Abstract
Psychosocial stress, specifically social isolation, is an important risk factor for the development of a variety of psychological and physiological disorders. Changes in immune function have been hypothesized to mediate this relationship. The current study used the prairie vole (Microtus ochrogaster) model of isolation-induced depressive-like behavior to test whether social isolation led to changes in innate immune function. Specifically, we used hemolytic complement (CH50) and bacteria killing assays to assess complement activity, in paired or singly housed male and female prairie voles. Further, in a second experiment we tested whether females exposed to an additional short-term social stressor, a resident intruder trial, would show changes in immune function as well as enhanced HPA activity as indicated by elevated plasma corticosterone levels. Socially isolated animals, regardless of sex, had significantly reduced CH50s and bacteria killing ability. Socially isolated females exposed to a resident intruder stressor also showed reduced CH50s and bacteria killing ability as well as significant increases in aggressive behavior, however, they did not show elevated circulating corticosterone levels. Collectively, these data will help inform our understanding of the relationship between social isolation and physiological and psychological health.
Keywords: CH50, corticosterone, hemolytic complement, psychosocial stress, aggression, Microtus ochrogaster
Introduction
The negative effect of psychosocial stress on health outcomes has been well documented (Kendler et al., 1999; Marmot et al., 1991; Uchino et al., 1996). Social isolation, in particular, is a powerful risk factor for the development of variety of physiological and psychological disorders (Cacioppo et al., 2002; Cacioppo et al., 2006; Uchino et al., 1996). The health risks associated with social isolation have been suggested to be similar in magnitude to those incurred by smoking, obesity, and other major biomedical and psychosocial risk factors (House, 2001; House et al., 1988). As such, social isolation is not linked to a single disease pathway, but is an independent risk factor for a wide range of disease states including cancers, cardiovascular diseases, diabetes, mood disorders, and infectious diseases (Cacioppo et al., 2002; Hawkley et al., 2006; Uchino et al., 1996).
In the last several decades it has become clear that alterations in the immune system may be an epidemiological link between psychosocial stress and the development of a variety of disease states (Kiecolt-Glaser et al., 2002; Uchino, 2006; Uchino et al., 1996), and the innate branch of the immune system is of particular importance in this relationship. For instance, psychological stress elevates inflammatory markers in humans (Steptoe et al., 2007). Individuals that have been diagnosed with depression, which is often triggered by social stress, have shown elevated levels of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) in their plasma and cerebral spinal fluid (Raison et al., 2006). These and other data focused on immunity and mental health have led to the hypothesis that inflammatory processes may link psychosocial stress with the development of depressive disorders (Dantzer, 2006; Dantzer et al., 2008; Raison et al., 2006).
Lack of social support may also affect the pathogenesis of specific physiological conditions, such as cancer (Lutgendorf et al., 2005). Social isolation is also associated with increased susceptibility to respiratory viruses and other infections, as well an increases in the duration of illness and wound healing in both humans and rodents (Clausing et al., 1994; Cohen et al., 1997; Cohen et al., 1991; Glasper and DeVries, 2005; Hawkley and Cacioppo, 2003).
The prairie vole (Microtus ochrogaster) has been suggested as a potential model for studying the role of social stress, and social isolation in particular, in the development of psychological and physiological disorders (Bosch et al., 2009; Grippo, 2011; McNeal et al., 2014; Peuler et al., 2012). Prairie voles are ideal for this research because, unlike traditional rodent models, their social behavior is similar to that of humans and non-human primates, including: social monogamy, bi-parental and allo-parental care of offspring, and the formation of strong social bonds (Carter and Getz, 1993). In this system, social stressors, including social isolation from family members or a pair bonded mate, are sufficient to produce changes in behavior and physiology similar to those observed in humans with depression and cardiovascular conditions (e.g., learned helplessness, anhedonia, dysregulation of the HPA axis, cardiac and autonomic dysfunction) (Bosch et al., 2009; Grippo et al., 2010; Grippo et al., 2007b; Grippo et al., 2007c; Grippo et al., 2008; McNeal et al., 2014; Peuler et al., 2012).
Much less in known about the effects of social isolation on immune function in prairie voles, and comparatively less progress has been made toward the investigation of the potential role of immune system dysregulation in the development of isolation-induced behavioral and physiological dysfunction in this model. However, previous studies have reported that individually-housed prairie voles that are housed at high-density (10.96 animals/m3) have greater serum levels of immunoglobulin (IgG) and greater splenic masses than individually-housed animals held in low-density rooms (0.18 animals/m3) (Nelson et al., 1996). These results are not surprising considering prairie voles are highly social, often living in extended family groups, and can be found at high density in their natural habitat (Getz and Carter, 1996; Getz et al., 1993). Social isolation is stressful for prairie voles, and living at low density may be additionally so, such that immune function is affected. An additional study found that when housed singly, monogamous prairie voles and polygynous meadow and montane voles do not differ in splenocyte proliferation in response to a T cell mitogen (Concanavalin A) (Klein et al., 1997). When housed with conspecifics of either sex, however, prairie voles consistently had a greater proliferative response than did meadow voles, suggesting that perhaps isolation is immunosuppressive in prairie voles, but not in meadow voles, a far less social species (Klein et al., 1997).
Given the previous findings from behavioral and physiological research using the prairie vole, the aim of the current study was to test the hypothesis that social isolation negatively affects immune function in the monogamous prairie vole, and that additional short-term social stress would exacerbate immune dysfunction. Specifically, in two experiments we compared innate immune function in socially isolated versus pair-housed control animals via two in vitro measures: hemolytic complement (CH50) and bacterial killing assays. We chose these measures because the complement system is a critical component of innate humoral immunity (Théroux and Martel, 2006; Thurman and Holers, 2006). The major functions of the complement system include 1) the recognition and elimination of pathogens via direct killing (Thurman and Holers, 2006) and 2) initiation of the inflammatory response (Frank and Fries, 1991; Théroux and Martel, 2006).
