Grove wrote the first draft of the manuscript. Drs. Stephan Taylor and Gregory Dalack provided valuable edits to the manuscript and all authors have approved the final manuscript.
Conflict of Interest
Dr. Stephan Taylor has a research contract with St. Jude Medical and research support from Neuronetics. All other authors declare they have no conflicts of interest.
References
- Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, Gao F, Smith CL, Shafa R, Aeali B, Carnevale J, Pan H, Papageorgis P, Ponte JF, Sivaraman V, Tsuang MT, Thiagalingam S. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum. Mol. Genet. 2006;15(21):3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Rashid NA, Lim J, Lam M, Chong SA, Keefe RS, Lee J. Unraveling the relationship between obesity, schizophrenia and cognition. Schizophr. Res. 2013 doi: 10.1016/j.schres.2013.09.020. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. [Google Scholar]
- Barbey AK, Colom R, Grafman J. Dorsolateral prefrontal contributions to human intelligence. Neuropsychologia. 2013;51(7):1361–1369. doi: 10.1016/j.neuropsychologia.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bonetti PO. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. Arterioscler. Thromb. Vasc. Biol. 2002;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- Burghardt KJ, Ellingrod VL. Detection of metabolic syndrome in schizophrenia and implications for antipsychotic therapy : is there a role for folate? Mol. Diagn. Ther. 2013;17(1):21–30. doi: 10.1007/s40291-013-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceaser A, Csernansky JG, Barch DM. COMT influences on prefrontal and striatal blood oxygenation level-dependent responses during working memory among individuals with schizophrenia, their siblings, and healthy controls. Cogn. Neuropsychiatry. 2013;18(4):257–283. doi: 10.1080/13546805.2012.698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dede DS, Yavuz B, Yavuz BB, Cankurtaran M, Halil M, Ulger Z, Cankurtaran ES, Aytemir K, Kabakci G, Ariogul S. Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? J. Am. Geriatr. Soc. 2007;55(10):1613–1617. doi: 10.1111/j.1532-5415.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- Ellingrod VL, Miller DD, Taylor SF, Moline J, Holman T, Kerr J. Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C variants. Schizophr. Res. 2008;98(1–3):47–54. doi: 10.1016/j.schres.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingrod VL, Taylor SF, Brook RD, Evans SJ, Zollner SK, Grove TB, Gardner KM, Bly MJ, Pop-Busui R, Dalack G. Dietary, lifestyle and pharmacogenetic factors associated with arteriole endothelial-dependent vasodilatation in schizophrenia patients treated with atypical antipsychotics (AAPs) Schizophr. Res. 2011;130(1–3):20–26. doi: 10.1016/j.schres.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingrod VL, Taylor SF, Dalack G, Grove TB, Bly MJ, Brook RD, Zollner SK, Pop-Busui R. Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. J. Clin. Psychopharmacol. 2012;32(2):261–265. doi: 10.1097/JCP.0b013e3182485888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J. Psychiatr. Res. 2013;47(11):1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using SPSS. London, England: SAGE; 2009. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, dministration Booklet. American Psychiatric Press; 1997. [Google Scholar]
- Friedman JI, Wallenstein S, Moshier E, Parrella M, White L, Bowler S, Gottlieb S, Harvey PD, McGinn TG, Flanagan L, Davis KL. The effects of hypertension and body mass index on cognition in schizophrenia. Am. J. Psychiatry. 2010;167(10):1232–1239. doi: 10.1176/appi.ajp.2010.09091328. [DOI] [PubMed] [Google Scholar]
- Goor DA, Sheffy J, Schnall RP, Arditti A, Caspi A, Bragdon EE, Sheps DS. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin. Cardiol. 2004;27(3):137–141. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Green MFKRS, Braff DL, Mintz J. Neurocognitive Deficits and Functional Outcome in Schizophrenia: Are We Measuring the “Right Stuff”? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Halligan SC, Murtagh B, Lennon RJ, Pumper GM, Mathew V, Higano ST, Lerman A. Effect of long-term hormone replacement therapy on coronary endothelial function in postmenopausal women. Mayo Clin. Proc. 2004;79(12):1514–1520. doi: 10.4065/79.12.1514. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Parrella M, White L, Mohs RC, Davidson M, Davis KL. Convergence of cognitive and adaptive decline in late-life schizophrenia. Schizophr. Res. 1999;35(1):77–84. doi: 10.1016/s0920-9964(98)00109-1. