Abstract
Background
Sub-anesthetic doses of the NMDA antagonist ketamine have been shown to model the formation and stability of delusion in human subjects. The later has been predicted to be due to aberrant prediction error resulting in enhanced destabilization of beliefs. To extend the scope of this model, we investigated the effect of administration of low dose systemic ketamine on memory in a rodent model of memory reconsolidation.
Methods
Systemic ketamine was administered either prior to or immediately following auditory fear memory reactivation in rats. Memory strength was assessed by measuring freezing behavior 24 hours later. Follow up experiments were designed to investigate an effect of pre-reactivation ketamine on short-term memory (STM), closely related memories, and basolateral amygdala (BLA) specific destabilization mechanisms.
Results
Rats given pre-reactivation, but not post-reactivation, ketamine showed larger freezing responses 24 hours later compared to vehicle. This enhancement was not observed 3 hours after the memory reactivation, nor was it seen in a closely related contextual memory. Prior inhibition of a known destabilization mechanism in the BLA blocked the effect of pre-reactivation ketamine.
Conclusions
Pre- but not post-reactivation ketamine enhances fear memory. These data together with recent data in human subjects supports a model of delusion fixity that proposes that aberrant prediction errors result in enhanced destabilization and strengthening of delusional belief.
Keywords: Schizophrenia, ketamine, fear conditioning, reconsolidation
Introduction
Delusions are fixed false beliefs. They are a characteristic of schizophrenia. Many delusions respond well to drugs that block dopamine D2 receptors in striatum. However, up to 50% of patients experience residual delusions (Curson et al., 1985; Kane, 1996). This is devastating for patients and their families. The public health impact is significant (Lindenmayer, 2000); those for whom current medications are ineffective are more likely to be hospitalized long term, to have poor functional outcome and to engage in suicidal behavior (Meltzer and Okayli, 1995). Patients with treatment-refractory delusions do not show the typical pattern of striatal dopamine disruption (Demjaha et al., 2012), suggesting that other brain regions and neurotransmitters may be involved. There is a clear need for new treatments, inspired not by serendipity but by the development of a precise understanding of the neurocognitive mechanisms of delusion.
Delusions are, however, challenging to study empirically – the sufferer often denies any problem (Gibbs and David, 2003) and they don’t present for clinical attention until their delusions are fully formed (Corlett et al., 2007). Experimental models provide a unique experimental window onto an otherwise inaccessible disease process (Corlett et al., 2007). Ketamine, the NMDA glutamate receptor antagonist compound, transiently and reversibly engenders delusion-like ideas in healthy human volunteers (Pomarol-Clotet et al., 2006). Likewise, ketamine administration in animal models can generate behavioral and neural disruptions that mimic human psychosis with face and construct validity (Corlett et al., 2007). In this report we used the ketamine model in a study of rodent learning and memory, to examine a phenomenon hitherto under explored in clinical and preclinical neuroscience, the fixity of delusions.
Setting aside philosophical questions regarding whether animals have beliefs (Dennett, 1995), pre-clinical behavioral neuroscience has furnished psychiatry with candidate processes that might contribute to the formation, updating and maintenance of beliefs in humans (Dickinson, 2001). One such process, prediction error, represents the mismatch between our expectancy in a given situation and what we experience (Rescorla, 1972). It is signaled by dopamine and glutamate neural activity in the brain (Lavin et al., 2005). By reducing the mismatch between expectancy and experience we improve our ability to anticipate the causal structure of the environment and we form causal beliefs (Dickinson, 2001).
Using functional magnetic resonance imaging (fMRI) we established a neurobiological marker for prediction error in right dorsolateral prefrontal cortex (Corlett et al., 2004; Fletcher et al., 2001; Turner et al., 2004). We used this marker to implicate aberrant prediction error in the genesis of endogenous delusions (Corlett et al., 2006) as well as those induced by ketamine in healthy volunteers (Corlett et al., 2006). We argue when prediction errors are signaled internally and inappropriately (Grace, 1991), individuals attend to and learn about irrelevant events. Delusions result as explanations for such aberrant experiences (Corlett et al., 2010).
We recently argued that the fixity of delusions might also be driven by prediction error, if we consider what happens to memories when they need to be updated (Corlett et al., 2009). The surprising (or prediction error driven) recall of a consolidated memory renders it labile and sensitive to disruption (Misanin et al., 1968; Nader et al., 2000). This process of memory reconsolidation is in competition with extinction learning (formation of a new competing memory that overrides the initial one) and the balance between the two may be mediated by prediction error (Eisenhardt and Menzel, 2007; Pedreira et al., 2004). Reconsolidation based memory strengthening (Lee, 2008) or reminder learning is mediated by positive prediction errors (Eisenhardt and Menzel, 2007; Lee, 2008; Pedreira et al., 2004).
Aberrant prediction error signaling should engender reminding of the initial memory and inappropriate strengthening – leading to exceptionally strong delusional memories (Corlett et al., 2009). Reactivating fear memories in human subjects under the influence of ketamine enhances subsequent expression of those memories (Corlett et al., 2013). Vulnerability to this enhancement is predicted by the neural prediction error signal and the severity of ketamine induced psychosis (Corlett et al., 2013), providing initial support for the model – delusions are formed and maintained due to aberrant prediction errors (Corlett et al., 2009). In the present study we sought to replicate and extend this finding in a preclinical setting.