Specifically, we predicted that socially isolated prairie voles (versus pair-housed controls), regardless of sex, would exhibit reduced hemolysis and reduced bacterial killing ability. We also predicted that isolated prairie voles would show elevated basal and stressor-induced corticosterone levels, as well as increased agonistic behaviors during a short-term social stressor. This work was undertaken as a first step to determine the association between social stressors and immune dysfunction.
Materials and Methods
Animals and Housing Conditions
Fifty-seven adult (60−90 days of age, 35−45 g body mass) male and female prairie voles, descendants of a wild stock caught near Champaign, Illinois, were used for the present experiments. All prairie voles were maintained on a 14/10 h light/dark cycle (lights on at 0630h), with a mean ± standard error (SEM) ambient temperature of 25±2°C and relative humidity of 40±5%. Animals were allowed food (Purina rabbit chow) and tap water ad libitum. Handling, cage changing, and measuring of body weight (weekly) were matched among all groups during the experimental protocols.
Prairie vole offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs until the commencement of the experimental protocol when animals were between 60 and 90 days of age. For all procedures described here, only one animal from each sibling pair was studied to avoid the possibility of the dependent measures being influenced by shared genetic factors. All procedures were conducted in accordance with the current National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the local university Institutional Animal Care and Use Committees.
Experimental Design
Experiment 1
Male and female prairie voles were randomly assigned to two independent groups: social isolation (n=13 males and n=9 females) or paired control conditions (n=9 males and n=10 females). Socially isolated animals were separated from their respective siblings and housed individually for 4 weeks. The socially isolated animal did not receive auditory, visual or olfactory cues from the sibling from which it was separated. Paired control animals were continually housed with their respective siblings of the same sex during this period. Following the 4-week period of social isolation or continued pairing, plasma was collected from each animal for the analysis of circulating basal corticosterone levels and innate immune function (detailed methods described in the following sections).
Experiment 2
Female prairie voles were randomly assigned to one of two independent groups: social isolation (n=8) or paired control conditions (n=8). Animals were either socially isolated or remained paired for 4 weeks, as described in Experiment 1. Following the 4-week period of social isolation or continued pairing, all animals were exposed to a 5-minute resident-intruder paradigm, in which the experimental animal was the resident (detailed methods described below). Ten minutes following the end of the resident-intruder paradigm, plasma was collected from each animal for the analysis of circulating stressor-induced corticosterone levels and innate immune function (detailed methods described in the following sections). Our previous work has suggested that female prairie voles may be more sensitive to social isolation than males (Grippo et al., 2007b). Therefore for this aspect of the study we chose to focus on female animals.
Resident-Intruder Paradigm
A resident-intruder test (Bosch et al., 2004; Grippo et al., 2007a) was conducted in Experiment 2 following 4 weeks of either social isolation or continued pairing. An unfamiliar animal of the same sex and approximate body weight (intruder) was placed into the cage of each paired or socially isolated animal (resident) for 5 minutes (for the paired group, the experimental animal’s sibling was first removed from the home cage). Aggressive behaviors (aggressive grooming or posture, lunging, swiping, sniffing, and attack behavior) were digitally video recorded and scored by two trained, experimentally-blind observers (Mitchell et al., 2003). All resident intruder trials occurred during the light period, between 0930h and 1130h.
Blood Sampling
All animals were anesthetized with a combination of ketamine (67 mg/kg, sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, sc; NLS Animal Health, Owings Mills, MD) during the light period (between 0930h and 1130h). Blood was sampled within 2 minutes of the anesthetic injection, from the periorbital sinus via a heparanized capillary tube, and was collected during a period not exceeding 1.5 minutes. The blood was placed immediately on ice, and subsequently centrifuged at 4° C, at 3500 rpm, for 15 minutes to obtain plasma. Plasma aliquots were stored at −80° C until assayed for circulating corticosterone and innate immune function (complement activity and bacterial killing ability).
Corticosterone Assay
Plasma levels of corticosterone were determined using a commercially available enzyme-linked immunosorbent assay kit (Enzo Life Sciences, ADI-900-097, Farmingdale, NY), which has been validated previously by our laboratory for use in prairie voles (McNeal et al., 2014). Plasma was diluted in assay buffer as necessary (1:500 ratio) to produce results reliably within the linear portion of the standard curve. The minimum detection limit for this assay is 27.0 pg/ml, and inter- and intra-assay coefficients of variation are less than 5%. Cross-reactivity with other steroids or peptides is less than 1.7%.
Hemolytic Complement Assay
To measure hemolytic complement activity we followed previously published methods (Greives et al., 2006). Briefly, plasma was diluted 1:75 in dextrose-gelatin veronal buffer (VB) (BioWhittaker, Walkersville, MD) and added in duplicate to 96-well round-bottomed microplates. VB was then added to remaining empty wells, and plasma was serially diluted twofold to a final concentration of 1:150. The final amount of sample in each well was 40 µl, to which 25 µl each of a 0.6% suspension of washed sheep red blood cells (in VB) (SRBC; MP Biomedicals, Irvine, CA) and a 1:40 dilution of rabbit anti-SRBC antibody in VB (Sigma-Aldrich Inc., St. Louis, MO) were added. Lysis wells of 0% and 100% were created by adding 65 µl VB or water, respectively, and 25 µl of 0.6% washed SRBC. The plates were shaken at ~180 rpm on a plate shaker for 5 minutes, incubated for 1.5 hours at 37°C, and then centrifuged for 5 min at 500 rpm at room temperature. Sixty µl of the supernatant from each well was removed, added to a new U-shaped microplate and read at 405 nm. Hemolytic complement activity was expressed as CH50, where a CH50 unit is the reciprocal of the dilution that caused 50% of antibody sensitized SRBCs to lyse. A higher CH50 is correlated with greater lytic ability of an animal’s complement system.
