Abstract
Loneliness is strongly linked to poor health. Recent research suggests that appetite dysregulation provides one potential pathway through which loneliness and other forms of social disconnection influence health. Obesity may alter the link between loneliness and appetite-relevant hormones, one unexplored possibility. We examined the relationships between loneliness and both post-meal ghrelin and hunger, and tested whether these links differed for people with a higher versus lower body mass index (BMI; kg/m2). During this double-blind randomized crossover study, women (N = 42) ate a high saturated fat meal at the beginning of one full-day visit and a high oleic sunflower oil meal at the beginning of the other. Loneliness was assessed once with a commonly used loneliness questionnaire. Ghrelin was sampled before the meal and post-meal at 2 and 7 hours. Self-reported hunger was measured before the meal, immediately post-meal, and then 2, 4, and 7 hours later. Lonelier women had larger postprandial ghrelin and hunger increases compared with less lonely women, but only among participants with a lower BMI. Loneliness and postprandial ghrelin and hunger were unrelated among participants with a higher BMI. These effects were consistent across both meals. These data suggest that ghrelin, an important appetite-regulation hormone, and hunger may link loneliness to weight gain and its corresponding negative health effects among non-obese people.
Keywords: loneliness, ghrelin, hunger, obesity, interpersonal relationships
1. Introduction
The desire for close and caring relationships motivates a wide range of human behavior (Leary and Cox, 2008; Maslow, 1968). This need to belong likely stems from the importance of group living for humans’ survival throughout their evolutionary past; being allied with a network of people who were mutually invested in each other’s welfare increased the likelihood of survival and the potential to thrive (Tooby and Cosmides, 1996). Over time, this ultimately developed into a basic need to form close and caring bonds with other people (Baumeister and Leary, 1995).
Because the need for social connection is fundamental to human nature, the failure to fulfill this need should have negative mental and physical health consequences. Indeed, loneliness, an interpersonally stressful state of perceived social disconnection, is strongly linked to poor health (Hawkley and Cacioppo, 2010). For example, lonely people had 45% lower odds of survival compared with those who were not lonely, even after accounting for important sociodemographic and health-relevant risk factors (Holt-Lunstad et al., 2010). In addition, lonelier people experienced more chronic diseases, reported worse physical health, and were more likely to develop coronary heart disease than those who were less lonely (Sugisawa et al., 1994; Thurston and Kubzansky, 2009).
Appetite dysregulation is one potential pathway through which loneliness and other forms of social disconnection may influence health. A person’s appetite and eating behavior are strongly linked to obesity (Arora and Anubhuti, 2006), which contributes to a host of medical problems, including type 2 diabetes, cardiovascular disease, and premature mortality (Billington et al., 2000). Ghrelin, an appetite-stimulating hormone, fuels food consumption (Klok et al., 2007). For instance, pre-meal hunger increases are related to similar rises in ghrelin (Cummings et al., 2004). Furthermore, ghrelin reliably surges before a meal and decreases after eating (Cummings et al., 2001). One novel study demonstrated that a ghrelin injection caused people to feel hungrier and consume more food compared with saline (Wren et al., 2001).
Recent research supports the argument that social disconnection may be implicated in appetite dysregulation. Specifically, non-obese women who experienced more interpersonal stressors had higher ghrelin and lower leptin, an appetite-suppressing hormone, than non-obese women who experienced fewer interpersonal stressors (Jaremka et al., 2014b). Supporting the hormone data, non-obese women who experienced more interpersonal stressors had a typical diet that was significantly higher in calories, fat, carbohydrates, protein, sugar, sodium, and fiber, and marginally higher in cholesterol, vegetables (but not fruits), vitamin A, and vitamin C. These data demonstrate that interpersonal stressors are linked to appetite-relevant hormones and food consumption. However, it is unclear if these results extend to other forms of social disconnection, such as loneliness.
Obesity may alter the link between social disconnection and ghrelin, one unexplored possibility. Obese people have lower fasting ghrelin levels than those who are overweight or a healthy weight (Buss et al., 2014; Klok et al., 2007; Shiiya et al., 2002). They also have lower postprandial ghrelin compared with normal weight people (Carlson et al., 2009; le Roux et al., 2005). Importantly, a recent study demonstrated that ghrelin was linked to caloric intake and hedonic eating among overweight but not obese people (Buss et al., 2014). Taken together, these data suggest that ghrelin may differentially affect obese compared with non-obese people. Accordingly, the link between social disconnection and ghrelin may be attenuated among obese people. Based on this rationale, we examined whether loneliness would be related to post-prandial ghrelin and hunger increases for people with a lower body mass index (BMI; kg/m2), but not among people with a higher BMI. We conducted secondary analyses from a parent study about fast-food-type-meals and fatigue to investigate these possibilities.