Our understanding of reconsolidation in rodents is much greater and more nuanced than that in human subjects. From preclinical work, we know memory reconsolidation is composed of at least two separate processes, destabilization, and re-stabilization (Lee, 2008). The latter process depends on similar processes to consolidation [such as protein synthesis and gene transcription (Tronson and Taylor, 2007)], however, memory reconsolidation is molecularly distinct from consolidation (Lee et al., 2004). The former, destabilization, process is dependent on prediction error (Sevenster et al., 2012, 2013; Winters et al., 2009) and GluN2B containing NMDA receptor activity (Ben Mamou et al 2006; Milton et al 2013). We examined the effect of ketamine on auditory fear reconsolidation in rodents, which is known to be critically dependent on the basolateral amygdala (BLA). We predicted an effect of ketamine on destabilization. Previous research has shown that while manipulations of re-stabilization are effective when administered prior to or immediately following memory reactivation (i.e. Nader et al 1999; Lee 2008), manipulations of memory destabilization are effective when administered prior to, but not immediately following memory retrieval (Ben Mamou et al., 2006). Following our model predictions (Corlett et al., 2009) and human data (Corlett et al., 2013), we hypothesized that ketamine would enhance fear memory when administered prior to, but not immediately following fear memory reconsolidation.
Methods
Subjects
Adult Sprague-Dawley male rats weighing about 300g (Charles River) were pair-housed and maintained on a 12-h light–dark cycle, with food and water provided ad libitum. All procedures were in accordance with the Yale Animal Resources Center, and were approved by the Yale Institutional Animal Care and Use Committee.
Apparatus
Context A
Med-Associates chambers lit by near-infrared light with inserted white plastic floors and rounded walls, and wiped down with 1% ascetic acid
Context B
The apparatus was changed by lighting it with diffuse white light, removing the plastic inserts rectangular plexiglass, acrylic and stainless steel aluminum walls, metal grid floors and wiped down with 70% ethanol
Fear Conditioning (Training)
Twenty-four hours following two days of exposure to Context A animals were trained in Context B. Following 180 seconds of acclimatization three 30 seconds tones (5000Hz 75dB) co-terminated with a mild foot shock (1” 0.7mA); the intertrial interval was 60 seconds and 30 seconds following the final shock they were removed from the chamber. To avoid ceiling effects following surgery in experiment #4 the shock intensity was reduced to 0.5mA.
Fear Conditioning (Reactivation)
Twenty-four hours after training animals were placed in Context A. After 180 seconds of acclimatization a 30 second tone was presented. Animals were removed from the context 30 seconds after the tone. All drug manipulations were performed either prior to, or immediately following this reactivation session.
Fear Conditioning (Testing)
Twenty-four hours after reactivation animals were returned to Context A and exposed to three 30 second tone presentation (ITI 60 seconds) following 180 seconds of acclimatization (post-reactivation long-term memory test [PR-LTM]). Tests of contextual fear were performed in the same context as training, Context B. For these test animals were placed in the chamber for 5 minutes.
Measurement of Freezing Behavior
The default settings for the Med-Associates automated freezing software have been calibrated for detecting mice (Anagnostaras et al., 2010). Using a similar approach we determined that to detect freezing behavior in rats optimal freezing was defined as the Motion Index being less than 75 for at least 15 frames (0.5 seconds). Auditory evoked freezing behavior was scored continuously for each of the three tones and analyzed as average percent. Context evoked freezing behavior was scored continuously for the duration of the test and analyzed as percent of total time.
Surgery
Rats were anesthetized with a combination of 87.5 mg/kg ketamine and 5 mg/kg xylazine. Rats were administered 5 ml of lactated Ringer's solution and 5 mg/kg of the analgesic Rimadyl before implantation with bilateral intracranial cannulae (3.0 mm posterior, 5.2 mm lateral and 8.0 mm ventral of bregma. 22 gauge; Plastics One) and given seven days to recover.
Drugs
Ketamine (dissolved in sterile saline,10mg/kg: Henry Schein, Melville, NY) was administered intraperitoneally (i.p.). In Experiment #4 ifenprodil (2µg/µl for two minutes at 0.25µl/minute: Sigma-Aldrich, St. Louis, MO) or vehicle (sterile saline) was infused into the BLA.