Bacterial Killing Assay
We used an ex vivo bacterial killing assay as a functional assessment of the innate immune system’s ability to clear a relevant pathogen [modified from (Matson et al., 2006)]. This assay quantifies the relative number of Escherichia coli (E. coli) colony forming units (CFU) that grow after incubation with plasma. The strain of E-coli (ATCC#8739) used in this study was complement dependent, meaning that this test relied on complement activity to kill the bacteria (Demas et al., 2011). Briefly, lyophilized E. coli (Epower™, ATCC #8739, Microbiologics, St. Cloud, MN; 1 pellet = 107 CFU) was added to 40 ml 1 M sterile PBS warmed to 37°C to create a bacterial stock solution. This solution was activated by incubation for 30 min at 37°C. The stock bacteria solution (500,000 CFU/ml) was diluted 1:10 with sterile 1 M PBS to create a 50,000 CFU/ml working solution. Meanwhile, plasma samples were diluted 1:20 in glutamine enriched CO2-independent media (Invitrogen Corp., Carlsbad, CA). For each sample, the bacterial working solution was added at a 1:10 ratio to the diluted plasma sample. To generate a positive control (i.e., solution containing only media and bacteria), the bacterial working solution was diluted 1:10 with glutamine enriched CO2-independent media. The diluted samples and the positive control were incubated for 30 min at 37°C to induce bacterial killing. After incubation, 50 µl of the samples and the positive control were added to tryptic soy agar plates in duplicate. All plates were stored upside down overnight at 37°C. Following incubation, colony numbers were counted on each plate, and duplicates were averaged. Bactericidal capacity was calculated as a percent of bacteria killed relative to the positive control plates in which no killing occurred.
