Abstract
Across human societies and many nonhuman animals, males have greater interest in uncommitted sex (more unrestricted sociosexuality) than do females. Testosterone shows positive associations with male-typical sociosexual behavior in nonhuman animals. Yet, it remains unclear whether the human sex difference in sociosexual psychology (attitudes and desires) is mediated by testosterone, whether any relationships between testosterone and sociosexuality differ between men and women, and what the nature of these possible relationships might be. In studies to resolve these questions, we examined relationships between salivary testosterone concentrations and sociosexual psychology and behavior in men and women. We measured testosterone in all men in our sample, but only in those women taking oral contraception (OC-using women) in order to reduce the influence of ovulatory cycle variation in ovarian hormone production. We found that OC-using women did not differ from normally-ovulating women in sociosexual psychology or behavior, but that circulating testosterone mediated the sex difference in human sociosexuality and predicted sociosexual psychology in men but not OC-using women. Moreover, when sociosexual psychology was controlled, men’s sociosexual behavior (number of sexual partners) was negatively related to testosterone, suggesting that testosterone drives sociosexual psychology in men and is inhibited when those desires are fulfilled. This more complex relationship between androgen and male sexuality may reconcile some conflicting prior reports.
Keywords: androgen, sex differences, sexual behavior, sociosexuality, testosterone
Introduction
Across societies, men report greater interest than women in uncommitted sex – that is, less restricted sociosexuality (Charles & Alexander, 2011; Edelstein, Chopik, & Kean, 2011; Lippa, 2009; Penke & Asendorpf, 2008; Schmitt, 2005; Simpson & Gangestad, 1991), but there is also a high degree of overlap between the sexes (Gangestad & Simpson, 2000). Between- and within-sex variation in sociosexuality may be mediated partly by testosterone (T). In nonhuman animals, androgens such as T mediate male-typical sociosexual behavior (Hart, 1974; Martin & Baum, 1986; Money & Ehrhardt, 1971; Xu et al., 2012; Yang & Shah, 2014; Zuloaga, Puts, Jordan, & Breedlove, 2008). Testosterone has also been investigated in relation to human sociosexuality with mixed results (Cashdan, 1995; Charles & Alexander, 2011; Edelstein et al., 2011; McIntyre et al., 2006; van Anders, Brotto, Farrell, & Yule, 2009; van Anders, Hamilton, & Watson, 2007b). One reason for these discrepancies may be that participants’ gender varies across studies, and T may be differently related to sociosexuality in men and women (see below).
Another source of variability may be different means of assessing sociosexuality. Sociosexual behaviors likely depend not only on desires and attitudes, but also on extrinsic factors such as opportunity (Edelstein et al., 2011; McIntyre et al., 2006; Ostovich & Sabini, 2005). For such reasons, recent research suggests that sociosexual behaviors should be treated as distinct from desires and attitudes (Jackson & Kirkpatrick, 2007; Lukaszewski, Larson, Gildersleeve, Roney, & Haselton, 2014; Ostovich & Sabini, 2005; Penke & Asendorpf, 2008; Webster & Bryan, 2007). Consequently, some authors have omitted behavioral items from the Sociosexual Orientation Inventory (SOI; Simpson & Gangestad, 1991), producing a composite from the remaining items (Lukaszewski et al., 2014; Ostovich & Sabini, 2005), while others have produced separate composites of the behavioral items and the items related to sociosexual attitudes and desires (Charles & Alexander, 2011; Webster & Bryan, 2007). Using the latter approach, Charles and Alexander (2011) found no significant relationships between T and behavioral and attitudinal composites in either men or women. Other authors have analyzed the SOI items separately. Adopting this approach, van Anders et al. (2007b) found a relationship between women’s T levels and responses on two SOI items related to sociosexual behavior, but no relationships between men’s T and total SOI score or scores on any individual items.
Penke and Aspendorpf (2008) emphasized the heterogeneous nature of the items on the original SOI (Simpson & Gangestad, 1991) and devised a 9-item Revised Sociosexual Orientation Inventory (SOI-R) with separate Attitude, Desire, and Behavior subscales comprising 3 items each. Using the SOI-R, Edelstein et al. (2011) found overall significant positive relationships between T and men’s scores on the sociosexual Attitude and Desire, but not Behavior, subscales, and no relationships between T and sociosexuality subscales in their full sample of women. However, after considering a potential moderating role of relationship status, Edelstein et al. (2011) found that sociosexual Behavior was positively associated with testosterone levels among partnered, but not single, women, and both Edelstein et al. (2011) and McIntyre et al. (2006) found that T was positively linked to sociosexuality among partnered, but not single, men. Other studies have found positive relationships between men’s T and sociosexual behaviors, including numbers of past sex partners (Bogaert & Fisher, 1995; Peters, Simmons, & Rhodes, 2008; Pollet, van der Meij, Cobey, & Buunk, 2011), and polygynous vs. monogamous marriage (Gray, 2003). Alvergne, Faurie, and Raymond (2009) found a positive relationship between T and polygyny in Senegalese men under 50 years of age and a negative relationship in men over 50 years of age.
Associations between T, sociosexual attitudes and desires (henceforth, “sociosexual psychology”), and sociosexual behaviors are complicated by the fact that causality is likely to be bidirectional (Archer, 2006; see also Mazur & Booth, 1998). Testosterone may lead to more unrestricted sociosexual psychology, which may then influence sociosexual behaviors (e.g., Mantzoros, Georgiadis, & Trichopoulos, 1995), but sexual behavior can also influence T levels (Goldey & van Anders, 2014). For example, T levels have been found to increase during sexual interest and arousal in men (Hellhammer, Hubert, & Schurmeyer, 1985; Lopez, Hay, & Conklin, 2009; Roney, Lukaszewski, & Simmons, 2007; van der Meij, Buunk, van de Sande, & Salvador, 2008) and after sexual activity in both sexes (Dabbs & Mohammed, 1992; van Anders, Hamilton, Schmidt, & Watson, 2007a). Although T levels did not remain elevated the day after sexual activity in women (van Anders et al., 2007a), Sakaguchi et al. (2007) found that Japanese men who reported regular sexual activity had lower T levels. Sakaguchi et al. hypothesized that frequent sexual behavior may exert negative feedback on T levels. Indeed, within-subjects studies have reported that periods of sexual abstinence are associated with increases in men’s T (Exton et al., 2001; Kraemer et al., 1976).
A functional perspective is likely to be useful in clarifying these complex relationships. Growing evidence indicates that T mediates men’s allocation of effort directed toward competition for mates (Ellison, 2001). For example, men’s T levels rise in the presence of potential mates (Roney et al., 2007) and in anticipation of intrasexual competition, often remaining elevated in the winners (Booth, Shelley, Mazur, Tharp, & Kittok, 1989; Mazur & Lamb, 1980; Mazur, Susman, & Edelbrock, 1997; Oxford, Ponzi, & Geary, 2010). Conversely, men’s T levels decrease in committed romantic relationships (Booth & Dabbs, 1993; Gangestad, Thornhill, & Garver-Apgar, 2010; Gettler, McDade, & Kuzawa, 2011c; Gray, 2003; Gray, Kahlenberg, Barrett, Lipson, & Ellison, 2002) and after having children (Gettler, McDade, Agustin, & Kuzawa, 2011a; Gettler, McDade, Feranil, & Kuzawa, 2011b; Gray et al., 2002; Gray, Yang, & Pope, 2006; Kuzawa, Gettler, Muller, McDade, & Feranil, 2009; Muller, Marlowe, Bugumba, & Ellison, 2009; Storey, Walsh, Quinton, & Wynne-Edwards, 2000). Such findings suggest that T levels rise in response to opportunities to augment mating success and decline in response to alternative fitness opportunities.