Histological Assessment
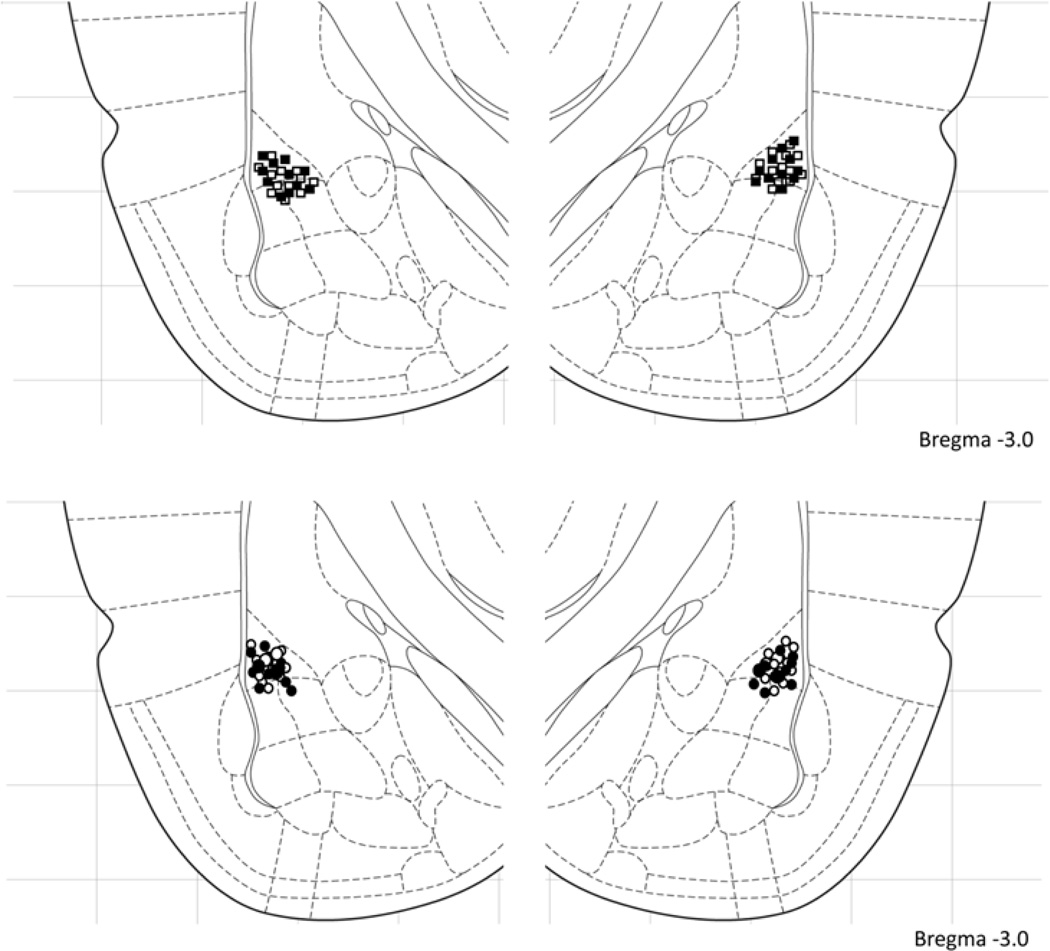
At the termination of the experiment, rats anesthetized (90mg/kg sodium pentobarbital, i.p.) and stored in 10% formalin/20% sucrose. Their brains were sectioned at 50µm thickness and examined with light microscopy for cannula placement. After histological verification, only animals that had cannula into the BLA were included in the present report (Fig. 2). Verification of infusion location was made by an experimenter blind to the experimental condition and behavioral performance of the animal.
Figure 2. Cannula placement in the amygdala.
Empty squares (Ifenprodil – Vehicle), filled squared (Ifenprodil – Ketamine), empty circles (Vehicle – Vehicle) and filled circles (Vehicle – Ketamine) indicate the location of the injecting cannula tip for the experiment depicted in figure 1D. All placements were within 0.5mm rostral-caudal of the section shown. [Figure adapted from Paxinos & Watson (Paxinos, 2005)]
Results
Twenty-four hours after training animals were separated into five groups Vehicle Pre-Reactivation, Vehicle Post-Reactivation, Ketamine Pre-Reactivation, Ketamine Post-Reactivation and Ketamine No Reactivation. Since no significant differences were observed between Vehicle Pre-Reactivation and Vehicle Post-Reactivation they were combined and will be referred to simply as Vehicle.
The first three groups (Vehicle, Ketamine Pre-Reactivation, Ketamine Post-Reactivation) were placed in context A and after 180 seconds of acclimatization were presented with a 30 second tone. Injections (10mg/kg ketamine or saline vehicle) were either given 15 minutes prior to being placed in the chamber or immediately after being removed. Ketamine No Reactivation animals remained in their home cage following their injection. Twenty-four hours later freezing was automatically scored.
