Abstract
Seasonal responses of many animal species are triggered by changes in daylength and its transduction into a neuroendocrine signal by the pineal gland through the nocturnal duration of melatonin (MEL) release. The precise central sites necessary to receive, transduce, and relay the short day (SD) fall-winter MEL signals into seasonal responses and changes in physiology and behavior are unclear. In Siberian hamsters, SDs trigger decreases in body and lipid mass, testicular regression and pelage color changes. Several candidate genes and their central sites of expression have been proposed as components of the MEL transduction system with considerable recent focus on the arcuate nucleus (ARC) and its component, the dorsomedial posterior arcuate nucleus (dmpARC). This site has been postulated as a critical relay of SD information through the modulation of a variety of neurochemicals/receptors important for the control of energy balance. Here the necessity of an intact dmpARC for SD responses was tested by making electrolytic lesions of the Siberian hamster dmpARC and then exposing them to either long days (LD) or SDs for 12 weeks. The SD typical decreases in body and fat mass, food intake, testicular volume, serum testosterone concentrations, pelage color change and increased UCP-1 protein expression (a proxy for brown adipose tissue thermogenesis) all occurred despite the lack of an intact dmpARC. Although the Siberian hamster dmpARC contains photoperiod-modulated constituents, these data demonstrate that an intact dmpARC is not necessary for SD responses and not integral to the seasonal energy- and reproductive-related responses measured here.
INTRODUCTION
Organisms inhabiting temperate zones are subject to environmental vicissitudes including, but not limited to, seasonal fluctuations in energy availability, temperature and precipitation. Seasonal changes in the environment have resulted in the evolution of mechanisms that are tuned to promote survival and reproduction over a wide range of conditions (Bronson, 1985; Lincoln and Short, 1980). Despite the myriad of environmental variables that could be cues to drive these mechanisms, photoperiod is the most noise-free cue and used by many temperate climate mammals to synchronize physiology and behavior to the environment (Turek and Campbell, 1979). Siberian hamsters (Phodopus sungorus) have been studied extensively in the laboratory as a model of seasonally-driven changes in physiology and behavior. These animals exhibit a remarkable suite of robust seasonal variations that include changes in reproduction, body and lipid mass, thermogenesis, aggression, immune function, pelage, and energy intake, all of which can be triggered by alterations in the photoperiod (for review see: (Bartness, et al., 2002; Prendergast, et al., 2002)). In Siberian hamsters specifically, as well as other small seasonal mammals, the photoperiod cue is converted into an endocrine signal via the retino-hypothalamic-pineal pathway that receives light/dark information resulting in the release of pineal melatonin (MEL; (Larsen, et al., 1998)). MEL is released in direct proportion to the length of the night, such that long daylengths (LD; summer-like) result in short durations of MEL release and short daylengths (SD; winter-like) result in long durations of MEL release.
Some of the central sites at which MEL drives the seasonal changes in physiology and behavior have been known for ~15–20 years, but the mechanism by which the MEL signal is transduced to the hypothalamus and other brain areas, and ultimately manifested in seasonal changes in their physiology and phenotype remains incompletely known. Early work uncovered several MEL binding sites within the hypothalamus and thalamus of the Siberian hamster (Duncan, et al., 1989; Weaver, et al., 1989). Functional tests of these sites of MEL binding and MEL1a-receptor mRNA (Song and Bartness, 2001; Song, et al., 1999; Song, et al., 2000) revealed the suprachiasmatic nucleus (SCN; (Bartness, et al., 1991; Song and Bartness, 1996; Song and Bartness, 1998)) and dorsomedial hypothalamic nucleus (DMH; (Leitner and Bartness, 2011)) are the only sites to date that are necessary for full expression of SD-like responses including gonadal regression, and decreases in body and white adipose tissue (WAT) mass. The thalamic paraventricular nucleus (PVT), by contrast, is necessary only for the body mass response (Purvis and Duncan, 1997). Several MEL binding/MEL1a-receptor mRNA expressing sites also are sufficient to trigger SD-like gonadal regression and decreases in body mass when MEL is applied site-specifically in a long SD-like duration including the SCN (Badura and Goldman, 1992; Leitner and Bartness, 2010), DMH (Leitner et al., 2010), subZona Incerta (subZI; (Leitner et al., 2010)) and the PVT (gonadal regression, not body mass decreases (Leitner et al., 2010)).