2. Method
2.1 Participants
Participants were recruited from two prior lab studies for a parent study about fast-food-type-meals and fatigue. Due to timing issues related to questionnaire collection, only women who participated in one of the two past studies comprised our analytic sample. One of these participants had diabetes and was thus excluded, leaving a total of 42 women. Due to the nature of the parent study, both breast cancer survivors (n=22) and non-cancer controls (n=20, women who had an initial abnormal mammogram) participated in the study. Survivors averaged 11.96 months (SD=4.15) since treatment completion. Individuals were ineligible if they had significant visual, auditory, or cognitive impairments, any prior history of cancer except basal or squamous cell skin carcinomas, symptomatic ischemic heart disease, chronic obstructive pulmonary disease, liver or kidney failure, or severe gastrointestinal problems. We also excluded women with major immune-mediated conditions, and anyone who abused alcohol or drugs or used medications with major immunological consequences. The women’s average age was 53.38 years (SD=9.02, range 31–75) and they were primarily white (79%). Additional sample characteristics can be found in Table 1.
Table 1.
Study sample characteristics.
| Characteristic | Category | Cancer survivors | Benign controls | ||
|---|---|---|---|---|---|
| Number(%) | Mean(SD) | Number(%) | Mean(SD) | ||
| Race | White | 17(77.3) | --- | 16(80.0) | --- |
| Black | 4(18.2) | --- | 4(20.0) | --- | |
| Other | 1(4.5) | --- | 0(0.0) | --- | |
|
| |||||
| Education | High school or below | 2(9.1) | --- | 3(15.0) | --- |
| Some college/College graduate | 16(72.7) | --- | 13(65.0) | --- | |
| Graduate/Professional training | 4(18.2) | --- | 4(20.0) | --- | |
|
| |||||
| Marital Status | Single | 4(18.2) | --- | 2(10.0) | --- |
| Married/Domestic partner | 13(59.0) | --- | 15(75.0) | --- | |
| Separated/divorced/widowed | 5(22.7) | --- | 3(15.0) | --- | |
|
| |||||
| Cancer Stage | 0 | 6(27.3) | --- | --- | --- |
| I | 7(31.8) | --- | --- | --- | |
| II | 7(31.8) | --- | --- | --- | |
| III | 2(9.1) | --- | --- | --- | |
|
| |||||
| Cancer Treatment | Surgery only | 3(13.6) | --- | --- | --- |
| Surgery and radiation | 8(36.4) | --- | --- | --- | |
| Surgery and chemotherapy | 6(27.3) | --- | --- | --- | |
| Surgery, chemotherapy, and radiation | 5(22.7) | --- | --- | --- | |
|
| |||||
| SERM Use | No | 14(64.0) | --- | --- | --- |
| Yes | 8(36.0) | --- | --- | --- | |
|
| |||||
| Menopausal Status | Pre | 4(18.2) | --- | 7(35.0) | --- |
| Post | 18(81.8) | --- | 13(65.0) | --- | |
|
| |||||
| HRT | No | 20(91.0) | --- | 18(90.0) | --- |
| Yes | 0(0) | --- | 0(0) | --- | |
| Missing | 2(9.0) | --- | 2(10) | --- | |
|
| |||||
| Months since Tx | N/A | --- | 11.96(4.15) | --- | --- |
|
| |||||
| Age | N/A | --- | 51.95(7.80) | --- | 54.95(10.16) |
|
| |||||
| Loneliness | N/A | --- | -.08(.68) | --- | .10(.85) |
|
| |||||
| BMI* | N/A | --- | 29.06(5.34) | --- | 26.72(4.07) |
Note. N = 42. Percentages reflect the proportion of participants within their respective group (cancer survivor vs. benign control). HRT = hormonal replacement therapy. SERM = selective estrogen receptor modulator.
The numbers reflect the BMI measurement taken at the first study visit.
2.2 Study Procedure
Participants completed two full-day study visits at the Clinical Research Center (CRC), a hospital research unit. During this double-blind randomized crossover study, women ate a high saturated fat meal at the beginning of one visit and a high oleic sunflower oil meal at the beginning of the other. Visits were spaced 1–4 weeks apart, and the meal order was randomized between visits.
Women were told to avoid alcohol use within one day prior and strenuous physical activity within two days prior to their study visits (Lairon et al., 2007). Participants were also instructed to stop taking aspirin, vitamins, antioxidants, and any other dietary supplements for 7 days prior to each admission. On the day before each of the study visits, participants received three standardized meals from the CRC’s metabolic kitchen, reducing any variability associated with recent food intake.
At each admission, women arrived after fasting for 12 hours and a catheter was inserted in their arm. Following a short relaxation period, women had 20 minutes to eat the high saturated fat or high oleic sunflower oil meal; they were required to eat the entire meal. Ghrelin was sampled before the meal and post-meal at 2 and 7 hours. Self-reported hunger was assessed before the meal, immediately post-meal, and then 2, 4, and 7 hours later. This research was approved by The Ohio State University (OSU) Institutional Review Board; participants provided written informed consent before participating.