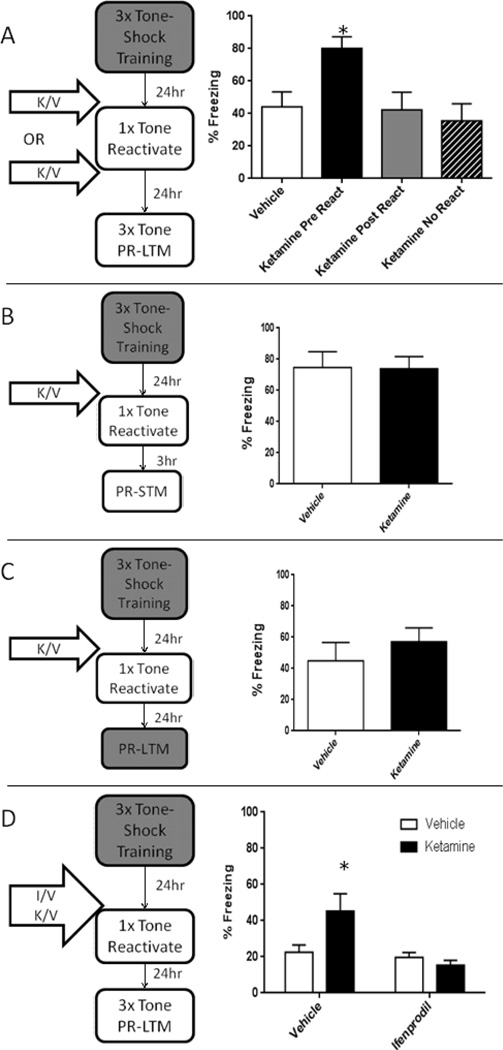
A one-way ANOVA found a significant effect of group (Figure 1A: F(3,44) = 4.45, p = 0.008). Dunnet’s multiple comparison showed that the Ketamine Pre-Reactivation group (p=0.005) but not the Ketamine Post-Reactivation (p=0.764) nor the Ketamine No Reactivation (p=0.624) group demonstrated elevated freezing compared to the Vehicle group. This suggests that systemic ketamine is enhancing destabilization of fear memory.
Figure 1. Ketamine strengthens reactivated memories in a manner dependent on BLA GluN2B.
Low dose systemic ketamine increased freezing in PR-LTM 24hrs later only when administered prior to fear memory reactivation (A). p< 0.05 compared to vehicle. One way ANOVA F(3,44) = 4.45, p = 0.008
Enhancements in freezing due to systemic ketamine prior to auditory fear memory reactivation are due to enhanced reconsolidation as there are no differences in PR-STM 3hrs following reactivation (B ); t(14) = 0.06, p = 0.95) and specific to the reactivated memory as there are no differences in freezing to the training context 24hrs following reactivation (C; t(22) = 0.86, p = 0.41)
Blocking a known mechanisms of fear memory destabilization blocks the enhancing effect of systemic ketamine (D). * p< 0.05 compared to vehicle. Ifenprodil/vehicle infusions were completed 15min prior to ketamine/vehicle injections. Two-way ANOVA; ME Ifenprodil F(1,47) = 8.16, p=0.006; ME Ketamine F(1,47) = 2.60, p=0.11; Interaction F(1,47) = 5.56, p=0.026.
The lack of an enhancement effect when there is no memory reactivation suggests that ketamine’s effects are specifically on reconsolidation mechanisms. It is unlikely that this increase in freezing is due a new association with the subjective effects of the drug. This dose of ketamine has been shown to produce conditioned place preference (Burgdorf et al., 2013) suggesting that it has appetitive, rather than aversive properties. Effects of manipulations of destabilization, like re-stabilization, should be seen in PR-LTM but not post-reactivation short term memory tests (PR-STM). Twenty-four hours following training, a different group of rats were given either 10mg/kg ketamine or vehicle injections 15 minutes prior to memory reactivation. Responses during reactivation were not significantly different. Three hours later there was no difference in freezing between vehicle- and ketamine-treated animals (Figure 1B t(14)=0.06, p = 0.95) . Typically, reactivation of a memory induces lability of directly, but not indirectly reactivated memories (Debiec et al., 2006; Doyere et al., 2007). Twenty-four hours after animals received either 10mg/kg ketamine or vehicle 15 minutes prior to auditory fear memory reactivation, we found no difference in a closely related fear memory of the training context (Figure 1C t(22) = 0.86, p = 0.41). These findings strengthen the interpretation that ketamine is enhancing the specific destabilization of a reactivated memory and strengthening that memory rather than altering the engagement of extinction learning.
To confirm that our ketamine manipulation was enhancing BLA specific destabilization mechanisms, we took advantage of prior research that has shown that pre-reactivation intra-BLA ifenprodil is capable of blocking the destabilization of auditory fear memory (Ben Mamou et al., 2006; Milton et al., 2013). Twenty-four hours after training, ifenprodil (2µg/µl for two minutes at 0.25µl/minute) or vehicle (sterile saline) was infused into the BLA 15 minutes prior to ketamine (10mg/kg i.p.) or vehicle injection. Fifteen minutes later all rats were placed in the chamber and were presented with a single 30 second tone. Data collected the next day, using a two-way ANOVA, showed a significant main effect of ifenprodil (Figure 1D F(1,47) = 8.16, p=0.006) but not ketamine (F(1,47) = 2.60, p=0.11) and a significant interaction (F(1,47) = 5.56, p=0.026). Dunnet’s multiple comparison showed that the Vehicle-Ketamine group (p=0.035) but not the Ifenprodil-Vehicle (p=0.568) nor the Ifenprodil-Ketamine (p=0.173) group were significantly greater than the Vehicle-Vehicle group. Thus, by blocking a known destabilization mechanism we were able to prevent ketamine’s effect on fear memory reconsolidation. This further supports our proposed model that ketamine enhances memory by specifically enhancing the destabilization of auditory fear memory.