Data Analysis
Data are presented as means ± SEM for all analyses and figures. A value of P < 0.05 was considered to be statistically significant. Data were analyzed using standard statistical computing software (SPSS version 19, IBM Corporation, Armonk, New York). Student’s t-tests were used for all between-groups comparisons, with a Bonferroni correction for multiple comparisons. Cohen’s d was calculated to determine effect size for each significant t-test comparison (Cohen, 1988). A z-test was used to analyze behaviors during the resident-intruder paradigm in Experiment 2. Pearson’s r correlation coefficients were used to compute relevant correlations between corticosterone and immune function.
Results
Hemolytic Complement Activity
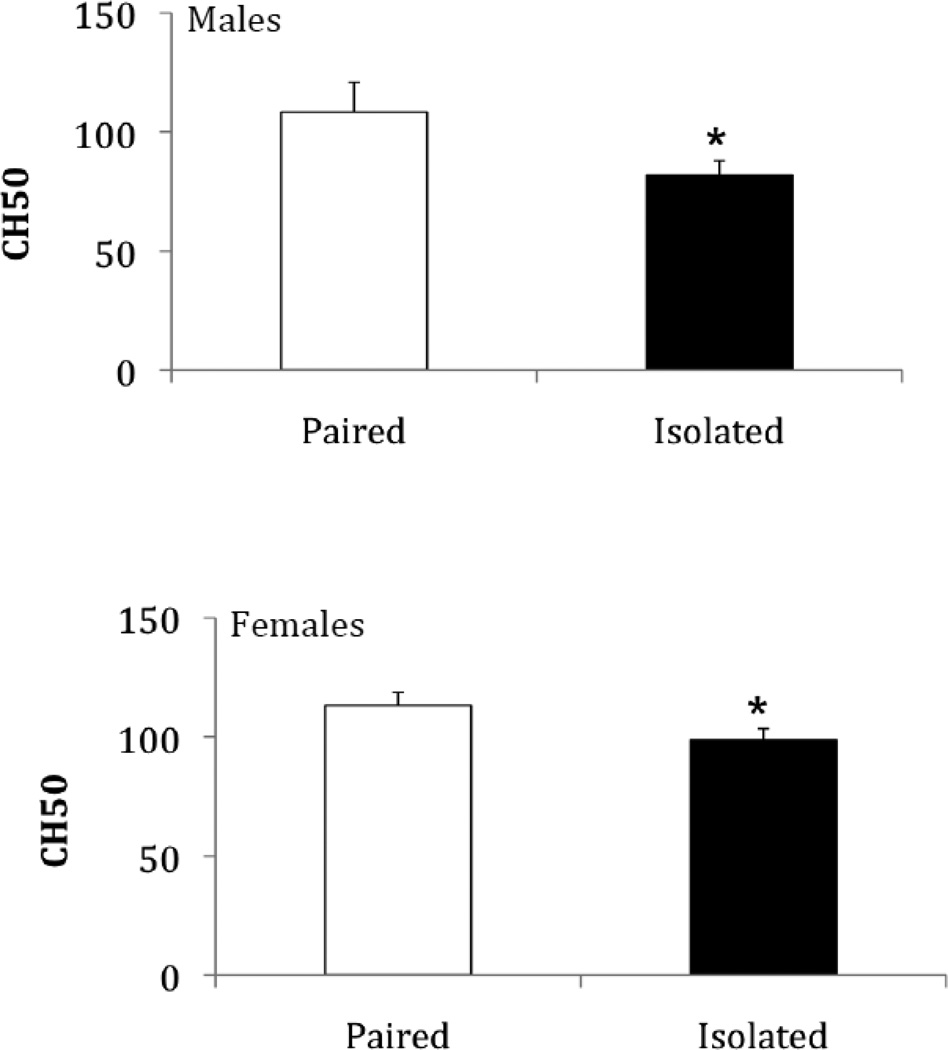
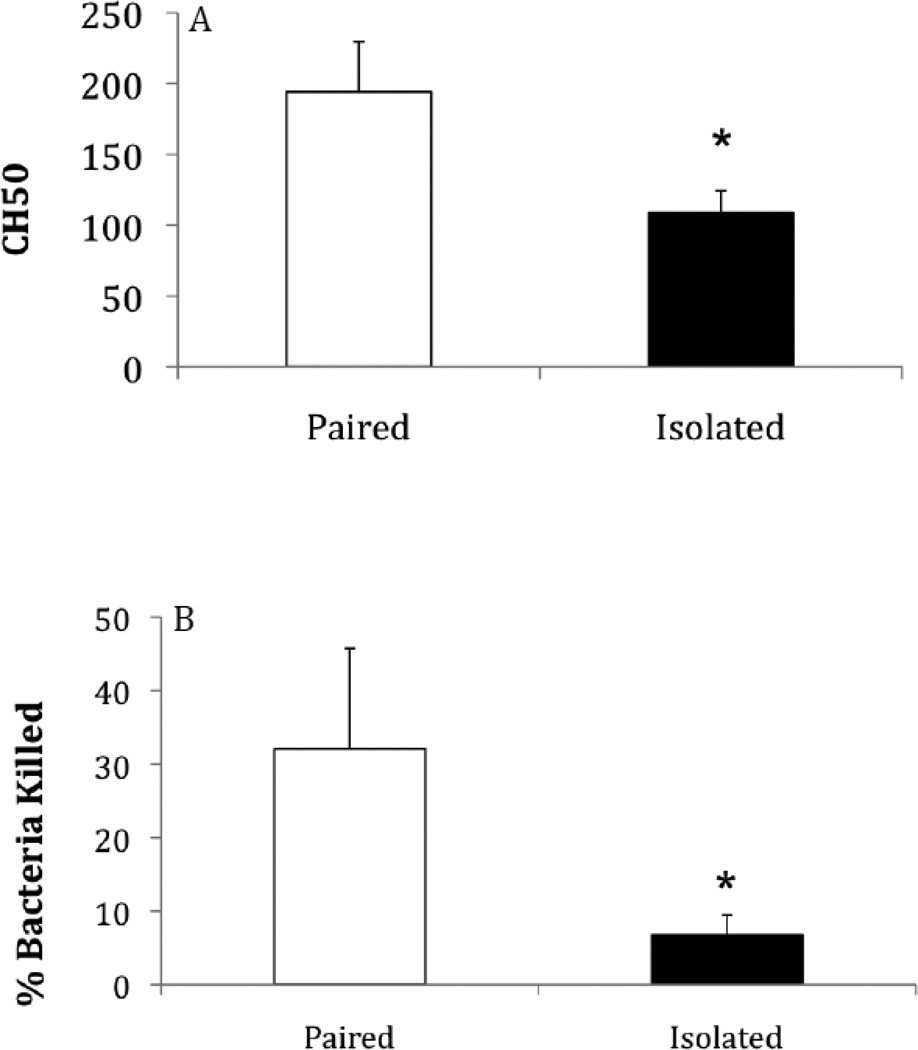
Social isolation (compared to pairing) was associated with a reduction in complement activity in both males and females (Figure 1), as well as in females following the resident-intruder paradigm (Figure 3A). Relative to paired control animals, CH50 levels were significantly reduced in socially isolated males [t(20) = 2.04, p < 0.03, Cohen’s d = 0.91], socially isolated females [t(17) = 1.95, p < 0.03, Cohen’s d = 0.95], and socially isolated females following the resident-intruder test [t(14) = 2.20, p < 0.02, Cohen’s d = 1.18].
Figure 1.
Mean (+ SEM) CH50 values following 4 weeks of either social isolation or paired control conditions in males and females. *P < 0.05 vs. respective paired control value.
Figure 3.
Mean (+ SEM) CH50 values (A), and percentage of bacteria killed (B), in females following 4 weeks of either social isolation or paired control conditions and a 5-minute resident intruder test.