That men’s T levels decline in committed romantic relationships and after having children highlights the opportunity costs of increased T: the resulting mating effort interferes with pair-bonding and parental care. In addition, T carries with it several other potential costs, including increased risk of injury resulting from aggressive and/or risky behaviors, increased energy consumption, oxidative stress, and suppression of some types of immune function (Archer, 2004, 2006, 2009; Bouman, Heineman, & Faas, 2005; McIntyre et al., 2006; Wingfield, Lynn, & Soma, 2001). Testosterone production should thus be regulated in relation to these fitness costs and the benefits of increased mating success. However, what constitutes optimal investment in mating depends in part on individual men’s ability to win mates and the presence of alternative targets of reproductive effort, such as existing mates and offspring—variables that are reflected in men’s sociosexual psychology (Gangestad & Simpson, 2000; Lukaszewski et al., 2014). Hence, if a man’s level of uncommitted mating is low relative to his sociosexual psychology, then this indicates suboptimal investment in mating effort, and T levels should be elevated. Conversely, high sociosexual behavior relative to desires indicates excess investment in mating effort, and T levels should be reduced.
In sum, T may motivate sociosexual behavior, but the realization of these desires should tend to reduce T levels in order to mitigate the costs of high T. Such negative feedback not only makes adaptive sense; it could also explain inconsistent past results. If T increases sociosexual behavior through promoting favorable attitudes and desires, but sociosexual behavior in turn decreases T levels (Fig. 1), then the result might be overall weak associations between T and both sociosexual psychology and behavior. This hypothesis makes specific predictions regarding relationships between men’s sociosexuality and T in cross-sectional samples: 1) When sociosexual psychology is statistically held constant, sociosexual behavior should negatively predict T, and 2) when sociosexual behavior is statistically held constant, sociosexual psychology should positively predict T. In the present research, we tested these predictions. If relationships between T and sociosexual behavior are bidirectional in a feedback loop, then these relationships should be apparent whether T is measured at the beginning or end of the interval over which behavioral patterns are assessed. In Study 1, we assayed T from saliva collected prior to the one-year interval over which sociosexual behavior (specifically, sex partner number) is assessed by the SOI-R, and in Study 2, we obtained behavioral data for the year preceding the time of saliva collection.
Fig. 1.
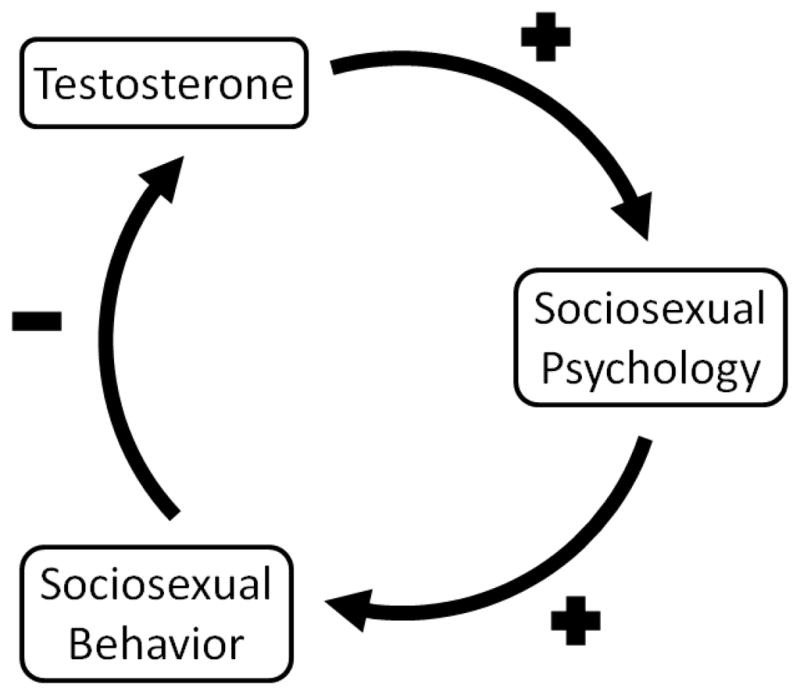
Hypothesized negative feedback—mediated by sociosexual psychology—between testosterone and sociosexual behavior.
We also tested whether 3) T levels mediate the sex difference in sociosexual psychology, and whether 4) associations between T and sociosexual psychology differ by sex. Women do not face the same tradeoff between mating effort and investment in mates and offspring that men do. The payoffs of high mating effort are potentially much larger for males, who can benefit reproductively from access to a large number of partners, whereas women generally produce only one child at a time regardless of their number of mates. Therefore, factors that influence reproductive strategies are expected to have a larger impact on the balance between mating effort and investment on the part of men compared to women (Del Giudice & Belsky, 2010). While T may mediate the allocation of men’s reproductive effort between mating and investment, we should expect that its effects would be less pronounced in women.
Study 1
Methods
Participants
Sixty-one male (19.2±1.4y) and 126 female (19.3±1.5y; 52 taking oral contraception) students from a Midwestern United States university participated in this institutional review board-approved study as part of a larger study of human sex differences. Reported ethnicities were 91.4% White, 3.6% Asian, 2.1% Hispanic or Latino, 1.2% Black or African American, 0.6% American Indian or Alaska Native, and 1.2% Other. Each participant attended three laboratory sessions as described below.
Saliva Collection
To minimize menstrual cycle-related hormonal changes, we examined the influence of T in women taking oral contraception (OC), whose cyclic hormonal variation is suppressed. In previous research (Edelstein et al., 2011; R. Edelstein, personal communication, May 31, 2013), hormonal contraception has not affected relationships between testosterone and sociosexuality (but see Discussion). We scheduled women taking OC and men for both a morning and an evening session (the first two of three sessions), approximately one week apart, as described in Puts et al. (2010). Men and OC-using women were randomly allocated to attend their rst session during the morning or evening, with their second session taking place at the other time of day. Morning sessions began between 0820 h and 1000 h, and evening sessions began between 1720 h and 1900 h. The difference between start times of morning and evening sessions was 8.9 (±1.0) h. Normally-cycling women were scheduled for two laboratory sessions between 1300 h and 1600 h according to self-reported menstrual cycle data to coincide with the late follicular phase and the midluteal phase (see Puts et al., 2013), but saliva collected during these sessions was not assayed for T.
Participants were instructed not to eat, drink (except water), smoke, chew gum, or brush their teeth for one hour before their sessions. Participants rinsed their mouths with water before chewing a piece of sugar-free Trident® gum to stimulate salivation. Some gums may influence levels of T in saliva samples depending on brand, flavor (Schultheiss, 2013; van Anders, 2010), and the specific antibodies used in the hormonal assays. However, we used sugar-free Trident® gum, which is inert in the salivary hormonal assays used in the present study (Neuroendocrinology Assay Laboratory at the University of Western Ontario, Canada, unpublished data). Participants collected approximately 9 mL of saliva in a sodium azide-coated polystyrene tube. The tube was then capped, left at room temperature for 18–24h to allow mucins to settle, and frozen at −20°C until analysis.