Discussion
We determined the effect of memory reactivation under ketamine infusion in rodents. Reactivation of an aversive memory under ketamine was associated with elevated responding to that cue 24 hours later. This effect was blocked by an infusion of ifenprodil into the BLA. Given that ifenprodil blocks memory destabilization (Ben Mamou et al., 2006; Milton et al., 2013), we suggest that ketamine strengthens memories reactivated in its presence by enhancing the destabilization of those memories. Memories that are frequently destabilized and reactivated are laid down more strongly (Inda et al., 2011). Destabilization is also dependent on prediction error (Sevenster et al., 2012, 2013). Memories are only destabilized if their reactivation entails some updating, signaled by prediction error (Eisenhardt and Menzel, 2007; Pedreira et al., 2004; Sevenster et al., 2012, 2013; Winters et al., 2009). Our previous human work demonstrated that ketamine engages aberrant prediction errors in human subjects (Corlett et al., 2006), that ketamine strengthens reactivated memories (Corlett et al., 2013) and that neural prediction error signals correlate with that strengthening effect (Corlett et al., 2013). Here we replicated that effect in rodents. We also extend that work by implicating GluN2B receptors in the amygdala as an important mechanism in the strengthening effect of ketamine on reactivated memories.
In rodent, primate and human work (Li et al., 2011; Roesch et al., 2012), the amygdala has been shown to code a prediction error signal that guides attentional allocation and associability [the degree to which predictive stimuli are entered into associative relationships (Le Pelley, 2004; Mackintosh, 1975; Pearce and Hall, 1980)] in cortical regions (particularly the anterior cingulate and dorsolateral prefrontal cortices) as an unsigned prediction error signal (Klavir et al., 2013). Ifenprodil infusions into the BLA block these prediction error signals (Cole and McNally, 2009). We suggest that our ifenprodil infusions in the BLA blocked the aberrant prediction error signals engendered by ketamine and thereby curtailed its memory strengthening effect.
Despite replicating our prior human work (Corlett et al., 2013), it is perhaps surprising that ketamine [a non-competitive NMDA receptor antagonist with known amnestic effects (Fletcher and Honey, 2006)] appears to enhance the strength of reactivated memories. Initial pre-clinical studies of pharmacological effects on reconsolidation have demonstrated that NMDA receptor antagonism impairs or prevents memory reconsolidation (Lee and Everitt, 2008; Milton et al., 2008).
Three observations might address this apparent paradox
First, many studies in which NMDA antagonism blocked reconsolidation and erased memories, the receptor manipulations were made after memory reactivation (Lee and Everitt, 2008; Milton et al., 2008), not before as done in the current experiment. Here we studied the effects of ketamine on memory destabilization. Previous studies with NMDA antagonists administered after memory reactivation established the effects of NMDA blockade on memories that were already destabilized and updated. Post reactivation NMDA antagonism should only impact memory restabilization and not destabilization.
Second, most preclinical studies that blocked NMDA receptors and degraded memories employed MK-801 or Phencyclidine as NMDA antagonists (Lee and Everitt, 2008; Milton et al., 2008). These drugs have a much higher potency at the NMDA receptor and presumably have very different effects on neural activity and connectivity as a result (Anticevic et al., 2012). We should note that there are studies where MK-801 was administered prior to memory reactivation where effects on re-stabilization have been observed (Lee et al., 2006). The activity of MK-801 at NMDA receptors is higher than that of ketamine (MacDonald et al., 1991). One explanation of the difference between our study and prior work could be this differential engagement of NMDA receptors by the two drugs. Our prior computational work suggests an inverted U relationship between NMDA blockade and neural network dis-inhibition (Murray et al., 2014). This could lead to differential effects of the two drugs, ketamine strengthening and MK-801 degrading reactivated memories. Dose dependent effects of NMDA blockade with memantine (an NMDA receptor antagonist weaker still than ketamine) prior to reactivation have been reported in day-old chicks (Samartgis et al., 2012).
Finally, recent work has highlighted the role of the subcomponents of the NMDA receptor in discerning its role in particular processes as well as the impact of pharmacological manipulations on those processes. NMDA receptors are tetrameric structures assembled from two obligatory GluN1 subunits and two GluN2A (formerly NR2A) or GluN2B (formerly NR2B) subunits (Jimenez-Sanchez et al., 2014). Milton and colleagues showed that GluN2A subunits are selectively involved in re-stabilizing memories and GluN2B are important for memory de-stabilization (Milton et al., 2013). It is unclear at which subunit ketamine has preferential activity. Some work has suggested a preferential effect of ketamine on GluN2A in inducing its effects on prefrontal cortical gamma oscillations, in the absence of a role for GluN2B in this signaling change (Kocsis, 2012). Preferential effects of ketamine on GluN2A would account for the deleterious impact of post-reactivation ketamine on memory stabilization (Milton et al., 2013). However, in visual cortex, ketamine slowed down high frequency gamma oscillations thought to underpin top-down effects of experience on perception, as did selective antagonism of GluN2B (Anver et al., 2011). Likewise, GluN2B is crucial for the effects of ketamine on working memory maintenance (Wang et al., 2013). It is notable that ketamine also impacts inhibitory GABA currents (McNally et al., 2011), and may induce presynaptic glutamate release (Jackson et al., 2004; Moghaddam et al., 1997). Taken together, it is possible that in some task contexts and some brain regions, ketamine might induce glutamate spillover and actually stimulate GluN2B, this spillover would mediate the strengthening effect we observed and its reversal with ifenprodil which preferentially antagonizes GluN2B (Williams, 2001).