Bacterial Killing Assay
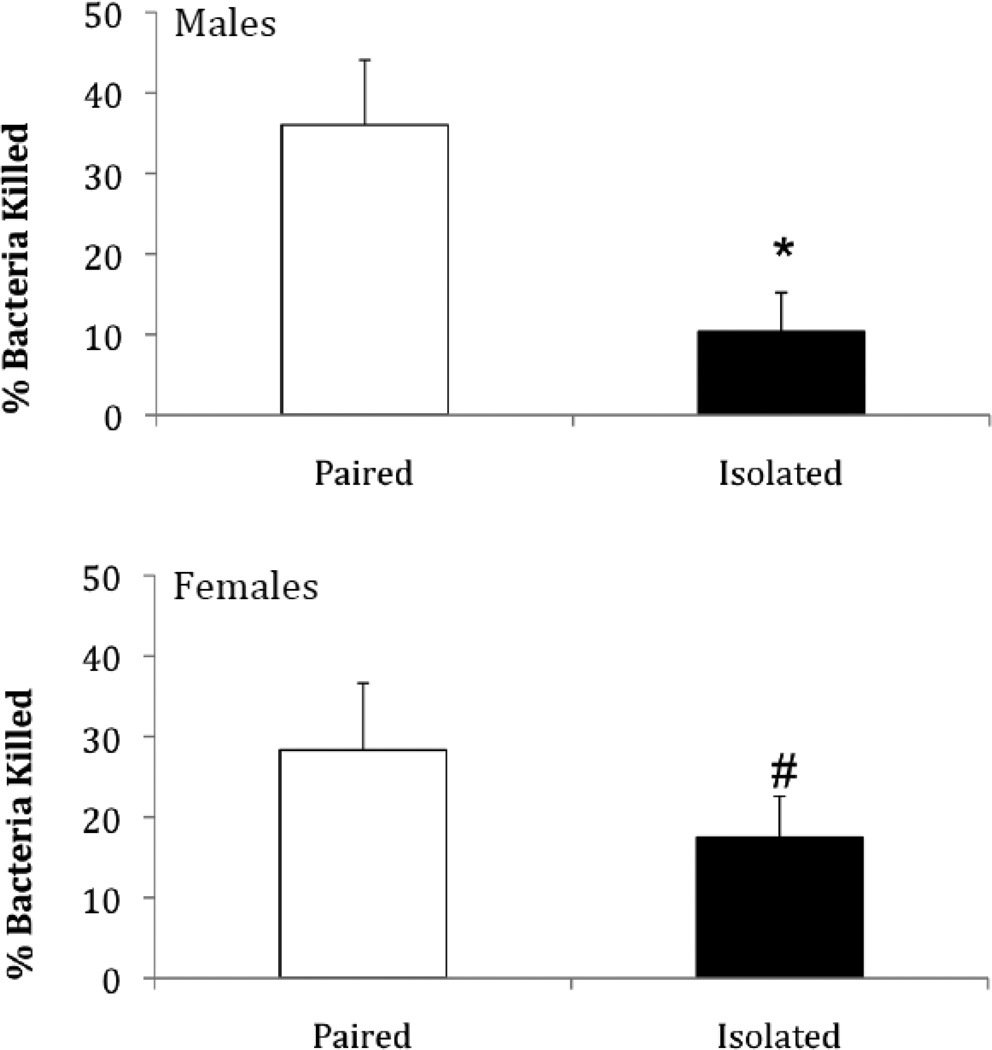
Social isolation (compared to pairing) was associated with a reduction in bacterial killing ability in both males and females (Figure 2), as well as in females following the resident-intruder paradigm (Figure 3B). Relative to paired control animals, the percentage of bacteria killed was reduced in socially isolated males [t(20) = 2.91, p < 0.004, Cohen’s d = 1.30]. Socially isolated females also showed a reduction in BKA, but it did not quite meet significance [t(17) = 1.48, p < 0.06, Cohen’s d = 0.72]. Socially isolated females did show a significant reduction in BKA when tested following the resident-intruder test [t(14) = 1.83, p < 0.05, Cohen’s d = 0.98].
Figure 2.
Mean (+ SEM) percentage of bacteria killed following 4 weeks of either social isolation or paired control conditions in males and females. *P < 0.05 vs. respective paired control value; #P < 0.06 vs. respective paired control value.
Agonistic Behaviors
Social isolation (compared to pairing) was associated with a significantly greater likelihood of attacking the intruder during the resident-intruder paradigm, however it did not affect the number or duration of specific aggressive behaviors. A z-test indicated a significant difference between the proportion of animals in the socially isolated group vs. the paired group that attacked the intruder [5/8 (62.5%) of socially isolated animals vs. 1/8 (12.5%) of paired animals, z = 2.07, p < 0.05]. Student’s t-tests indicated no significant differences between paired and socially isolated animals in either the number or duration of lunges, swipes, sniffs, or grooming episodes (p > 0.05 for all comparisons; data not shown).
Corticosterone
Social isolation did not significantly affect circulating corticosterone levels, relative to paired control conditions, in either Experiments 1 or 2 (p > 0.05 in all cases; Table 1). However, corticosterone levels were significantly higher in both paired and socially isolated females that were exposed to the resident-intruder test, relative to females that did not experience agonistic interactions [Student’s t-tests with a Bonferroni correction; paired, t(14) = 2.77, p < 0.007, Cohen’s d =1.48 ; socially isolated, t(14) = 1.98, p < 0.03, Cohen’s d = 1.06 ; Table 1].
Table 1.
Circulating corticosterone (± SEM) levels following social isolation or pairing alone (basal levels) and following the resident-intruder test (RI; stressor-induced levels).
| Paired | Socially Isolated | |
|---|---|---|
| Males, Isolation/Pairing Alone (Basal Levels; Experiment 1 | 535.6 ± 112.2 | 367.2 ± 55.6 |
| Females, Isolation/Pairing Alone (Basal Levels; Experiment 1) | 513.2 ± 134.9 | 610.2 ± 96.0 |
| Females, Isolation/Pairing + 5-Min RI (Stressor-Induced Levels; Experiment 2) | 922.3 ± 59.6* | 992.75 ± 167.7* |
P < 0.05 vs. respective females exposed to isolation/pairing alone (Experiment 1).
Correlations
Although the sample sizes in this study were small, we computed correlations between corticosterone and each BKA and CH50 to gain an understanding of the general associations among these dependent measures. All Pearson’s r, R2, and probability values for Experiments 1 and 2 are reported in Table 2.
Table 2.