Testosterone Assays
We obtained salivary unbound testosterone concentrations through radioimmunoassay. Following a double ether extraction, samples were assayed in duplicate using a Coat-A-Count kit for total testosterone (Diagnostic Products, Los Angeles, CA), modified for saliva. Assays were performed separately for men and women in two batches for each sex. Sensitivity was 5–10 pg/mL, and the average intra-assay coefficient of variation (CV) was 6.3%. Assay concentrations (log-transformed to correct skew) correlated highly across duplicates (rs>.97, Ps<.0001) and sessions (r95=.91, P<.0001) and showed expected decreases from morning to afternoon (paired t-tests: men: t57=7.5, P<.0001; women: t36=5.6, P<.0001; Table 1). Here, we are not interested in morning or afternoon testosterone levels specifically, but rather in between-participant differences in average hormonal concentrations that may relate to sociosexual behavior and sociosexual psychology. Because the average of morning and afternoon testosterone concentrations should provide a better estimate of each participant’s average level, and because testosterone concentrations correlated strongly across duplicates and sessions, all concentrations were averaged for each participant. Untransformed testosterone concentrations (Table 1) compared well with previously published concentrations collected across the day and assayed using this kit (Liening, Stanton, Saini, & Schultheiss, 2010: men: 108.1 ± 33.9 pg/mL; OC-using women: 15.7 ± 5.9 pg/mL).
Table 1.
Descriptive statistics (mean ± SD) for raw (untransformed) data.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Women (non-OC) (N=74) | Women (OC) (N=52) | Men (N=61) | Men (N=62) | |
| AM T (pg/mL) | --- | 18.8 ± 11.2 (N=43) | 113.0 ± 44.9 (N=57) | 164.9 ± 62.4 |
| PM T (pg/mL) | --- | 11.6 ± 7.1 (N=45) | 72.8 ± 33.5 (N=58) | --- |
| SOI-R Attitude | 3.1 ± 2.0 | 3.4 ± 1.6 | 4.8 ± 2.6 | 7.5 ± 1.4 |
| SOI-R Desire | 2.9 ± 1.5 | 2.9 ± 1.4 | 5.0 ± 1.7 | 6.5 ± 1.4 |
| SOI-R Behavior | 1.8 ± 1.0 | 1.9 ± 1.0 | 1.9 ± 1.2 | 3.9 ± 1.6 |
| No. sex partners1 | 2.1 ± 1.2 | 2.1 ± 0.7 | 2.2 ± 1.5 | 3.9 ± 1.7 |
| SOI-R Total | 2.6 ± 1.1 | 2.7 ± 1.0 | 3.9 ± 1.5 | 6.0 ± 1.1 |
| Relationship status | --- | --- | --- | 1.7 ± 0.7 |
| Min. since waking | --- | --- | --- | 135.0 ± 74.9 |
Response to Item 1 on the SOI-R.
Sexuality assessment
Sexual orientation was assessed during the first session using the sexual attraction and fantasy dimensions of the Kinsey Scale (Kinsey, Pomeroy, & Martin, 1948), which is a 7-point scale used to identify the statement that best describes the respondent’s sexual feelings and fantasies at present. For sexual attraction, possible responses ranged from “I am attracted to men only, never to women” to “I am attracted to women only, never to men”, and for sexual fantasy, responses ranged from “always to a man, never a woman” to “always to a woman, never a man”. Zero represents exclusive heterosexual orientation, and 6 represents exclusive homosexual orientation. We excluded from further analysis one female and two male participants whose responses on the sexual attraction or fantasy dimensions were >2. This exclusion did not alter the results.
During a third and final session 1.19 (±0.28) years after their previous session, participants completed the Revised Sociosexual Orientation Inventory (SOI-R) (Penke & Asendorpf, 2008), a nine-item instrument targeting sociosexual attitudes, desires and behaviors. For each item, participants indicated their level of agreement on 9-point scales with labels that varied by item (Penke & Asendorpf, 2008). Internal reliability was high (Cronbach’s α >.73) for each subscale, and for all subscales combined. We distinguished between sociosexual behaviors and the psychology that might motivate them (see, e.g., Jackson & Kirkpatrick, 2007; Lukaszewski et al., 2014; Ostovich & Sabini, 2005; Penke & Asendorpf, 2008; Webster & Bryan, 2007) by producing both a “sociosexual behavior” variable (the average response on the three items comprising the Behavior subscale of the SOI-R) and a “sociosexual psychology” variable (the average of the Attitude and Desire subscales of the SOI-R). Although scores on all three subscales intercorrelate (Edelstein et al., 2011; Penke & Asendorpf, 2008), it is important to distinguish between sociosexual behavior and sociosexual psychology when exploring their relationships with T. This is because both theory and prior empirical work suggest that T may positively influence attitudes and desires, whereas behavior may negatively influence T. The distinct hypothesized causal relationships with T necessitate treating sociosexual behavior and sociosexual psychology separately in order to explore these relationships.
Results
Sociosexual attitudes, behaviors and desires intercorrelated significantly (Table 2), and did not differ between women on and off hormonal contraception (t-tests: all P>.24; see also Charles & Alexander, 2011). As expected, testosterone (t108=−21.0, P<.0001) and sociosexual psychology (t182=−7.8, P<.0001) showed large sex differences (Table 1). In a multiple regression model in which predictors were first standardized (mean=0, SD=1) to reduce multicolinearity, testosterone was differently related to sociosexual psychology in men and OC-using women (i.e., testosterone and sex significantly interacted), and testosterone predicted sociosexual psychology, but sex did not (Table 3; model F3,109=16.46, R2=.31, P<.0001). A bootstrap test (number of resamples=5000, P<.05; Preacher & Hayes, 2004) confirmed that the sex difference in sociosexual psychology was mediated by testosterone.
Table 2.
Intercorrelations between subscales of the SOI-R.
| Study 1 | Study 2 | ||
|---|---|---|---|
| Women (N=126) | Men (N=61) | Men (N=62) | |
| Attitude-Behavior1 | .56** | .46** | .29* |
| Attitude-Desire | .18* | .52** | .36** |
| Behavior1-Desire | .18* | .51** | .28* |
Log-transformed in Study 1.
P < .05,
P < .01
Table 3.