Prior work on memory destabilization and updating has focused on the role of voltage-gated calcium channels. Blocking these channels with nimodipine attenuates prediction error driven memory updating in rodents (De Oliveira Alvares et al., 2013). Likewise, nimodipine reverses the psychotomimetic effects of ketamine in human volunteers (Krupitsky et al., 2001). Ifenprodil may also regulate voltage-gated calcium channel signaling in the amygdala (Delaney et al., 2012). Future work with more specific pharmacological tools will establish the role of GluN2B and voltage-gated calcium channels in the impact of ketamine on reactivated memories.
These prediction error driven effects of reactivation may also increase the precision of the memory, the more it is retrieved the more the relevant elements are consolidated (De Oliveira Alvares et al., 2013). However, repeated retrieval can also distort the content of the memory (Estes, 1997). In a computational model of episodic memory formation and retrieval, elevating the magnitude of prediction error can encourage errors in episodic memory relevant to psychosis – the confusion of which agents were involved in an episode (Hoffman et al., 2011). Our results suggest that GluN2B subunits and calcium signaling in the amygdala may be a candidate mechanism for this effect.
The present observations may also be relevant to the apparent anti-depressant effects of NMDA antagonism (Berman et al., 2000). Some authors have suggested that GluN2B sub-units mediate those effects (Jimenez-Sanchez et al., 2014). Given that ketamine is administered to patients in a clinical setting, this work, taken with our previous human work, suggests some caution with regards to the psychological state of the subjects during the infusion: if their depressive schema are activated, we might expect strengthening. We have observed such effects in at least two subjects with a history of trauma (Niciu et al., 2013). Likewise, post-traumatic stress disorder could be worse in subjects administered with ketamine proximal to the trauma (Schonenberg et al., 2008), although contradictory results also exist (McGhee et al., 2008). Given that ketamine enhances the destabilization of memories reactivated in its presence, we might even turn this destabilization to our clinical advantage – for example by arranging for extinction therapy in the aftermath of a ketamine infusion (Monfils et al., 2009).
In conclusion, we present evidence for aberrant memory strengthening following memory reactivation during ketamine infusion. Antagonizing GluN2B sub-units in the BLA blocked this strengthening effect. We argue that this BLA manipulation blocked the prediction error driven destabilization of the fear memory and hence prevented its updating and strengthening. We believe this observation represents a first step towards an animal model of a hitherto under explored process in behavioral neuroscience, the fixity of delusional beliefs that attend psychotic mental illnesses like schizophrenia (Corlett et al., 2009; Corlett et al., 2010). GluN2B in the striatum and prefrontal cortex are also crucial for initial appetitive learning and its reversal (Brigman et al., 2013). Future work will establish the impact of ketamine on reactivated appetitive memories. In an initial human study, they too were strengthened when reactivated on ketamine (Corlett et al., 2013). Furthermore, future work will characterize the impact of memory reactivation during acute endogenous psychosis to establish the relevance of our model to clinical delusions. One such study has demonstrated an enhancement of the illusory truth effect (Begg et al., 1992) in psychotic patients (Moritz et al., 2012) – merely considering the truth-value associated with a delusion-related statement enhances subsequent memory and endorsement of that statement weeks later. We appreciate that this animal model is concerned with one aspect of delusions; their fixity rather than their bizarre contents, however, fixity (and associated distress) may be pathognomonic of delusions – as Emmanuelle Peters writes; when distinguishing delusions from other odd beliefs: “It is not what you believe but how you believe it” (Peters et al., 2004). There are animal learning phenomena that may be relevant to content, for example Kamin blocking (Kamin, 1969) assays the degree to which animals learn to ignore irrelevant stimuli and it is weaker in animals treated with amphetamine [a pharmacological model of psychosis (Crider et al., 1982)]. We feel ours is the first animal model to address the fixity and elasticity of delusions. A crucial next step will be to attempt to ameliorate this strengthening effect with antipsychotic drugs. With greater understanding of these aberrant strengthening mechanism we hope to design better treatments for fixed delusions that address the unmet clinical need of intractable fixed delusions despite adequate dosing with currently available antipsychotic drugs (Curson et al., 1985; Kane, 1996). Animal models, like ours, with face and construct validity for the symptoms at hand will facilitate that process.