Correlations between circulating corticosterone levels and immune measures following social isolation or pairing alone (Experiment 1) and following the resident-intruder test (RI; Experiment 2).
| Corticosterone vs. BKA | Corticosterone vs. CH50 | |
|---|---|---|
| Isolated Males | r = 0.13, p > 0.05, R2 = 0.02 | r = −0.41, p > 0.05, R2 = 0.17 |
| Paired Males | r = −0.82, p= 0.007, R2 = 0.67* | r = −0.29, p> 0.05, R2 = 0.08 |
| Isolated Females | r = −0.35, p> 0.05, R2 = 0.13 | r = −0.48, p> 0.05, R2 = 0.23 |
| Paired Females | r = −.70, p= 0.025, R2 = 0.49* | r = 0.26, p> 0.05, R2 = 0.07 |
| Isolated Females + 5 min RI | r = −0.08, p> 0.05, R2 = 0.01 | r = −0.16 p> 0.05, R2 = 0.03 |
| Paired Females + 5 min RI | r = −0.018, p> 0.05, R2 = 3.09 E−4 | r = 0.78, p=0.038, R2 = 0.61* |
P < 0.05.
Discussion
Social stressors, and social isolation in particular, have been consistently shown to negatively influence health (Cacioppo et al., 2002; Cacioppo et al., 2006; Uchino et al., 1996). Although it is likely that more than one system is affected, a large body of literature has implicated changes in immune function in particular as a link between social stressors and various negative health outcomes (Glaser and Kiecolt-Glaser, 2005; Uchino, 2006; Uchino et al., 1996). Prairie voles have been previously shown to suffer similar psychological and physiological effects of social isolation to humans (Bosch et al., 2009; Grippo et al., 2007b; Grippo et al., 2008; McNeal et al., 2014; Peuler et al., 2012). The purpose of the current study was to investigate the potential that, as in humans, social isolation impairs immune function in these rodents. Our hypothesis that social isolation results in deficits in innate immunity was supported. In both sexes, social isolation was associated with a significant decrease in complement activity (Figure 1). Our data suggest, therefore, that both socially isolated male and socially isolated female voles had impairment of the pathway required to destroy foreign cells. Further, in a more functional test of innate immunity, the bacterial killing assay, social isolation reduced the ability of plasma to kill E. coli colonies in both sexes, however the data only reached significance in males (Figure 2). Similarly, under stressor-induced conditions in females (resident-intruder stressor), impairments in both complement activity and bacterial killing ability also were observed (Figure 3).
The present findings are consistent with a vast amount of evidence indicating an association between chronic social stressors and alterations in immune function in both humans and animal models (Glaser and Kiecolt-Glaser, 2005; Kim and Maes, 2003; Nguyen et al., 1998; Raison et al., 2006; Slavich et al., 2010). For example, chronic mild stress not only induces depressive-like behavior (anhedonia) in Flinders lines of rats, but also results in decreased CH50 responses (Ayensu et al., 1995). Similarly, guinea pigs and calves subjected to social stressors also show reduced complement activity (Purdy et al., 1991; Stefanski and Hendrichs, 1996). In humans, higher Beck Depression Inventory Scores have been correlated with reduced total hemolytic complement activity (CH50), as well as reduced T-cell count and elevated IL-1 levels (Kimmel et al., 2002). Chronic social stress in humans has been associated with the later development of depressive disorders (Hammen, 2005; Kendler et al., 1999), and individuals with depression show elevated levels of inflammatory markers such as the cytokines IL-6 and TNF-α, as well as C-reactive protein (CRP) (Raison et al., 2006). IL-6 is the most important inducer of acute phase proteins, such as CRP, which are important for the activation of the complement system (Baumann and Gauldie, 1994; Maier and Watkins, 1998). However, other data from humans suggest that some individuals suffering from major depression show increased activation of aspects of the complement pathway (Song et al., 1994). This discrepancy may be the result of differential responses to chronic and acute stressors.
Prairie voles are characterized by significantly higher levels of circulating glucocorticoids than other rodents such as mice (Klein et al., 1996). As such, it has be suggested that they are glucocorticoid-resistant to these high circulating levels because they do not show reduced immune responses as a result, unlike glucocorticoid-sensitive species such as mice (Klein et al., 1996). It is possible, however, that long-term exposure to stressor-induced elevated levels of glucocorticoids may have led to the deficits in complement activity in socially isolated prairie voles. In fact, both in vivo and in vitro studies have found that these steroids inhibit both the classical and alternative pathways of the complement system (Atkinson and Frank, 1973). Further, glucocorticoids may inhibit the lytic action of the complement system (Gewurz et al., 1965; Jennings and Taylor, 1964; Packard and Weiler, 1983). As such, a negative effect of social isolation on complement activity, including negative correlations between circulating glucocorticoid levels and CH50 and BKA, could be expected. Consistent with previous studies (Grippo et al., 2007b), we did not observe significant differences in corticosterone levels between housing groups. Social isolation produces alterations in several physiological parameters in prairie voles, but this treatment alone does not significantly elevate circulating levels of corticosterone in either males or females (Grippo et al., 2007b). Interestingly, socially isolated prairie voles previously have shown a heightened endocrine response (e.g., elevated corticosterone) to acute stressors relative to paired animals (Grippo et al., 2007b; McNeal et al., 2014). In contrast to these previous findings, we did not replicate this effect in the present study, as female prairie voles that were exposed to an intruder had significantly elevated corticosterone levels relative to animals not exposed to an intruder regardless of whether they had been previously socially isolated or paired (Table 1).
Although we did not detect any significant main effects of chronic social isolation on circulating corticosterone levels, we investigated the general association between corticosterone and each BKA and CH50 levels in the present experiments. Interesting associations were observed between corticosterone and these immune measures. In Experiment 1, a negative association was observed between circulating corticosterone and BKA levels in both paired males and females, but not isolated in animals. It is unclear why this relationship was only observed in paired animals. Although we did not find any significant differences in the corticosterone levels between groups at the time point that we measured, it is possible that corticosterone was in fact elevated in isolated animals at other time points during their 4-week isolation period. Alternatively, it is possible that chronic sympathetic hyper-activation [as a function of increased sympathetic drive and/or down-regulation of parasympathetic drive, which has been observed in previous studies of social isolation (Grippo et al., 2007c)] may have led to an overall reduction in immune activity such that there was no longer an effect of glucocorticoid exposure on complement activity.