Multiple regression predicting sociosexual psychology in Study 1 participants.
| β | t | p | |
|---|---|---|---|
| Testosterone | .53 | 2.71 | .008 |
| Sex | −.05 | −.11 | .916 |
| Testosterone × Sex | .24 | 2.89 | .005 |
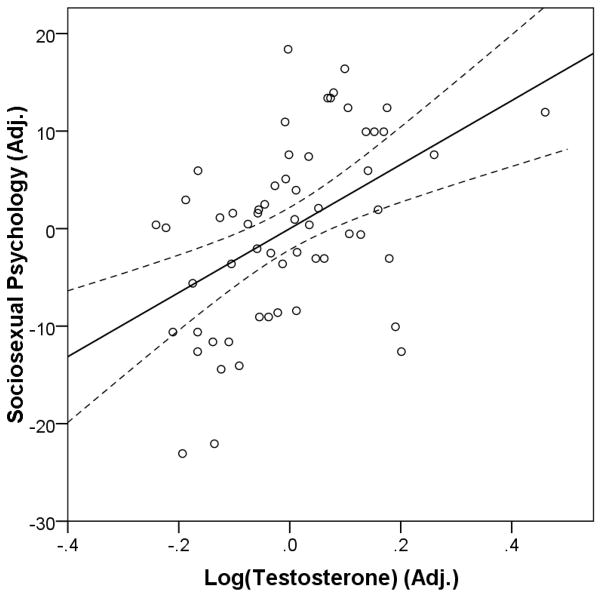
In a separate regression model run on men, men with more unrestricted sociosexual psychology had higher testosterone levels (F1,57=8.97, β=.37, t=3.00, P=.004, see also Table 4). When testosterone’s relationship with sociosexual psychology was controlled, sociosexual behaviors (log-transformed to correct skew) negatively predicted testosterone (multiple regression model: F2,56=7.27, R2=.21, P=.002; psychology: β=.54, t=3.81, P<.001; behavior: β=−.32, t=−2.22, P=.030). We then investigated whether any particular behavioral item was driving the negative association with testosterone by replacing sociosexual behavior with each individual behavioral item in the multiple regression model above. Behaviors that most strongly negatively predict T should be those most representative of mating success. On the SOI-R, number of sexual partners in the last 12 months (Item 1) best measures men’s mating success (see, e.g., Faurie, Pontier, & Raymond, 2004; Hill et al., 2013; Hodges-Simeon, Gaulin, & Puts, 2011), whereas the other behavioral items do not measure mating success per se. Items 1 (log-transformed to correct skew) and 3 (number of sex partners in whom the participant was not interested in a long-term committed relationship) were statistically significant predictors (P=.011 and .043, respectively), but Item 2 (number of partners with whom sex occurred only once) was not (P=.116). However, Item 1 significantly (P<.05) mediated the effect of Item 3, whereas Item 3 did not significantly mediate the effect of Item 1 (bootstrap test; bootstrap resamples = 5000; Preacher & Hayes, 2008). Therefore, the negative relationship between sociosexual behavior and T was attributable to the influence of SOI-R Item 1 (multiple regression model: F2,56=8.42, R2=.23, P<.001; Table 5). After controlling for partner number, the positive relationship between T and sociosexual psychology was even more evident (Fig. 2).
Table 4.
Zero-order correlations with log-transformed testosterone concentrations.
| Study 1 | Study 2 | ||
|---|---|---|---|
| Women (N = 51) | Men (N = 59) | Men (N=62) | |
| SOI-R Attitude | −.03 | .33* | .21+ |
| SOI-R Desire | .12 | .31* | .16 |
| SOI-R Attitude + Desire | .05 | .37** | .23+ |
| SOI-R Behavior1 | .05 | −.02 | −.01 |
| Number of sex partners1 | .01 | −.06 | −.16 |
| SOI-R Total | .06 | .29* | .16 |
| Relationship status | --- | --- | −.29* |
| Min. since waking2 | --- | --- | −.22+ |
Log-transformed in Study 1.
N=61.
P<.1,
P<.05,
P<.01
Table 5.
Multiple regression predicting testosterone in Study 1 male participants.
| β | t | P | |
|---|---|---|---|
| No. sex partners1 | −.37 | −2.63 | .011 |
| Sociosexual psychology | .57 | 4.08 | .0001 |
Response to Item 1 on the SOI-R.
Fig. 2.
Men’s testosterone levels predicted their sociosexual psychology (combined Attitude and Desire subscales of the SOI-R), r(59)=.48, P=.0001. Sociosexual psychology and testosterone are conditioned on number of sexual partners in the past year. Dashed lines represent 95% confidence interval for least-squares regression line.
In separate regression models run on OC-using women, sociosexual psychology was unrelated to testosterone (F1,49=.12, β=.05, t=.35, P=.727). Sociosexual behaviors (log-transformed to correct skew) were also unrelated to testosterone alone (F1,49=.11, β=.05, t=.33, P=.746) and when controlling for sociosexual psychology (multiple regression model: F2,49=.14, R2<.01, P=.929; psychology: β=.04, t=.21, P=.837; behavior: β=.03, t=.16, P=.873).
Controlling for session order (morning or evening session first) did not influence any of the above results, and session order was not a significant predictor when added to any model.
Study 2
We attempted to replicate the results from men in Study 1 in a new sample, this time including time since waking, which negatively predicts T levels (Liening et al., 2010) and relationship status, which also influences men’s T levels (Booth & Dabbs, 1993; Burnham et al., 2003; Gettler et al., 2011c; Gray, 2003; Gray et al., 2002) and has been found to interact with sociosexuality in predicting men’s T levels (Edelstein et al., 2011; McIntyre et al., 2006). We also collected data on men’s peer- and self-rated social status (attractiveness, leadership, dominance, likeability), using these data to explore the mode by which T might relate to men’s sociosexual behavior.
Methods
Participants
In order to obtain evaluations by familiar peers, we recruited members of two fraternities (N = 63, mean age = 19.9, SD = 1.2) and two sororities (N = 72, mean age = 19.4, SD = 1.0) from a large university in the northeastern United States. Each fraternity was socially affiliated with one of the sororities, with members attending joint social functions at least three times per month. Male participants were paid US$15, and female participants were paid US$10. All methods were approved by the university’s institutional review board.
Procedures
We collected data at male participants’ residence (fraternity) houses using a series of stations. The first station acquired informed consent; other stations collected saliva, photographs, answers to online questionnaires, and data not used in this study (see Doll et al., 2014; Hill et al., 2013). Each male participant provided two saliva samples via passive drool 19.2 ± 6.9 min apart between 900 h and 1330 h. We aliquotted 0.5 mL from each sample into a cryovial and froze it at −20°C until hormone analysis. Participants provided facial photographs and completed online questionnaires between saliva collections. Online instruments included the SOI-R (items answered on 9-point scales, as in study 1) and questions on time since waking, sexual attraction and fantasy dimensions of the Kinsey sexual orientation questionnaire (as in study 1), and current relationship status (options “not seeing anyone” [N=27], “casual relationship” [N=27], and “serious relationship” [N=8] coded with increasing commitment level as 0–2, respectively). With the exception of one participant who reported 1 on the sexual attraction dimension, all male participants reported exclusive heterosexuality (0 on both sexual attraction and fantasy). On the SOI-R, internal reliabilities were high (Cronbach’s α >.79 for each subscale, and for all subscales combined). Male participants were also asked what percentage of men they could defeat in a physical fight, how good a leader they were, and how attractive they were. All rating tasks used an 11-point Likert scale (0–10) except for the question “What percentage of men your age could you beat in a physical fight?” for which choices were listed from 0% to 100% in increments of 10%.
Ratings
One week after the initial data collection, we returned to the residences, at which time male participants were sequentially shown facial photographs of all other male participants from their fraternity and asked what percentage of men each could beat in a physical fight, how good a leader he was, and how much they liked him. The same scales used for self-assessments were used for assessment of other male participants. Order of rating tasks and presentation order of stimuli were randomized. For each participant, all of the stimuli for a particular dimension were rated before moving on to the next dimension. As before, participants were seated privately.