Acknowledgements
Role of the funding source
Supported by PHS DA015222, the Ribicoff Research Facilities, the CT Department of Mental Health. Dr. Corlett is also supported by the Connecticut State Department of Mental Health and Addiction Services, the National Center for PTSD, an IMHRO / Janssen Rising Star Translational Research Award and CTSA Grant Number UL1 TR000142 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We thank Dr. Mary Torregrossa and members of the Division of Molecular Psychiatry for valuable feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MH – Designed the studies, Conducted the studies, Wrote the manuscript
JRT – Designed the studies, Wrote the manuscript
PRC - Designed the studies, Wrote the manuscript
Disclosures: The authors report no biomedical financial conflicts of interest
References
- Anagnostaras SG, Wood SC, Shuman T, Cai DJ, Leduc AD, Zurn KR, Zurn JB, Sage JR, Herrera GM. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Frontiers in behavioral neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, Ramani R, Smith MA, Wang XJ, Krystal JH, Corlett PR. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anver H, Ward PD, Magony A, Vreugdenhil M. NMDA receptor hypofunction phase couples independent gamma-oscillations in the rat visual cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(2):519–528. doi: 10.1038/npp.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg IM, Anas A, Farinacci S. Dissociation of Processes in Belief - Source Recollection, Statement Familiarity, and the Illusion of Truth. Journal of Experimental Psychology-General. 1992;121(4):446–458. [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nature neuroscience. 2006;9(10):1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nature neuroscience. 2013;16(8):1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(5):729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, McNally GP. Complementary roles for amygdala and periaqueductal gray in temporal-difference fear learning. Learning & memory. 2009;16(1):1–7. doi: 10.1101/lm.1120509. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Aitken MR, Dickinson A, Shanks DR, Honey GD, Honey RA, Robbins TW, Bullmore ET, Fletcher PC. Prediction error during retrospective revaluation of causal associations in humans: fMRI evidence in favor of an associative model of learning. Neuron. 2004;44(5):877–888. doi: 10.1016/j.neuron.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Cambridge V, Gardner JM, Piggot JS, Turner DC, Everitt JC, Arana FS, Morgan HL, Milton AL, Lee JL, Aitken MR, Dickinson A, Everitt BJ, Absalom AR, Adapa R, Subramanian N, Taylor JR, Krystal JH, Fletcher PC. Ketamine effects on memory reconsolidation favor a learning model of delusions. PloS one. 2013;8(6):e65088. doi: 10.1371/journal.pone.0065088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Aitken MR, Dickinson A, Shanks DR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Robbins TW, Bullmore ET, Fletcher PC. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Archives of general psychiatry. 2006;63(6):611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Fletcher PC. From prediction error to psychosis: ketamine as a pharmacological model of delusions. Journal of psychopharmacology. 2007;21(3):238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Krystal JH, Taylor JR, Fletcher PC. Why do delusions persist? Frontiers in human neuroscience. 2009;3:12. doi: 10.3389/neuro.09.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Taylor JR, Wang XJ, Fletcher PC, Krystal JH. Toward a neurobiology of delusions. Progress in neurobiology. 2010;92(3):345–369. doi: 10.1016/j.pneurobio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider A, Solomon PR, McMahon MA. Disruption of selective attention in the rat following chronic damphetamine administration: relationship to schizophrenic attention disorder. Biological psychiatry. 1982;17(3):351–361. [PubMed] [Google Scholar]
- Curson DA, Barnes TR, Bamber RW, Platt SD, Hirsch SR, Duffy JC. Long-term depot maintenance of chronic schizophrenic out-patients: the seven year follow-up of the Medical Research Council fluphenazine/placebo trial. III. Relapse postponement or relapse prevention? The implications for long-term outcome. The British journal of psychiatry : the journal of mental science. 1985;146:474–480. doi: 10.1192/bjp.146.5.474. [DOI] [PubMed] [Google Scholar]
- De Oliveira Alvares L, Crestani AP, Cassini LF, Haubrich J, Santana F, Quillfeldt JA. Reactivation enables memory updating, precision-keeping and strengthening: exploring the possible biological roles of reconsolidation. Neuroscience. 2013;244:42–48. doi: 10.1016/j.neuroscience.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Power JM, Sah P. Ifenprodil reduces excitatory synaptic transmission by blocking presynaptic P/Q type calcium channels. Journal of neurophysiology. 2012;107(6):1571–1575. doi: 10.1152/jn.01066.2011. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. The American journal of psychiatry. 2012;169(11):1203–1210. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- Dennett D. Do animals have beliefs. In: Roitblat HL, Meyer JA, editors. Comparative Approaches to Cognitive Science. Cambrudge Massachusetts; London, England: MIT Press; 1995. [Google Scholar]
- Dickinson A. The 28th Bartlett Memorial Lecture. Causal learning: an associative analysis. Q J Exp Psychol B. 2001;54(1):3–25. doi: 10.1080/02724990042000010. [DOI] [PubMed] [Google Scholar]
- Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nature neuroscience. 2007;10(4):414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Eisenhardt D, Menzel R. Extinction learning, reconsolidation and the internal reinforcement hypothesis. Neurobiol Learn Mem. 2007;87(2):167–173. doi: 10.1016/j.nlm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Estes WK. Processes of memory loss, recovery, and distortion. Psychological review. 1997;104(1):148–169. doi: 10.1037/0033-295x.104.1.148. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Anderson JM, Shanks DR, Honey R, Carpenter TA, Donovan T, Papadakis N, Bullmore ET. Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nature neuroscience. 2001;4(10):1043–1048. doi: 10.1038/nn733. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Honey GD. Schizophrenia, ketamine and cannabis: evidence of overlapping memory deficits. Trends in cognitive sciences. 2006;10(4):167–174. doi: 10.1016/j.tics.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Gibbs AA, David AS. Delusion formation and insight in the context of affective disturbance. Epidemiologia e psichiatria sociale. 2003;12(3):167–174. doi: 10.1017/s1121189x00002943. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Grasemann U, Gueorguieva R, Quinlan D, Lane D, Miikkulainen R. Using computational patients to evaluate illness mechanisms in schizophrenia. Biological psychiatry. 2011;69(10):997–1005. doi: 10.1016/j.biopsych.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(5):1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(22):8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Sanchez L, Campa L, Auberson YP, Adell A. The Role of GluN2A and GluN2B Subunits on the Effects of NMDA Receptor Antagonists in Modeling Schizophrenia and Treating Refractory Depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin L. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and Aversive Behavior. New York: Appleton-Century-Crofts; 1969. [Google Scholar]