In contrast to Experiment 1, a positive association between corticosterone levels and CH50 was observed in paired (but not socially isolated) females that underwent resident-intruder trials (Experiment 2). The resident-intruder trial was an acute social stressor, which seems to have had different effects on this relationship than the chronic isolation stressor alone. It is possible that the positive relationship between CH50 and corticosterone levels in these animals could be due to the pro-inflammatory action of acute elevations in glucocorticoid secretion (Sapolsky et al., 2000) or the type of stressor itself, as psychosocial stress has been found to prevent the anti-inflammatory effects of glucocorticoids (Miller et al., 2002; Sheridan et al., 2000). Similar to the findings in Experiment 1, there is no effect in socially isolated animals. However, these data are not unprecedented as an acute stressor (forced swim test) in isolated prairie voles did not reduce immunoglobulin levels in these animals, although corticosterone levels were elevated post-test (DeVries et al., 1997). Furthermore, the effects of stress on immune function are not always suppressive; for example, stressor-induced secretion of glucocortioicds can suppress acquired immunity (e.g., antibody responses) but can enhance specific components of the innate immune responses (Chester et al., 2010). In any case it is clear that the social environment to which the prairie voles were exposed significantly affected the functioning of their innate immunity, and these changes could have implications for disease susceptibility.
Socially isolated and paired female animals also differed in their behavioral response to an intruder; socially isolated females were significantly more likely to attack the intruder than paired animals. It has been long known that social isolation can induce aggressive behavior in many species, especially rodent systems (Valzelli, 1973). It is quite possible that the mechanisms that underlie this behavioral shift are similar to those that mediate other behavioral changes that occur after periods of protracted isolation (e.g., depressive-like behaviors, anxiety-like behaviors, etc.). For example, socially isolated rodents have altered HPA axis and sympathetic nervous system activity (Grippo et al., 2007c; Sánchez et al., 1995). Heightened aggression has been observed in rats that exhibit elevated sympathetic activity in response to stressors (Koolhaas, 2008). It has been well established that prairie voles that have been socially isolated show increased sympathetic tone and a reduction of parasympathetic activation both at baseline and when exposed to physical stressors (Grippo et al., 2007c; McNeal et al., 2014). Interestingly, sympathetic nervous system activation is known to affect immune function, and some studies suggest that chronic sympathetic activation may be immunosuppressive (McClelland, 1982; McClelland et al., 1980; Prass et al., 2003).
In conclusion, social isolation is a significant risk factor for the development of a variety of physiological and psychological disorders. Changes in immune function have been postulated to be a mechanism that may underlie the relationship between social isolation and some disease states. Therefore, these studies will enhance our understanding of the physiological changes that occur during extended periods of social stress in a rodent model system of isolation-induced dysfunction. Collectively, these data will help inform our understanding of the relationship between social isolation and physiological and psychological health. Future studies will benefit from investigating the role of pro-inflammatory cytokines and other inflammatory mediators in the development of behavioral and physiological changes as a function of social stressors.
Highlights.
Socially isolated prairie voles have reduced innate immune function.
Isolated animals of both sexes have reduced hemolytic complement activity (CH50).
Plasma from isolated animals has reduced bacteria killing ability (BKA) in vitro.
Isolated animals did not display elevated circulating corticosterone levels.
An acute social stressor enhances aggression in isolated females.
Acknowledgements
The investigators would like to thank Christina Bishop, Joshua Wardwell, and Neal McNeal for assistance. This research was supported by the following funding sources: National Institutes of Health [MH-77581 (AJG), HL-112350 (AJG), HL-108475 (M-ALS)]; NSF IOS 0543798 (GED), NSF Graduate Research Fellowship (EDC), and Northern Illinois University (AJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Atkinson JP, Frank MM. Effect of cortisone therapy on serum complement components. J. Immunol. 1973;111:1061–1066. [PubMed] [Google Scholar]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol. Behav. 1995;57:165–169. doi: 10.1016/0031-9384(94)00204-i. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol. Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Bosch O, Krömer S, Brunton P, Neumann I. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch O, Nair H, Ahern T, Neumann I, Young L. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom. Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol. Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Sci. Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Chester EM, Bonu T, Demas GE. Social defeat differentially affects immune responses in Siberian hamsters (Phodopus sungorus) Physiol. Behav. 2010;101:53–58. doi: 10.1016/j.physbeh.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Clausing P, Bocker T, Diekgerdes J, Gärtner K, Güttner J, Haemisch A, Veckenstedt A, Weimer A. Social isolation modifies the response of mice to experimental Mengo virus infection. J. Exper. Anim. Sci. 1994;36:37–54. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. second ed. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. J.A.M.A. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS. Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J. Anim. Ecol. 2011;80:710–730. doi: 10.1111/j.1365-2656.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Gerber JM, Richardson HN, Moffatt CA, Demas GE, Taymans SE, Nelson RJ. Stress affects corticosteroid and immunoglobulin concentrations in male house mice (Mus musculus) and prairie voles (Microtus ochrogaster) Comp. Biochem. Physiol. A-Physiol. 1997;118:655–663. doi: 10.1016/s0300-9629(97)87355-0. [DOI] [PubMed] [Google Scholar]
- Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol. Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am. Sci. 1996:56–62. [Google Scholar]
- Getz LL, McGuire B, Pizzutio T, Hofman JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J. Mammal. 1993;74:44–48. [Google Scholar]