2.3 Standardized Pre-Study Meals
Equations from the Dietary Reference Intakes were used to determine total kcal requirements for each participant based on age, height, weight, and physical activity (Food and Nutrition Board, 2002). Macronutrient targets (as percent of total energy) for the pre-study meals were 54.9 + 2.68% carbohydrate, 27.6 + 2.13% fat, and 17.6 + 0.95% protein. The fat content was 9.10 + 1.20 % saturated fats, 9.43 + 1.55% monounsaturated fats, and 7.26 + 1.25% polyunsaturated fats. Participants ate their last meal no later than 7:30 pm the night before admission to the CRC; the dinner was light and low in fat (Lairon et al., 2007). Compliance was good: women consumed 91.83 + 8.41% of their pre-study meals.
2.4 Research Meals
Both the high saturated fat and the high oleic sunflower oil meals included eggs, turkey sausage, biscuits, and gravy for a total of 930 kcals, with 60 grams of fat, 59 grams of carbohydrates, and 36 grams of protein (percent of total kcals = 60, 25, 15, respectively). In line with prior research (Poppitt et al., 2008), the saturated:unsaturated fatty acid ratio varied between the meals; the high saturated fat meal contained 16.84 g palmitic and 13.5 g oleic (ratio=1.93) oil, compared to 8.64 g palmitic and 31.21 g oleic oil for the high oleic sunflower oil meal (ratio=0.67). The composition of the research meals was based on the parent study; some human studies have suggested that high saturated fat meals may fuel fatigue-inducing inflammatory responses, although others have not found these effects (Herieka and Erridge, 2014; Manning et al., 2008; Poppitt et al., 2008).
2.5 Questionnaires and Interviews
Loneliness was measured with the 8-item New York University Loneliness (NYUL) scale (Rubenstein and Shaver, 1982), which assessed the extent to which participants felt chronically alone and socially isolated. Individual items are measured on different metrics. Accordingly, each item was z-scored prior to creating the scale average (Rubenstein and Shaver, 1982). The NYUL scale demonstrates convergent validity with other loneliness measures and has good internal consistency (Rubenstein and Shaver, 1982; Russell, 1996). The NYUL was collected as part of a prior lab study that occurred an average of 5.50 (SD = 4.93) months before participation in the current investigation (see the participant section for additional details). Loneliness is relatively stable over time (Cacioppo et al., 2002; Kiecolt-Glaser et al., 2003). Accordingly, loneliness within the context of the present study could represent a pre-existing vulnerability for appetite dysregulation, a current risk factor, or a combination of the two, all of which are interesting possibilities.
Hunger was measured multiple times at each visit with a scale that was modeled after prior research (Flint et al., 2000; Harthoorn and Dransfield, 2008). Women were asked to rate how they felt at the current moment. The items were “How hungry are you?”, “How strong is your desire to eat?”, “How full do you feel?” (reversed), and “How satiated do you feel with the amount you have eaten? (reversed)” The scale demonstrated excellent reliability, α = .92.
The mood disorders module of the Structured Clinical Interview for DSM-IV Axis I disorders - Nonpatient version (SCID-NP) measured current syndromal depression. The SCID-NP is designed for rapid and valid DSM-IV diagnoses by clinically-trained interviewers (First et al., 1997). The SCID-NP was included to account for potential relationships between loneliness and syndromal depression (Jaremka et al., 2014a). Participants’ syndromal depression diagnosis was assessed at the study visit during which the loneliness data were collected.
Participants reported how many hours they slept the night before each CRC visit and their age. They also had their height and weight measured at the first visit.
2.6 Ghrelin Assay
All blood samples for a participant were collected via a catheter, frozen after collection, and analyzed within the same assay run. Determination for total ghrelin was made using the respective RIA kit per kit instructions with the following exception (Millipore Corporation, St. Charles, MO 63304). Ethylenediaminetetraacetic acid (EDTA) plasma is specified in the kit instructions. We only had EDTA plasma available for a small subset of participants, but we had heparin plasma available for everyone. Accordingly, we wanted to ensure that the results of the ghrelin assay using our heparin plasma samples would be similar to that of EDTA plasma samples. Following a conversation with the kit manufacturer, we conducted a pilot study to assess potential differences between EDTA and heparin plasma ghrelin levels. No significant differences were detected across plasma types, and thus heparin plasma samples were used for all participants. The intra-assay CV was 5.12% and inter-assay CV was 6.5%; sensitivity was 93 pg/ml.
2.7 Data Analytic Strategy
Linear mixed models were used to account for correlations within a participant, both within and across meals. A random subject-specific meal and time effect captured the within-subject correlations across meals (high saturated fat versus high oleic sunflower oil) and also across sample times (measured in hours post-meal). All analyses were conducted in SPSS version 19.0 (IBM, New York).
We investigated whether the combination of loneliness and BMI predicted postprandial ghrelin and hunger both in terms of average levels and their trajectory over time. Specifically, we tested whether loneliness (continuous), BMI (continuous), time (continuous), and the 3-way loneliness by BMI by time interaction (and all corresponding 2-way interactions) predicted postprandial ghrelin and hunger, controlling for pre-meal levels. Due to the complexity of the analytic model and our limited sample size, we first investigated a model with no covariates except for baseline ghrelin or hunger levels. For the primary analysis, we added potential confounds that were selected based on their theoretical and empirical relationships to loneliness, ghrelin, and hunger (Di Francesco et al., 2006; Knutson et al., 2007; Koliaki et al., 2010; Taheri et al., 2004). The adjusted models included age, syndromal depression, meal type, and sleep duration; we also added cancer status as a covariate due to the design of the parent study.