Female participants were brought individually into the lab, sequentially shown facial photographs of all male participants from their affiliated fraternity on a computer, and asked to rate on a 10-point Likert scale how much they liked each man and how attractive each was for a “short-term, purely sexual relationship, such as a one night stand” and for a “long-term, committed relationship, such as marriage.” Rating task and stimulus presentation order were randomized as above.
Interclass correlations were computed to determine inter-rater reliability. For each male participant, some data were missing because male participants rated other members, but not themselves. Thus, to compute inter-rater reliability in a way not likely to bias existing ratings, we imputed a random value from the pool of observed ratings for each missing value. Average two-way agreement interclass correlations were computed for each variable for each fraternity, as members of one fraternity did not rate members of the other fraternity. Six female participants’ ratings were removed because they were missing more than 16 of 34 trials. All interclass correlations were high (>0.8).
Testosterone Assays
We assessed salivary unbound testosterone concentrations through enzyme immunoassay (EIA). Samples were assayed in duplicate using Enzyme Immunoassay Kits (Salimetrics, State College, PA). Sensitivity was <1.0 pg/mL, and the intra-assay CV was 2.5%. Duplicate assay concentrations correlated r64=.97, P<.0001 and consequently were averaged. Untransformed testosterone concentrations (Table 1) compared well with previously published concentrations assayed using this kit (Josephs, Sellers, Newman, & Mehta, 2006; Mehta & Josephs, 2006; Newman, Sellers, & Josephs, 2005).
Results
In a multiple regression predicting log-transformed testosterone (F5,55=3.83, R2=.26, P<.005), testosterone was again positively related to sociosexual psychology and negatively related to sex partner number (SOI-R Item 1; Table 6). In this model, sociosexual psychology and relationship status were first standardized to reduce multicolinearity, and then their interaction was calculated. Testosterone was lower in committed relationships and when measured later in relation to the time of waking, but sociosexual psychology and relationship status did not significantly interact. In separate multiple regression models replacing Item 1 in the model above with Items 2 and 3 (the remaining items on the behavior subscale), neither Item 2 nor Item 3 was a significant predictor (both P>.5), and thus neither significantly mediated the effect of sex partner number, as in study 1 (bootstrap test; bootstraps = 5000; Preacher & Hayes, 2008).
Table 6.
Multiple regression predicting testosterone in Study 2 male participants.
| β | t | P | |
|---|---|---|---|
| No. sex partners1 | −.27 | −2.15 | .036 |
| Relationship status | −.31 | −2.49 | .016 |
| Sociosexual psychology | .31 | 2.27 | .027 |
| Sociosexual psychology × Relationship status | −.16 | −1.26 | .214 |
| Time since waking | −.25 | −2.09 | .041 |
Response to Item 1 on the SOI-R.
In order to further explore our failure to replicate previous findings (Edelstein et al., 2011; McIntyre et al., 2006) of an interaction between relationship status and sociosexuality in predicting T, we recoded relationship status into single=0 vs. partnered (casual or serious relationship)=1. We then standardized relationship status, computed its interaction with standardized sociosexual psychology, and replaced the corresponding variables in the multiple regression model described in Table 6. Once again, the model was statistically significant (F5,55=3.29, R2=.23, P=.011), as were sociosexual psychology (β=.35, t=2.39, P=.020) and sex partner number (β=−.28, t=−2.15, P=.036), but again sociosexual psychology and relationship status did not significantly interact (β=−.13, t=−.96, P=.340). Time since waking (β=−.22, t=−1.87, P=.066) and relationship status were (β=−.22, t=−1.77, P=.082) marginally significant negative predictors of T.
We then explored relationships between sociosexuality and T in partnered men and single men separately by entering sociosexual psychology, partner number, and time since waking into separate multiple regression models to predict T. In partnered men, this model was statistically significant (F3,30=5.67, R2=.36, P=.003), as were sociosexual psychology (β=.31, t=2.09, P=.045), partner number (β=−.39, t=−2.55, P=.016), and time since waking (β=−.38, t=−2.53, P=.017); however, in single men, this model was not statistically significant (F3,23=.49, R2=.36, P=.696), nor were sociosexual psychology (β=.27, t=1.12, P=.272), partner number (β=−.10, t=−.41, P=.686), or time since waking (β=−.07, t=−.35, P=.733).
Next, we explored whether the negative association between number of sex partners and testosterone in the multiple regression model described in Table 6 was due to more attractive, high status or well-liked men having lower testosterone. We examined as potential mediators female peer-rated attractiveness for short- and long-term relationships, male peer-rated physical dominance and leadership abilities, and how well participants were liked by male and female peers. None of these significantly mediated the relationship between number of sex partners and testosterone; 95% confidence intervals included zero for indirect effects through all proposed mediators (bootstrap test; bootstraps = 5000; Preacher & Hayes, 2008).
We performed a similar set of mediation analyses to explore whether increased number of sex partners with decreasing testosterone was due to a tendency for men with lower testosterone to engage in more self-serving exaggeration. We first standardized each male participant’s self-rated attractiveness, fighting ability, and leadership ability, as well as his male peer-rated fighting and leadership abilities and his female peer-rated long- and short-term attractiveness. The last two of these correlated highly (r58=.93, P<.0001) and were thus averaged. We then computed the difference between standardized self- and peer-ratings for each attribute, such that positive values indicated a tendency to view oneself as higher on that attribute than peers do. None of these difference scores significantly mediated the relationship between number of sex partners and testosterone.
Finally, to more exactly replicate the analysis from Study 1, we ran a multiple regression predicting log-transformed testosterone (F2,59=3.72, R2=.11, P=.030), including only sociosexual psychology and sex partner number as predictors. Sociosexual psychology was again a significant positive predictor (β=.31, t=2.40, P=.020), and sex partner number fell just short of statistical significance as a negative predictor (β=−.26, t=−1.99, P=.051).
Discussion
In Study 1, the sex difference in sociosexual psychology was fully mediated by testosterone, which was also differentially related to sociosexual psychology in men and OC-using women. Testosterone did not predict OC-using women’s sociosexual psychology, but it positively predicted men’s in both samples. This sex difference in the relationship between T and sociosexual psychology may reflect sex differences in reproductive strategies (Buss & Schmitt, 1993; Gangestad & Simpson, 2000). Because ancestral men could benefit reproductively from uncommitted mating with a high number of sexual partners in way that ancestral women could not, mechanisms that encourage uncommitted mating in men may do so to a lesser degree or not at all in women (Del Giudice & Belsky, 2011). At a proximate level, perhaps sociosexual responsiveness to testosterone in adulthood requires threshold testosterone concentrations or early organizational effects of sex hormones, such as high fetal testosterone. Such organizational effects are necessary for later activational effects of sex hormones across a wide variety of traits and species (Nelson, 2005; Phoenix, Goy, Gerall, & Young, 1959).