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: A meta-analysis of intervention studies. Atherosclerosis. 2014;239(1):21–30. doi: 10.1016/j.atherosclerosis.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Keefe R. Brief Assessment of Cognition in Schizophrenia (BACS) Duke University Medical Center; 1999. [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr. Res. 2008;102(1–3):108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, Group MSC. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- Kontis D, Theochari E, Fryssira H, Kleisas S, Sofocleous C, Andreopoulou A, Kalogerakou S, Gazi A, Boniatsi L, Chaidemenos A, Tsaltas E. COMT and MTHFR polymorphisms interaction on cognition in schizophrenia: an exploratory study. Neurosci. Lett. 2013;537:17–22. doi: 10.1016/j.neulet.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J. Am. Coll. Cardiol. 2007;49(13):1413–1426. doi: 10.1016/j.jacc.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Lavie P, Shlitner A, Sheffy J, Schnall RP. Peripheral arterial tonometry: a novel and sensitive non-invasive monitor of brief arousals during sleep. Isr. Med. Assoc. J. 2000;2(3):246–247. [PubMed] [Google Scholar]
- LeBlanc M, Kulle B, Sundet K, Agartz I, Melle I, Djurovic S, Frigessi A, Andreassen OA. Genome-wide study identifies PTPRO and WDR72 and FOXQ1-SUMO1P1 interaction associated with neurocognitive function. J. Psychiatr. Res. 2012;46(2):271–278. doi: 10.1016/j.jpsychires.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Maia-de-Oliveira JP, Abrao J, Evora PR, Zuardi AW, Crippa JA, Belmonte-de-Abreu P, Baker GB, Dursun SM, Hallak JE. The effects of sodium nitroprusside treatment on cognitive deficits in schizophrenia: a pilot study. J. Clin. Psychopharmacol. 2015;35(1):83–85. doi: 10.1097/JCP.0000000000000258. [DOI] [PubMed] [Google Scholar]
- Matthys KE, Bult H. Nitric oxide function in atherosclerosis. Mediators Inflamm. 1997;6(1):3–21. doi: 10.1080/09629359791875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, Patel JK, Keefe RS, Stroup TS, Lieberman JA. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr. Res. 2005;80(1):9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008;40(3):180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- Pearson TA. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. Circulation. 2002;106(3):388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Dekker JM, Nijpels G, Stehouwer CD, Kappelle LJ, Biessels GJ. The metabolic syndrome, atherosclerosis and cognitive functioning in a non-demented population: the Hoorn Study. Atherosclerosis. 2011;219(2):839–845. doi: 10.1016/j.atherosclerosis.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Brohawn DG, Friedman JS, Dyckman KA, Thakkar KN, Agam Y, Vangel MG, Goff DC, Manoach DS. MTHFR 677C>T effects on anterior cingulate structure and function during response monitoring in schizophrenia: a preliminary study. Brain Imaging Behav. 2011a;5(1):65–75. doi: 10.1007/s11682-010-9111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, White T, Clark VP, Fries J, Andreasen NC, Goff DC, Manoach DS. MTHFR 677C --> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val --> Met. Proc. Natl. Acad. Sci. U.S.A. 2008a;105(45):17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Nitenson AZ, Agam Y, Isom M, Friedman JS, Dyckman KA, Brohawn DG, Smoller JW, Goff DC, Manoach DS. A hypomethylating variant of MTHFR, 677C>T, blunts the neural response to errors in patients with schizophrenia and healthy individuals. PLoS One. 2011b;6(9):e25253. doi: 10.1371/journal.pone.0025253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, Wong DH, Halsted CH, Goff DC. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr. Res. 2007;92(1–3):181–188. doi: 10.1016/j.schres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, Halsted CH, Goff DC. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008b;147B(6):990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, Hinderliter A, Sherwood A. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom. Med. 2007;69(6):578–586. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Warden DR, Johnston C, Refsum H, Smith AD. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am. J. Med. Genet. B Neuropsychiatr.. Genet. 2008;147B(6):996–999. doi: 10.1002/ajmg.b.30700. [DOI] [PubMed] [Google Scholar]
- Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr. Metab. Cardiovasc. Dis. 2010;20(2):140–146. doi: 10.1016/j.numecd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Yang O, Li J, Kong J. The Endothelium as a Target for the Treatment of Heart Failure. Cell Biochem. Biophys. 2015 doi: 10.1007/s12013-015-0526-7. [DOI] [PubMed] [Google Scholar]