Following published recommendations (Aiken and West, 1991), signi cant 3-way interactions were decomposed into the 2-way loneliness by time interactions for people with a higher versus lower BMI (computed at ±1 SD from the mean). If a 2-way interaction was signi cant, simple slopes tests examined the effect of time for people who were more versus less lonely (computed at ±1 SD from the mean). All of these follow-up analyses included loneliness, BMI, and time as continuous variables and focused on specific points of interest along each continuum, as noted above.
Finally, an ancillary analysis examined whether meal type moderated any of our effects. We tested this possibility by adding fixed effects for the 4-way loneliness by BMI by time by meal-type interaction, and all corresponding 3- and 2-way interactions.
3. Results
Due to the nature of the parent study, the current sample consisted of both post-treatment breast cancer survivors and non-cancer controls. Accordingly, we first investigated whether these groups differed on the key predictors in our study, loneliness and BMI. Cancer survivors and non-cancer controls did not differ significantly in terms of BMI, t(40) = −1.59, p = .121, or loneliness, t(39) = 0.73, p = .467, suggesting similarity across our groups. As described previously, all of the adjusted analyses include cancer status as a covariate to further account for the sample composition of the parent study.
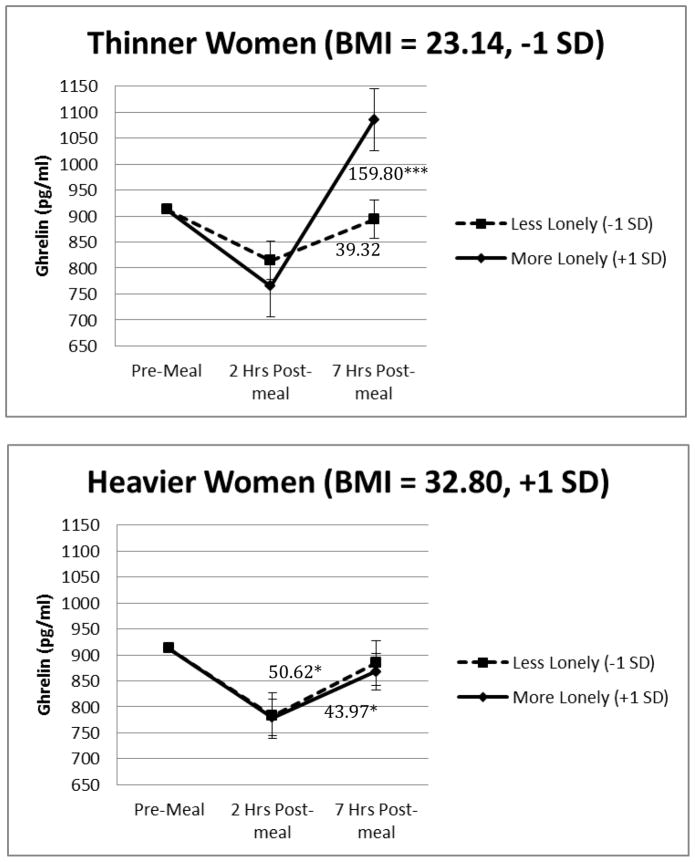
Preliminary analyses demonstrated that the 3-way loneliness by BMI by time interaction significantly predicted postprandial ghrelin in a model that was unadjusted except for baseline levels, F(1,36) = 5.22, p = .028. Importantly, the results remained the same in the primary analysis that included age, sleep duration, meal type, syndromal depression, and cancer status as covariates, F(1,36) = 5.32, p = .027, see Table 2. On average, postprandial ghrelin increased throughout the day. Furthermore, there was a signi cant 2-way loneliness by time interaction among people with a lower BMI, F(1,35) = 6.54, p = .015. As shown in Figure 1, among people with a lower BMI, those who were lonelier had larger ghrelin increases over time than those who were less lonely. The 2-way loneliness by time interaction for people with a higher BMI was non-significant (F(1,38) = 0.07, p = .798), demonstrating that loneliness did not affect changes in ghrelin over time for people with a higher BMI. Finally, we tested whether meal type moderated our effects. The 4-way loneliness by BMI by time by meal type interaction and the lower order 3- and 2-way interactions involving meal type were non-significant, p >.243, with the following exceptions. There was a significant meal by time interaction (F(1,36) = 8.99, p = .005), such that postprandial ghrelin increases were larger throughout the day after people ate the high saturated fat compared with the high oleic sunflower oil meal. There was also a significant meal by loneliness interaction (F(1,27) = 4.97, p = .034), such that the relationship between loneliness and average postprandial ghrelin levels was negative for the high saturated fat meal compared with a positive relationship after the high oleic sunflower oil meal. These meal interactions were not replicated with the hunger data and are thus not discussed further.