Functionally, men’s testosterone may represent a trigger mediating the allocation of reproductive effort from investment in a mate and/or offspring to seeking additional mating opportunities (Archer, 2006; McIntyre et al., 2006). It is also possible that the relationship between sociosexual psychology and testosterone is bidirectional in men; Testosterone may not only increase interest in and positive attitudes toward uncommitted sex—thinking about uncommitted sex may also increase men’s testosterone levels. However, while abundant research indicates that exposure to sexual stimuli raises men’s testosterone levels (Roney et al., 2007; Roney, Simmons, & Lukaszewski, 2010), merely thinking about sex does not appear sufficient to do so (Goldey & van Anders, 2014; Goldey & van Anders, 2012).
Finally, controlling for sociosexual psychology, testosterone negatively predicted men’s sociosexual behavior, particularly their number of sex partners in the previous year. This was true regardless of whether testosterone was measured before (Study 1) or at the end of (Study 2) the interval over which number of sex partners was reported. This was also true across samples of male undergraduates that differed markedly in sociosexual attitudes, behavior, and desires, as well as number of reported sexual partners (Cohen’s d=1.0–1.3), likely reflecting differences between fraternity members (Study 2) and a broader sample of undergraduate men (Study 1). One explanation for this negative relationship between testosterone and sociosexual behavior is that testosterone may decrease men’s ability to acquire sexual partners. However, this relationship was not mediated by men’s attractiveness, dominance, leadership or likeability. Another explanation is that men with lower testosterone may be more prone to exaggerating their partner numbers. However, testosterone did not reflect general self-serving exaggeration, as it was unrelated to any exaggeration regarding attractiveness, dominance or leadership.
We favor a third possibility: men’s general satisfaction in the mating sphere decreases testosterone. Although T may increase mating opportunities through elevating sociosexual psychology (Edelstein et al., 2011; this study), intrasexual competitiveness (Archer, 1991, 2006; Mazur & Booth, 1998), and the production of the anatomical traits relevant to intrasexual competition (Puts, Jones, & DeBruine, 2012), there are numerous costs to high levels of T. These include interference with pair-bonding and parental care, increased energy consumption, oxidative stress, suppression of some types of immune function, and increased risk of injury resulting from aggressive and/or risky behaviors (Archer, 2004, 2006, 2009; Bouman et al., 2005; McIntyre et al., 2006; Wingfield et al., 2001). Men who achieve what, for them, represents a successful pattern of mating, whether through committed relationships or uncommitted sex, should lower these costs by decreasing T production. The present results thus point to negative feedback in which T promotes copulatory success, and copulatory success in turn down-regulates T production (Fig. 1).
We also replicated the finding that men in more committed relationships have lower testosterone (Booth & Dabbs, 1993; Burnham et al., 2003; Gray et al., 2002; McIntyre et al., 2006) and found partial support for previous findings that sociosexuality is related to T in partnered but not single men (Edelstein et al., 2011; McIntyre et al., 2006). Although we found no significant interaction between sociosexual psychology and relationship status in predicting testosterone using two coding schemes for relationship status, the interaction was in the predicted direction, and sociosexual psychology was significantly related to T in partnered but not single men. Functionally, such an effect of partnered status may reflect adaptations related to extra-pair mating (McIntyre et al., 2006). Both partnered and single men vary in their opportunities for uncommitted mating and hence the degree to which they could benefit reproductively from an interest in uncommitted mating and the mating effort that it promotes (Gangestad & Simpson, 2000; Lukaszewski et al., 2014). However, the costs of mating effort should be particularly great for partnered men, who would suffer a proportionate decrement to their investment and may lose their mate altogether. Consequently, in partnered men, sociosexual psychology may be particularly strongly tied to physiological factors such as T that track this cost/benefit tradeoff. In other words, T may be more strongly tied to uncommitted mating orientation in partnered men in order to promote extra-pair mating effort in those with high T and many extra-pair mating opportunities, and to decrease mating effort and promote investment by partnered men with low T and few such opportunities.
Limitations
Due to the cross-sectional nature of our data, we cannot definitively discriminate between whether T is a cause, consequence, or merely a correlate of sociosexual behavior or psychology. Total SOI-R and all three subscales tend to be stable over time, with measures taken a year apart correlating at r=0.7–0.8 (Penke & Asendorpf, 2008). Consequently, comparing results when T is sampled at the beginning (Study 1) versus at the end (Study 2) of the year over which participants reported their sociosexual behavior would not resolve the direction of causality. Each sampling regime used in the present research has advantages and disadvantages for testing the hypothesized relationships. Testosterone data collected prior to the interval over which partner number was assessed (Study 1) should be more useful in testing possible effects of T on sexual behavior, but relationships between T and sociosexual psychology should be attenuated in proportion to the length of the interval, despite the temporal stability of the measures. Testosterone data collected at the end of the interval over which partner number was assessed (Study 2) should be more useful in testing the influence of sexual behavior on T, and relationships between T and sociosexual psychology, but less valuable for testing an influence of T on behavior. The combination of these two approaches and strong agreement between their results increases confidence in the hypothesized relationships. Nevertheless, the correlational nature of our data, along with temporal separation between when T and behavior was measured, necessitate that caution be used in interpreting these results. Future longitudinal data in which changes in sociosexuality and behavior are related to changes in T may help clarify these relationships.
Another potential concern is that we measured T only in female participants who were taking OC, and that the observed relationships with T reflect effects of OC. However, while OC use lowers free (bioavailable) T, it does so to a similar degree across OC types (van der Vange, Blankenstein, Kloosterboer, Haspels, & Thijssen, 1990), particularly after the first cycle of use (Wiegratz et al., 2003). Furthermore, we found no differences in sociosexuality between normally cycling and OC-using women, and Edelstein and colleagues (Edelstein et al., 2011; R. Edelstein, personal communication, May 31, 2013) found no effect of OC use on relationships between sociosexuality and T. We view the inclusion of OC-using women as a strength of our methods, as it should reduce the influence of cyclic fluctuations in ovarian hormones. Nevertheless, it remains possible that any relationships between women’s T levels and sociosexuality were attenuated or otherwise influenced by our use of OC-using subjects (Goldey & van Anders, 2011), and future research should also explore these relationships in normally-cycling women.
Our samples were also modest in size and comprised students of a narrow age range around young adulthood. Mating motivation can be expected to be especially strong in this age range, and thus it will be important for future studies to explore the extent to which the present results generalize to other age groups and populations. In Study 2, we also collected some potentially useful data, such as relationship status, that were not available in Study 1. Given prior evidence, with some support from the present research, that this variable mediates relationships between T and sociosexuality, these data should be collected in future research. Finally, it will be important for future research to examine the extent to which these results generalize to other aspects sociosexual behavior, such as the frequency of sex and whether it occurred within the context of a long-term partnership, and these data should be collected over different time intervals.
Summary
We found that sociosexuality was related to testosterone in men, but not OC-using women. In two samples of men, we showed positive relationships between testosterone and interest in uncommitted sex and negative relationships between number of sex partners and testosterone, when both sociosexual psychology and number of sex partners were entered into a multiple regression model to predict testosterone. These results are consistent with a negative feedback loop, supported by prior empirical research and adaptive reasoning, in which testosterone increases desires for uncommitted sex and hence numbers of sexual partners, and in which the satisfaction of these desires lowers testosterone.
Highlights.
Testosterone (T) mediated the sex difference in sociosexual orientation.
T did not predict sociosexual orientation in women using oral contraception (OC).
T was differently related to sociosexual orientation in men and OC-using women.
Sociosexual orientation positively predicted T in two samples of men.