- Gewurz H, Wernick PR, Quie PG, Good RA. Effects of hydrocortisone succinate on the complement system. Nature. 1965;208:755–757. doi: 10.1038/208755a0. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glasper ER, DeVries AC. Social structure influences effects of pair-housing on wound healing. Brain Behav. Immun. 2005;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Greives T, McGlothlin J, Jawor J, Demas G, Ketterson E. Testosterone and innate immune function inversely covary in a wild population of breeding dark- eyed juncos (Junco hyemalis) Funct. Ecol. 2006;20:812–818. [Google Scholar]
- Grippo A, Sgoifo A, Mastorci F, McNeal N, Trahanas D. Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. Auton. Neurosci. 2010;156:44–50. doi: 10.1016/j.autneu.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ. The utility of animal models in understanding links between psychosocial processes and cardiovascular health. Soc. Personal. Psychol. Compass. 2011;5:164–179. doi: 10.1111/j.1751-9004.2011.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med. 2007a;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry. 2007c;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress. Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Ann. Rev. Clin. Psych. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Bosch JA, Engeland CG, Marucha PT, Cacioppo JT. Loneliness, dysphoria, stress, and immunity: a role for cytokines. In: Faith RE, Murgo AJ, Good RA, Plotnikoff NP, editors. Cytokines: Stress and Immunity. 2nd ed. Boca Raton, Florida: CRC Press; 2006. pp. 67–85. [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness and pathways to disease. Brain Behav. Immun. 2003;17:98–105. doi: 10.1016/s0889-1591(02)00073-9. [DOI] [PubMed] [Google Scholar]
- House JS. Social isolation kills, but how and why? Psychosom. Med. 2001;63:273–274. doi: 10.1097/00006842-200103000-00011. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jennings J, Taylor G. Effect of hydrocortisone hemi-succinate on immune lysis of sheep erythrocytes. Nature. 1964;203:661. doi: 10.1038/203661a0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiat. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J. Consult. Clin. Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Kim YK, Maes M. The role of the cytokine network in psychological stress. Acta Neuropsychiatrica. 2003;15:148–155. doi: 10.1034/j.1601-5215.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Kimmel PL, Patel SS, Peterson RA. Depression in African-American patients with kidney disease. Journal of the National Medical Association. 2002;94:92S–103S. [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, DeVries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm. Behav. 1997;32:30–39. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- Klein SL, Taymans SE, DeVries AC, Nelson RJ. Cellular immunity is not compromised by high serum corticosterone concentrations in prairie voles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1996;40:R1608. doi: 10.1152/ajpregu.1996.271.6.R1608. [DOI] [PubMed] [Google Scholar]
- Koolhaas J. Coping style and immunity in animals: making sense of individual variation. Brain Behav. Immun. 2008;22:662–667. doi: 10.1016/j.bbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil-servants-the Whitehall-II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Matson KD, Tieleman BI, Klasing KC. Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiol. Biochem. Zool. 2006;79:556–564. doi: 10.1086/501057. [DOI] [PubMed] [Google Scholar]
- McClelland DC. The need for power, sympathetic activation, and illness. Motive. Emotion. 1982;6:31–41. [Google Scholar]
- McClelland DC, Floor E, Davidson RJ, Saron C. Stressed power motivation, sympathetic activation, immune function, and illness. J. Hum. Stress. 1980;6:11–19. doi: 10.1080/0097840X.1980.9934531. [DOI] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, LaRocca M, Trahanas DM, Grippo AJ. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Fairhall S, Fletcher A, Redfern P. Effects of single and repeated electroconvulsive shock on the social and agonistic behaviour of resident rats. Neuropharmacology. 2003;44:911–925. doi: 10.1016/s0028-3908(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Fine JB, Demas G, Moffatt CA. Photoperiod and population density interact to reproductive and immune function in male prairie voles. Am. J. Physiol. 1996;270:R572–R576. doi: 10.1152/ajpregu.1996.270.3.R571. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. J. Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard B, Weiler J. Steroids inhibit activation of the alternative-amplification pathway of complement. Infect. Immun. 1983;40:1011–1014. doi: 10.1128/iai.40.3.1011-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuler JD, Scotti M-AL, Phelps LE, McNeal N, Grippo AJ. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol. Behav. 2012;106:476–484. doi: 10.1016/j.physbeh.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1–like immunostimulation. J. Exp. Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy CW, Richards AB, Foster GS. Market stress-associated changes in serum complement activity in feeder calves. Am. J. Vet. Res. 1991;52:1842–1847. [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M, Aguado F, Sánchez-Toscano F, Saphier D. Effects of prolonged social isolation on responses of neurons in the bed nucleus of the stria terminalis, preoptic area, and hypothalamic paraventricular nucleus to stimulation of the medial amygdala. Psychoneuroendocrinology. 1995;20:525–541. doi: 10.1016/0306-4530(94)00083-m. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sheridan J, Stark J, Avitsur R, Padgett D. Social disruption, immunity, and susceptibility to viral infection: role of glucocorticoid insensitivity and NGF. Ann. N.Y. Acad. Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. U S A. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Dinan T, Leonard B. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J. Affect. Disord. 1994;30:283–288. doi: 10.1016/0165-0327(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Hendrichs H. Social confrontation in male guinea pigs: behavior, experience, and complement activity. Physiol. Behav. 1996;60:235–241. doi: 10.1016/0031-9384(95)02269-4. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Théroux P, Martel C. Complement activity and pharmacological inhibition in cardiovascular disease. Can. J. Cardiol. 2006;22:18B–24B. doi: 10.1016/s0828-282x(06)70982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J. Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J. Behav. Med. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]