Table 2.
Complete statistics for the primary adjusted models predicting ghrelin levels.
| Outcome | b | Standard error | F | df | p value |
|---|---|---|---|---|---|
| Baseline ghrelin (pg/ml) | 0.51 | 0.08 | 39.98 | 1,69 | <.001 |
| Age | −3.41 | 2.79 | 1.50 | 1,21 | .234 |
| Sleep duration | 0.55 | 12.23 | 0.00 | 1,58 | .964 |
| Cancer status | −61.13 | 58.52 | 1.09 | 1,24 | .307 |
| Meal type | −10.41 | 18.86 | 0.30 | 1,26 | .586 |
| Syndromal depression | −62.11 | 125.54 | 0.25 | 1,21 | .626 |
| BMI (centered) | −6.30 | 5.60 | 1.27 | 1,23 | .272 |
| Time (centered) | 73.43 | 11.02 | 44.43 | 1,34 | <.001 |
| Loneliness (centered) | 20.40 | 44.16 | 0.21 | 1,22 | .649 |
| BMI by Time | −5.41 | 2.23 | 5.89 | 1,35 | .021 |
| BMI by Loneliness | −5.63 | 9.99 | 0.32 | 1,23 | .578 |
| Loneliness by Time | 37.94 | 17.44 | 4.73 | 1,35 | .036 |
| Loneliness by BMI by Time | −8.77 | 3.80 | 5.31 | 1,36 | .027 |
NOTE: b is the unstandardized beta coefficient. We report the coding of the loneliness, BMI, and time in order to facilitate interpretation of the interactions and corresponding main effects.
Figure 1.
Figures 1a and 1b. Loneliness, BMI, and time predicting ghrelin responses. The values depicted are the estimated marginal means obtained from a model that included baseline ghrelin levels, age, sleep duration meal type, cancer status, time (continuous), loneliness (continuous), BMI (continuous), the loneliness by BMI by time interaction and its corresponding 2-way interactions. The error bars refer to the standard error of the mean. *p<.05, ***p<.001
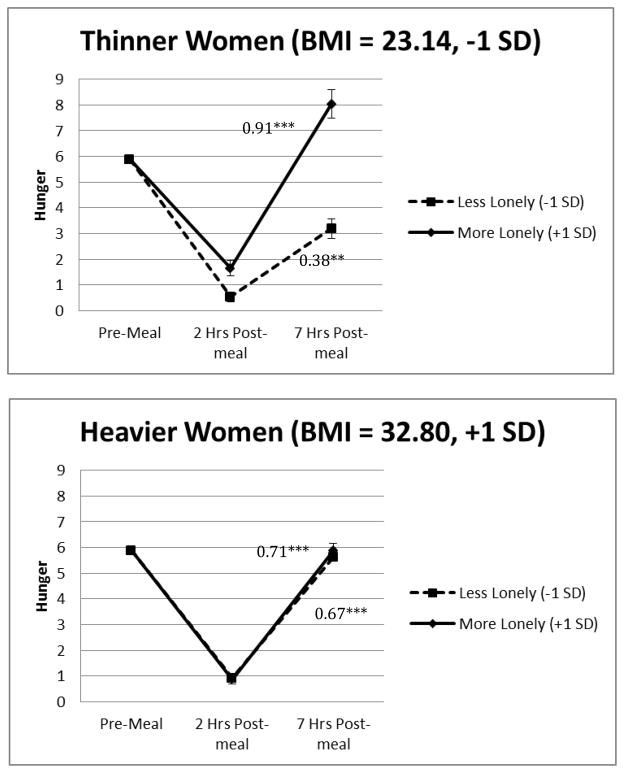
The hunger analyses revealed very similar loneliness-related results. Preliminary analyses demonstrated that the 3-way loneliness by BMI by time interaction significantly predicted postprandial hunger in a model that was unadjusted except for baseline levels, F(1,42) = 3.99, p = .052. Importantly, the results remained the same in the primary analysis that included age, sleep duration, meal type, syndromal depression, and cancer status as covariates, F(1,42) = 4.11, p = .049, see Table 3. On average, postprandial hunger increased throughout the day. Furthermore, there was a signi cant 2-way loneliness by time interaction among people with a lower BMI, F(1,41) = 6.48, p = .015. As shown in Figure 2, among people with a lower BMI, those who were lonelier had larger hunger increases over time than those who were less lonely. The 2-way loneliness by time interaction for people with a higher BMI was non-significant (F(1,40) = 0.12, p = .726), demonstrating that loneliness did not affect changes in hunger over time for people with a higher BMI. Finally, we tested whether meal type moderated our effects. The 4-way loneliness by BMI by time by meal type interaction and the lower order 3- and 2-way interactions involving meal type were non-significant, p >.175.
Table 3.