Controlling sociosexual orientation, sexual success negatively predicted T in men.
Acknowledgments
We thank Kim Wallen and three anonymous reviewers for their helpful comments on a previous draft of this paper. DAP was funded on NIH grant number T32MH070343-05 awarded to SMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvergne A, Faurie C, Raymond M. Variation in testosterone levels and male reproductive effort: insight from a polygynous human population. Horm Behav. 2009;56:491–497. doi: 10.1016/j.yhbeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Archer J. The influence of testosterone on human aggression. Br J Psychol. 1991;82(Pt 1):1–28. doi: 10.1111/j.2044-8295.1991.tb02379.x. [DOI] [PubMed] [Google Scholar]
- Archer J. Sex differences in aggression in real-world settings: a meta-analytic review. Review of General Psycholology. 2004;4:291–322. [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Archer J. Does sexual selection explain human sex differences in aggression? Behavioral and Brain Sciences. 2009;32:249–266. doi: 10.1017/S0140525X09990951. [DOI] [PubMed] [Google Scholar]
- Bogaert AF, Fisher WA. Predictors of university men’s number of sexual partners. Journal of Sex Research. 1995;32:119–130. [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men’s marriages. Social Forces. 1993;72:463–477. [Google Scholar]
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Hormones and Behavior. 1989;23:556–571. doi: 10.1016/0018-506x(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human Reproduction Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Burnham TC, Chapman JF, Gray PB, McIntyre MH, Lipson SF, Ellison PT. Men in committed, romantic relationships have lower testosterone. Hormones and Behavior. 2003;44:119–122. doi: 10.1016/s0018-506x(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Buss DM, Schmitt DP. Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Cashdan E. Hormones, sex, and status in women. Hormones and Behavior. 1995;29:354–366. doi: 10.1006/hbeh.1995.1025. [DOI] [PubMed] [Google Scholar]
- Charles NE, Alexander GM. The association between 2D:4D ratios and sociosexuality: a failure to replicate. Arch Sex Behav. 2011;40:587–595. doi: 10.1007/s10508-010-9715-z. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Mohammed S. Male and female salivary testosterone concentrations before and after sexual activity. Physiology and Behavior. 1992;52:195–197. doi: 10.1016/0031-9384(92)90453-9. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Belsky J. The development of life history strategies: toward a multi-stage theory. In: Buss DM, Hawley PH, editors. The Evolution of Personality and Individual Differences. Oxford: Oxford University Press; 2010. pp. 154–176. [Google Scholar]
- Del Giudice M, Belsky J. The development of life history strategies: Toward a multi-stage theory. In: Buss DM, Hawley PH, editors. The evolution of personality and individual differences. New York: Oxford University Press; 2011. pp. 154–176. [Google Scholar]
- Doll LM, Hill AK, Cárdenas RA, Welling LLM, Wheatley JR, Puts DA. How well do men’s faces and voices index mate quality and dominance? Human Nature. 2014;25:200–212. doi: 10.1007/s12110-014-9194-3. [DOI] [PubMed] [Google Scholar]
- Edelstein RS, Chopik WJ, Kean EL. Sociosexuality moderates the association between testosterone and relationship status in men and women. Horm Behav. 2011;60:248–255. doi: 10.1016/j.yhbeh.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Ellison PT. On fertile ground: A natural history of human reproduction. Cambridge, MA: Harvard University Press; 2001. [Google Scholar]
- Exton MS, Kruger TH, Bursch N, Haake P, Knapp W, Schedlowski M, et al. Endocrine response to masturbation-induced orgasm in healthy men following a 3-week sexual abstinence. World Journal of Urology. 2001;19:377–382. doi: 10.1007/s003450100222. [DOI] [PubMed] [Google Scholar]
- Faurie C, Pontier D, Raymond M. Student athletes claim to have more sexual partners than other students. Evolution and Human Behavior. 2004;25:1–8. [Google Scholar]
- Gangestad SW, Simpson JA. On the evolutionary psychology of human mating: Trade-offs and strategic pluralism. Behavioral and Brain Sciences. 2000;23:573–587. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R, Garver-Apgar CE. Men’s facial masculinity predicts changes in their female partners’ sexual interests across the ovulatory cycle, whereas men’s intelligence does not. Evolution and Human Behavior. 2010;31:412–424. [Google Scholar]
- Gettler LT, McDade TW, Agustin SS, Kuzawa CW. Short-term changes in fathers’ hormones during father-child play: Impacts of paternal attitudes and experience. Horm Behav. 2011a doi: 10.1016/j.yhbeh.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc Natl Acad Sci U S A. 2011b;108:16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Kuzawa CW. Cortisol and testosterone in Filipino young adult men: evidence for co-regulation of both hormones by fatherhood and relationship status. Am J Hum Biol. 2011c;23:609–620. doi: 10.1002/ajhb.21187. [DOI] [PubMed] [Google Scholar]
- Goldey K, van Anders S. Sexual Modulation of Testosterone: Insights for Humans from Across Species. Adaptive Human Behavior and Physiology. 2014:1–31. [Google Scholar]
- Goldey KL, van Anders SM. Sexy thoughts: effects of sexual cognitions on testosterone, cortisol, and arousal in women. Horm Behav. 2011;59:754–764. doi: 10.1016/j.yhbeh.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Goldey KL, van Anders SM. Sexual thoughts: links to testosterone and cortisol in men. Arch Sex Behav. 2012;41:1461–1470. doi: 10.1007/s10508-011-9858-6. [DOI] [PubMed] [Google Scholar]
- Gray PB. Marriage, parenting, and testosterone variation among Kenyan Swahili men. Am J Phys Anthropol. 2003;122:279–286. doi: 10.1002/ajpa.10293. [DOI] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior. 2002;23:193–201. [Google Scholar]
- Gray PB, Yang CF, Pope HG., Jr Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proc Biol Sci. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BJ. Gonadal androgen and sociosexual behavior of male mammals: a comparative analysis. Psychology Bulletin. 1974;81:383–400. doi: 10.1037/h0036568. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Hubert W, Schurmeyer T. Changes in saliva testosterone after psychological stimulation in men. Psychoneuroendocrinology. 1985;10:77–81. doi: 10.1016/0306-4530(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Hill AK, Hunt J, Welling LLM, Cárdenas RA, Rotella MA, Wheatley JR, et al. Quantifying the strength and form of sexual selection on men’s traits. Evolution and Human Behavior. 2013;34:334–341. [Google Scholar]
- Hodges-Simeon CR, Gaulin SJ, Puts DA. Voice correlates of mating success in men: examining “contests” versus “mate choice” modes of sexual selection. Archives of Sexual Behavior. 2011;40:551–557. doi: 10.1007/s10508-010-9625-0. [DOI] [PubMed] [Google Scholar]
- Jackson JJ, Kirkpatrick LA. The structure and measurement of human mating strategies: toward a multidimensional model of sociosexuality. Evolution and Human Behavior. 2007;28:382–391. [Google Scholar]
- Josephs RA, Sellers JG, Newman ML, Mehta PH. The mismatch effect: when testosterone and status are at odds. J Pers Soc Psychol. 2006;90:999–1013. doi: 10.1037/0022-3514.90.6.999. [DOI] [PubMed] [Google Scholar]