- Kane JM. Treatment-resistant schizophrenic patients. The Journal of clinical psychiatry. 1996;57(Suppl 9):35–40. [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron. 2013;80(5):1290–1300. doi: 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biological psychiatry. 2012;71(11):987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Romanova TN, Grinenko NI, Grinenko AY, Fletcher J, Petrakis IL, Krystal JH. Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25(6):936–947. doi: 10.1016/S0893-133X(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(20):5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pelley ME. The role of associative history in models of associative learning: a selective review and a hybrid model. The Quarterly journal of experimental psychology. B, Comparative and physiological psychology. 2004;57(3):193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nature neuroscience. 2008;11(11):1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiology of learning and memory. 2008;90(1):147–154. doi: 10.1016/j.nlm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304(5672):839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(39):10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nature neuroscience. 2011;14(10):1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP. Treatment refractory schizophrenia. The Psychiatric quarterly. 2000;71(4):373–384. doi: 10.1023/a:1004640408501. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. The Journal of physiology. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological review. 1975;82:276–298. [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH. The correlation between ketamine and posttraumatic stress disorder in burned service members. The Journal of trauma. 2008;64(2 Suppl):S195–S198. doi: 10.1097/TA.0b013e318160ba1d. Discussion S197–-198. [DOI] [PubMed] [Google Scholar]
- McNally JM, McCarley RW, McKenna JT, Yanagawa Y, Brown RE. Complex receptor mediation of acute ketamine application on in vitro gamma oscillations in mouse prefrontal cortex: modeling gamma band oscillation abnormalities in schizophrenia. Neuroscience. 2011;199:51–63. doi: 10.1016/j.neuroscience.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. The American journal of psychiatry. 1995;152(2):183–190. doi: 10.1176/ajp.152.2.183. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(33):8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ. Double dissociation of the requirement for GluN2B- and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(3):1109–1115. doi: 10.1523/JNEUROSCI.3273-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160(827):554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S, Kother U, Woodward TS, Veckenstedt R, Dechene A, Stahl C. Repetition is good? An Internet trial on the illusory truth effect in schizophrenia and nonclinical participants. Journal of behavior therapy and experimental psychiatry. 2012;43(4):1058–1063. doi: 10.1016/j.jbtep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJ. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cerebral cortex. 2014;24(4):859–872. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Grunschel BD, Corlett PR, Pittenger C, Bloch MH. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. Journal of psychopharmacology. 2013;27(7):651–654. doi: 10.1177/0269881113486718. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic; 2005. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological review. 1980;87(6):532–552. [PubMed] [Google Scholar]
- Pedreira ME, Perez-Cuesta LM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learning & memory. 2004;11(5):579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al Delusions Inventory (PDI) Schizophrenia bulletin. 2004;30(4):1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, McKenna PJ, Bullmore ET, Fletcher PC. Psychological effects of ketamine in healthy volunteers. Phenomenological study. The British journal of psychiatry : the journal of mental science. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Roesch MR, Esber GR, Li J, Daw ND, Schoenbaum G. Surprise! Neural correlates of Pearce-Hall and Rescorla-Wagner coexist within the brain. The European journal of neuroscience. 2012;35(7):1190–1200. doi: 10.1111/j.1460-9568.2011.07986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartgis JR, Schachte L, Hazi A, Crowe SF. Memantine facilitates memory consolidation and reconsolidation in the day-old chick. Neurobiology of learning and memory. 2012;97(4):380–385. doi: 10.1016/j.nlm.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Schonenberg M, Reichwald U, Domes G, Badke A, Hautzinger M. Ketamine aggravates symptoms of acute stress disorder in a naturalistic sample of accident victims. Journal of psychopharmacology. 2008;22(5):493–497. doi: 10.1177/0269881107082481. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiology of learning and memory. 2012;97(3):338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339(6121):830–833. doi: 10.1126/science.1231357. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8(4):262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Turner DC, Aitken MR, Shanks DR, Sahakian BJ, Robbins TW, Schwarzbauer C, Fletcher PC. The role of the lateral frontal cortex in causal associative learning: exploring preventative and super-learning. Cerebral cortex. 2004;14(8):872–880. doi: 10.1093/cercor/bhh046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77(4):736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Current drug targets. 2001;2(3):285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Winters BD, Tucci MC, DaCosta-Furtado M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learning & memory. 2009;16(9):545–553. doi: 10.1101/lm.1509909. [DOI] [PubMed] [Google Scholar]