Complete statistics for the primary adjusted models predicting hunger.
| Outcome | b | Standard error | F | df | p value |
|---|---|---|---|---|---|
| Baseline hunger | 0.07 | 0.06 | 1.25 | 1,68 | .267 |
| Age | −0.03 | 0.02 | 3.38 | 1,35 | .075 |
| Sleep duration | −0.00 | 0.08 | 0.00 | 1,66 | .971 |
| Cancer status | −0.06 | 0.30 | 0.04 | 1,33 | .841 |
| Meal type | −0.13 | 0.16 | 0.66 | 1,38 | .422 |
| Syndromal depression | −1.01 | 0.67 | 2.26 | 1,32 | .142 |
| BMI (centered) | −0.02 | 0.03 | 0.44 | 1,36 | .511 |
| Time (immediate post-meal = 0) | 0.67 | 0.05 | 177.89 | 1,40 | <.001 |
| Loneliness (centered) | 0.36 | 0.24 | 2.13 | 1,37 | .153 |
| BMI by Time | 0.00 | 0.01 | 0.22 | 1,41 | .645 |
| BMI by Loneliness | −0.08 | 0.06 | 2.05 | 1,38 | .161 |
| Loneliness by Time | 0.19 | 0.08 | 6.01 | 1,40 | .019 |
| Loneliness by BMI by Time | −0.03 | 0.02 | 4.11 | 1,42 | .049 |
NOTE: b is the unstandardized beta coefficient. We report the coding of the loneliness, BMI, and time in order to facilitate interpretation of the interactions and corresponding main effects.
Figure 2.
Figures 2a and 2b. Loneliness, BMI and time predicting hunger. The values depicted are the estimated marginal means obtained from a model that included baseline hunger levels, age, sleep duration meal type, cancer status, time (continuous), loneliness (continuous), BMI (continuous), the loneliness by BMI by time interaction and its corresponding 2-way interactions. The error bars refer to the standard error of the mean. *p<.05, **p<.01, ***p<.001
4. Discussion
The current study demonstrated that lonelier women had larger postprandial ghrelin and hunger increases compared with less lonely women, but only among participants with a lower BMI. Loneliness and postprandial ghrelin and hunger were unrelated among participants with a higher BMI. Furthermore, the data suggest that lonelier women with a lower BMI had higher ghrelin levels and felt hungrier at the end of the day than both lonelier and less lonely women with a higher BMI. The meal type consumed at breakfast did not alter the links among loneliness, ghrelin, and hunger. Accordingly, loneliness was related to thinner women’s postprandial ghrelin and hunger increases after they ate a high saturated fat versus and a high sunflower oil meal.
Loneliness and other forms of social disconnection are strongly linked to poor health (Hawkley and Cacioppo, 2010; Holt-Lunstad et al., 2010; Sugisawa et al., 1994; Thurston and Kubzansky, 2009). One key question is to determine what mechanisms are driving these negative health effects; appetite dysregulation is one promising avenue. A recent study demonstrated that non-obese women who experienced more interpersonal stressors had higher ghrelin and lower leptin than those who experienced fewer interpersonal stressors (Jaremka et al., 2014b). The current data demonstrated that loneliness, another form of social disconnection, is linked to postprandial appetite dysregulation among people with a lower (but not higher) BMI. Accordingly, the present study expands upon prior work by revealing that the links among social disconnection, in this case loneliness, and ghrelin and hunger were only evident for non-obese people. These findings raise the question of how social disconnection, ghrelin, hunger, obesity, and weight gain are related. The present data support the possibility that loneliness may lead to weight gain among non-obese people because of its effects on ghrelin and hunger. However, the same process may not apply to people who are already obese, suggesting that loneliness does not maintain obesity via elevated ghrelin and hunger.
The current research extends prior research in a few additional directions. First, the present study demonstrated that the ghrelin results extend to self-reported hunger. Furthermore, prior research and the current data suggest that different forms of social disconnection (i.e., interpersonal stressors and loneliness) have shared features, which influence ghrelin and hunger levels. Theoretically, these forms of social disconnection both threaten human beings’ basic need to belong (Baumeister and Leary, 1995), which may be the core underlying commonality driving the effects.
Other researchers have proposed additional complimentary risk factors for appetite dysregulation, unhealthy eating habits, and weight gain. For instance, depression can significantly alter food intake. In fact, one of the diagnostic criteria for major depressive disorder in the DSM-5 is diet-relevant: weight gain/loss or hyperphagia/hypophagia (American Psychiatric Association, 2013). In addition, depressed people have a 58% increased risk of becoming obese (Luppino et al., 2010). The current results demonstrated that the links among BMI, loneliness, ghrelin, and hunger were independent of syndromal depression. Furthermore, prior research has demonstrated that loneliness is a risk factor for the development of depression (Cacioppo et al., 2010, 2006; Jaremka et al., 2014a). Accordingly, loneliness and depression may be two risk factors that could either work independently or in tandem to influence ghrelin and self-reported hunger.