- Kinsey AC, Pomeroy WB, Martin CE. Sexual Behavior in the Human Male. Philadelphia: W. B. Saunders; 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Becker HB, Brodie HK, Doering CH, Moos RH, Hamburg DA. Orgasmic frequency and plasma testosterone levels in normal human males. Arch Sex Behav. 1976;5:125–132. doi: 10.1007/BF01541869. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Gettler LT, Muller MN, McDade TW, Feranil AB. Fatherhood, pairbonding and testosterone in the Philippines. Horm Behav. 2009;56:429–435. doi: 10.1016/j.yhbeh.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol Behav. 2010;99:8–16. doi: 10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lippa RA. Sex differences in sex drive, sociosexuality, and height across 53 nations: testing evolutionary and social structural theories. Arch Sex Behav. 2009;38:631–651. doi: 10.1007/s10508-007-9242-8. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Hay AC, Conklin PH. Attractive men induce testosterone and cortisol release in women. Horm Behav. 2009;56:84–92. doi: 10.1016/j.yhbeh.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Lukaszewski AW, Larson CM, Gildersleeve KA, Roney JR, Haselton MG. Condition-dependent calibration of men’s uncommitted mating orientation: Evidence from multiple samples. Evolution and Human Behavior. 2014;35:319–326. [Google Scholar]
- Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310:1289–1291. doi: 10.1136/bmj.310.6990.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JT, Baum MJ. Neonatal exposure of female ferrets to testosterone alters sociosexual preferences in adulthood. Psychoneuroendocrinology. 1986;11:167–176. doi: 10.1016/0306-4530(86)90051-x. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21:353–363. discussion 363–397. [PubMed] [Google Scholar]
- Mazur A, Lamb TA. Testosterone, status, and mood in human males. Hormones and Behavior. 1980;14:236–246. doi: 10.1016/0018-506x(80)90032-x. [DOI] [PubMed] [Google Scholar]
- Mazur A, Susman EJ, Edelbrock S. Sex difference in testosterone response to a video game contest. Evolution and Human Behavior. 1997;18:317–326. [Google Scholar]
- McIntyre M, Gangestad SW, Gray PB, Chapman JF, Burnham TC, O’Rourke MT, et al. Romantic involvement often reduces men’s testosterone levels--but not always: The moderating role of extrapair sexual interest. Journal of Personality and Social Psychology. 2006;91:642–651. doi: 10.1037/0022-3514.91.4.642. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone change after losing predicts the decision to compete again. Horm Behav. 2006;50:684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Money J, Ehrhardt AA. Fetal hormones and the brain: effect on sexual dimorphism of behavior -- a review. Arch Sex Behav. 1971;1:241–262. doi: 10.1007/BF01541686. [DOI] [PubMed] [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proc Biol Sci. 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ. An Introduction to Behavioral Endocrinology. 3. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Newman ML, Sellers JG, Josephs RA. Testosterone, cognition, and social status. Horm Behav. 2005;47:205–211. doi: 10.1016/j.yhbeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Ostovich JM, Sabini J. Timing of puberty and sexuality in men and women. Arch Sex Behav. 2005;34:197–206. doi: 10.1007/s10508-005-1797-7. [DOI] [PubMed] [Google Scholar]
- Oxford J, Ponzi D, Geary DC. Hormonal responses differ when playing violent video games against and ingroup and an outgroup. Evolution and Human Behavior. 2010;31:201–209. [Google Scholar]
- Penke L, Asendorpf JB. Beyond global sociosexual orientations: A more differentiated look at sociosexuality and its effects on courtship and romantic relationships. Journal of Personality and Social Psychology. 2008;95:1113–1135. doi: 10.1037/0022-3514.95.5.1113. [DOI] [PubMed] [Google Scholar]
- Peters M, Simmons LW, Rhodes G. Testosterone is associated with mating success but not attractiveness or masculinity in human males. Animal Behaviour. 2008;76:297–303. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pollet TV, van der Meij L, Cobey KD, Buunk AP. Testosterone levels and their associations with lifetime number of opposite sex partners and remarriage in a large sample of American elderly men and women. Horm Behav. 2011;60:72–77. doi: 10.1016/j.yhbeh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods Instruments & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Puts DA, Bailey DH, Cárdenas RA, Burriss RP, Welling LL, Wheatley JR, et al. Women’s attractiveness changes with estradiol and progesterone across the ovulatory cycle. Horm Behav. 2013;63:13–19. doi: 10.1016/j.yhbeh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Puts DA, Cárdenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Hormones and Behavior. 2010;58:282–289. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Puts DA, Jones BC, DeBruine LM. Sexual selection on human faces and voices. Journal of Sex Research. 2012;49:227–243. doi: 10.1080/00224499.2012.658924. [DOI] [PubMed] [Google Scholar]
- Roney JR, Lukaszewski AW, Simmons ZL. Rapid endocrine responses of young men to social interactions with young women. Horm Behav. 2007;52:326–333. doi: 10.1016/j.yhbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL, Lukaszewski AW. Androgen receptor gene sequence and basal cortisol concentrations predict men’s hormonal responses to potential mates. Proc Biol Sci. 2010;277:57–63. doi: 10.1098/rspb.2009.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K, Oki M, Honma S, Uehara H, Hasegawa T. The lower salivary testosterone levels among unmarried and married sexually active men. Journal of Ethology. 2007;25:223–229. [Google Scholar]
- Schmitt DP. Sociosexuality from Argentina to Zimbabwe: A 48-nation study of sex, culture, and strategies of human mating. Behavioral and Brain Sciences. 2005;28:247–275. doi: 10.1017/s0140525x05000051. discussion 275–311. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC. Effects of sugarless chewing gum as a stimulant on progesterone, cortisol, and testosterone concentrations assessed in saliva. International Journal of Psychophysiology. 2013;87:111–114. doi: 10.1016/j.ijpsycho.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Gangestad SW. Individual differences in sociosexuality: evidence for convergent and discriminant validity. J Pers Soc Psychol. 1991;60:870–883. doi: 10.1037//0022-3514.60.6.870. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evolution and Human Behavior. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- van Anders SM. Chewing gum has large effects on salivary testosterone, estradiol, and secretory immunoglobulin A assays in women and men. Psychoneuroendocrinology. 2010;35:305–309. doi: 10.1016/j.psyneuen.2009.06.009. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Brotto L, Farrell J, Yule M. Associations among physiological and subjective sexual response, sexual desire, and salivary steroid hormones in healthy premenopausal women. J Sex Med. 2009;6:739–751. doi: 10.1111/j.1743-6109.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Horm Behav. 2007a;51:477–482. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Watson NV. Multiple partners are associated with higher testosterone in North American men and women. Horm Behav. 2007b;51:454–459. doi: 10.1016/j.yhbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- van der Meij L, Buunk AP, van de Sande JP, Salvador A. The presence of a woman increases testosterone in aggressive dominant men. Horm Behav. 2008;54:640–644. doi: 10.1016/j.yhbeh.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Webster GD, Bryan A. Sociosexual attitudes and behaviors: Why two factors are better than one. Journal of Research in Personality. 2007;41:917–922. [Google Scholar]
- Wingfield JC, Lynn S, Soma KK. Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain, Behavior and Evolution. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, et al. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82:261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: What we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]