One intriguing avenue for future research is investigating why loneliness was related to ghrelin and hunger. One possibility is that feeling hungry in response to interpersonal stress is socially adaptive. The need for social connection is fundamental to human nature. Consequently, feeling socially disconnected should motivate people to try and bond with others in order to restore their sense of belonging. Furthermore, eating and social connection are intricately linked; eating was a highly social activity throughout human evolution (Wrangham, 2010), and today meals are often eaten with other people. In addition, recent research demonstrated that eating comfort food caused people to spontaneously think about their relationships, and simply thinking about comfort food decreased loneliness (Troisi and Gabriel, 2011). Consequently, people may feel hungrier when they feel socially disconnected because they have either implicitly or explicitly learned that eating helps them feel socially connected and/or provides them with an opportunity for social connection. One caveat is that any links between eating and social connection may be attenuated among obese people, perhaps because obesity puts people at risk for social stigmatization and depression (Luppino et al., 2010; Puhl and Brownell, 2003)
Exploration of the physiological mechanisms that link social disconnection to appetite dysregulation represent another critical avenue for research. One rodent study demonstrated that a β3-adrenergic antagonist attenuated the effects of chronic social defeat on leptin production (Chuang et al., 2010). In addition, direct stimulation of the sympathetic nervous system in rats elevated ghrelin levels (Mundinger, 2006). Accordingly, sympathetic nervous system activation provides one promising mechanism. However, research examining the links among social disconnection, ghrelin, and hunger is in its infancy, particularly among humans.
The current research examined total ghrelin as a primary outcome. However, active ghrelin is particularly important for regulating food intake (Cummings et al., 2004), and the link between total and active ghrelin among lonelier versus less lonely people in unknown. Accordingly, future research examining a combination of appetite-relevant hormones, including both total and active ghrelin, would answer important questions about the specificity of the current results.
The loneliness data were collected as part of a prior study, and thus it is unclear if the results reflect feelings of loneliness at the time that the ghrelin and hunger data were collected. However, loneliness is relatively stable over time; one common loneliness measure has a .73 test-retest reliability at both 2-month and 1-year intervals (Cacioppo et al., 2002). Accordingly, loneliness could represent a pre-existing vulnerability, a current risk factor, or a combination of the two, all of which are interesting possibilities that are important to further investigate.
The present study consisted of women who were primarily white, one limitation of the current results. The study hypotheses were also designed and tested after data collection for the study was complete. Accordingly, researchers may gain additional insight by designing studies to a priori test the relationships among loneliness, ghrelin, and hunger in more diverse samples. The current sample size was relatively small, another limitation. However, women were assessed multiple times throughout the day on two separate visits, providing repeated measures for every participant. The results of the current data are also consistent with prior work, further bolstering the reliability of the present findings. Due to the nature of the parent study, the present sample consisted of both breast cancer survivors and non-cancer controls. Accordingly, it is possible that something specific to being a cancer survivor was driving the link between loneliness and ghrelin or hunger. However, the primary analyses adjusted for cancer status, demonstrating that the links among loneliness, ghrelin, and hunger were independent of whether the women had cancer. Nonetheless, additional studies using different medical and healthy populations are needed to further generalize the findings. Demonstrating similar links between loneliness and ghrelin or hunger among a sample of healthy adults would bolster the reliability of the current findings.
In sum, lonelier women had larger postprandial ghrelin and hunger increases compared with less lonely women, but only among participants with a lower BMI. Loneliness and postprandial ghrelin and hunger were unrelated among participants with a higher BMI. These data suggest that ghrelin, an appetite-regulating hormone, and hunger may link interpersonal stress to weight gain and its corresponding negative health effects among non-obese people.
Highlights.
Loneliness was linked to larger post-meal ghrelin rises among women with a lower BMI
Loneliness was linked to larger post-meal hunger rises among women with a lower BMI
Loneliness and post-meal ghrelin was unrelated among women with a higher BMI
Loneliness and post-meal hunger was unrelated among women with a higher BMI
These effects were consistent across two different meal types.
Acknowledgments
Work on this project was supported in part by NIH grants R21 CA154054, K05 CA172296, P30 CA016058, and UL1RR025755, American Cancer Society Postdoctoral Fellowship Grants PF-11-007-01-CPPB and 121911-PF-12-040-01-CPPB, and a Pelotonia Postdoctoral Fellowship from the Ohio State University Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage Publications, Inc; Thousand Oaks, CA US: 1991. [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM. Vol. 5. American Psychiatric Publishing, Inc; 2013. [Google Scholar]
- Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity: A review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117:497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Billington CJ, Epstein LH, Goodwin NJ, Hill JO, Pi-Sunyer FX, Rolls BJ, Stern J, Wadden TA, Weinsier RL, Wilson GT, Wing RR, Yanovski SZ, Hubbard VS, Hoofnagle JH, Everhart J, Harrison B. Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- Buss J, Havel PJ, Epel E, Lin J, Blackburn E, Daubenmier J. Associations of ghrelin with eating behaviors, stress, metabolic factors, and telomere length among overweight and obese women: Preliminary evidence of attenuated ghrelin effects in obesity? Appetite. 2014;76:84–94. doi: 10.1016/j.appet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: Potential mechanisms. Psychosom Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging. 2010;25:453–463. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Carlson JJ, Turpin AA, Wiebke G, Hunt SC, Adams TD. Pre- and post-prandial appetite hormone levels in normal weight and severely obese women. Nutr Metab. 2009;6:32. doi: 10.1186/1743-7075-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A β3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry, Synaptic Development in Mood Disorders. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol - Endocrinol Metab. 2004;287:E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Di Francesco V, Zamboni M, Zoico E, Mazzali G, Dioli A, Omizzolo F, Bissoli L, Fantin F, Rizzotti P, Solerte SB, Micciolo R, Bosello O. Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: another reason for the “anorexia of aging. Am J Clin Nutr. 2006;83:1149–1152. doi: 10.1093/ajcn/83.5.1149. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-1 Clinician Version. American Psychiatric Press, Inc; Washington, DC: 1997. [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids [WWW Document] [accessed 5.1.14];Inst Med Natl Acad. 2002 doi: 10.1016/s0002-8223(02)90346-9. http://www.iom.edu/Reports/2002/Dietary-Reference-Intakes-for-Energy-Carbohydrate-Fiber-Fat-Fatty-Acids-Cholesterol-Protein-and-Amino-Acids.aspx. [DOI] [PubMed]
- Harthoorn LF, Dransfield E. Periprandial changes of the sympathetic–parasympathetic balance related to perceived satiety in humans. Eur J Appl Physiol. 2008;102:601–608. doi: 10.1007/s00421-007-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. 2014;58:136–146. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Andridge RR, Fagundes CP, Alfano CM, Povoski SP, Lipari AM, Agnese DM, Arnold MW, Farrar WB, Yee LD, Carson WE, III, Bekaii-Saab T, Martin EW, Jr, Schmidt CR, Kiecolt-Glaser JK. Pain, depression, and fatigue: Loneliness as a longitudinal risk factor. Health Psychol. 2014a;33:948–957. doi: 10.1037/a0034012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Belury MA, Andridge RR, Malarkey WB, Glaser R, Christian L, Emery CF, Kiecolt-Glaser JK. Interpersonal stressors predict ghrelin and leptin levels in women. Psychoneuroendocrinology. 2014b;48:178–188. doi: 10.1016/j.psyneuen.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliaki C, Kokkinos A, Tentolouris N, Katsilambros N. The effect of ingested macronutrients on postprandial ghrelin response: A critical review of existing literature data. Int J Pept. 2010;2010:e710852. doi: 10.1155/2010/710852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61:1145–1161. doi: 10.1038/sj.ejcn.1602749. [DOI] [PubMed] [Google Scholar]
- Leary MR, Cox CB. Belongingness motivation: A mainspring of social action. In: Gardner WL, Shah JY, editors. Handbook of Motivation Science. Guilford Press; New York, NY: 2008. pp. 27–40. [Google Scholar]
- Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90:1068–1071. doi: 10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Manning PJ, Sutherland WHF, McGrath MM, De Jong SA, Walker RJ, Williams MJA. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity. 2008;16:2046–2052. doi: 10.1038/oby.2008.334. [DOI] [PubMed] [Google Scholar]
- Maslow AH. Toward a Psychology of Being. 2. D. Van Nostrand; Oxford, England: 1968. [Google Scholar]
- Mundinger TO. Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, Chan YK, McArdle BH, Cooper GJS. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-α, and C-reactive protein to a high-fat dietary load. Nutrition. 2008;24:322–329. doi: 10.1016/j.nut.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Puhl RM, Brownell KD. Psychosocial origins of obesity stigma: Toward changing a powerful and pervasive bias. Obes Rev. 2003;4:213–227. doi: 10.1046/j.1467-789X.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein C, Shaver P. The Experience of Loneliness. In: Peplau LA, Perlman D, editors. Loneliness: A Sourcebook of Current Theory, Research and Therapy, Wiley Series on Personality Processes. John Wiley & Sons; 1982. pp. 207–223. [Google Scholar]
- Russell DW. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. J Pers Assess. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe SI, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jc.87.1.240. [DOI] [PubMed] [Google Scholar]
- Sugisawa H, Liang J, Liu X. Social networks, social support, and mortality among older people in Japan. J Gerontol. 1994;49:S3–S13. doi: 10.1093/geronj/49.1.S3. [DOI] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosom Med. 2009;71:836–842. doi: 10.1097/PSY.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. Friendship and the banker’s paradox: Other pathways to the evolution of adaptations for altruism. In: Runciman WG, Smith JM, Dunbar RIM, editors. Evolution of Social Behaviour Patterns in Primates and Man. Oxford University Press; New York, NY: 1996. pp. 119–143. [Google Scholar]
- Troisi JD, Gabriel S. Chicken soup really is good for the soul: “Comfort food” fulfills the need to belong. Psychol Sci. 2011;22:747–753. doi: 10.1177/0956797611407931. [DOI] [PubMed] [Google Scholar]
- Wrangham R. Catching fire: How cooking made us human. Basic Books; New York, NY: 2010. [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5992. doi: 10.1210/jc.86.12.5992. [DOI] [PubMed] [Google Scholar]