The advent of advanced DNA screening methods/tools sparked a new interest in the role of daylength in driving physiology and behavior. Many candidate genes and nuclei that express these genes have been suggested as contributors in the transduction of MEL binding at its target sites to changes in physiology and behavior (Barrett, et al., 2005; Greives, et al. 2007; Nilaweera, et al., 2008; Wagner, et al., 2007). One site that gained attention is the arcuate nucleus (ARC) – a nucleus important in the control of energy balance, among other regulatory responses (Barrett et al., 2005; Ellis, et al. 2008; Mason, et al., 2007; Tups, et al., 2004). A sub-area within the ARC is the dorsomedial posterior ARC (dmpARC), an area discovered when it was left, apparently, intact after neonatal monosodium glutamate (MSG) injections (Ebling, et al., 1998; Leitner and Bartness, 2008; Leitner and Bartness, 2009; Pelz, et al. 2008). The dmpARC exhibits alterations in gene expression for a variety of neurochemicals and receptors in a photoperiod-regulated manner (Barrett et al., 2005; Barrett, et al., 2009; Nilaweera, et al., 2009). Among the photoperiod-sensitive constituents of the dmpARC, are at least two that are important to food intake and energy expenditure; SDs upregulate the non-acronymic (68 kDa transcript) VGF and decrease expression of the histamine H3 receptor (H3R; (Barrett et al., 2005; Barrett et al., 2009; Ross, et al., 2005)). VGF has been suggested to be an important component for SD-induced reductions in food intake and body mass, and increases in brown adipose tissue (BAT) thermogenesis through increased uncoupling protein 1 (UCP-1) expression (Barrett et al., 2005; Jethwa, et al., 2007). This suggestion is based on the ability of chronic 3rd ventricular infusion of TLQP-21, a biologically active fragment of VGF, to trigger SD-like changes of these three seasonal responses in LD-housed Siberian hamsters (Barrett et al., 2005; Jethwa et al., 2007). Decreased H3R activity may cause increases in histamine release supporting a catabolic state, as inverse agonism of the H3R in LDs is able to decrease food intake akin to that seen in SDs (for review see: (Barrett et al., 2005; Hancock, 2003; Jethwa, et al., 2009)). In addition, SDs increase dmpARC patch clamp-measured electrophysiological activity mimicked by application of the H3R antagonist clobenproprit (Barrett et al., 2009). The dmpARC also could be involved in the SD-induced increases in white adipose tissue (WAT) lipolysis (Bowers, et al., 2005), because it is a component of the sympathetic outflow to WAT as revealed by viral tract tracing (Barrett et al., 2009). Thus, these convergent lines of evidence suggest that the dmpARC may be important and, as some suggest, integral to seasonal variations in energy intake and body mass loss of Siberian hamsters by possibly relaying information required for seasonal responses (i.e., reproduction and energy expenditure; (Barrett et al., 2005; Morgan, et al., 2006; Nilaweera et al., 2009)). It is noteworthy, however, that the dmpARC does not possess MEL1a-receptor mRNA (e.g., (Song et al., 2001)) suggesting that the photoperiod-driven responses of the dmpARC must be via other sites harboring these receptors.
Therefore we asked: Is a neurologically intact dmpARC necessary for SD responses? This question was addressed using discrete electrolytic lesions of the dmpARC, the completeness of which was assessed by Nissl staining and immunohistochemistry to label the high concentration of H3Rs that define the boundaries and center of the this nucleus (Barrett et al., 2005), and examination of several seasonal responses during and after 12 wks of SD exposure, including body mass, WAT pad mass, reproductive status, BAT UCP-1 protein, food intake, and pelage color.
MATERIALS AND METHODS
Animals and Housing Conditions
Fifty male Siberian hamsters (Phodopus sungorus) were obtained from our breeding colony. The breeding colony was established in 1988 with recent outbreeding described previously (Bowers, Festuccia, Song, Shi, Migliorini, and Bartness, 2004). Hamsters were weaned at ~21 days of age, housed in same-sex groups in polypropylene cages (48 × 27 × 15 cm), given access to food (5001, Purina, St. Louis, MO) and tap water ad libitum, and maintained in a 16L:8D photoperiod (LD; light offset 1900) until used in the following experiment. When the hamsters were ~3 months old they were transferred and singly-housed in polypropylene cages (27.8 × 17.5 × 13.0 cm) with corncob bedding (The Andersons, Maumee, OH) and one cotton nestlet (Ancare, Belmore, NY). Animals were given ad libitum access to food and tap water and housed at 22 °C ± 2 and 50 % humidity ± 10 in 16L:8D (light offset 1400) for two wks to acclimate to the new light offset. Animals remained in this lighting regimen unless noted otherwise.
Surgical Procedures
After the two wk acclimation to the new lighting regimen, food intake, body mass and estimated testis volume (ETV) were recorded. ETV is highly correlated to paired testis weight (Watson-Whitmyre and Stetson, 1985) and is the product of testis width squared multiplied by its length (W2 × L). ETV were measured while animals were under light anesthesia with isoflurane vapors (Aerrane, Baxter Healthcare Corporation, Deerfield, IL) using analog calipers. The Siberian hamsters were divided into two dmpARC treatment groups (sham or lesion) controlling for body mass, ETV and food intake. All surgeries occurred under isoflurane anesthesia. DC Lesions were made using a Lesion Making Device (C.H. Stoelting, Chicago, IL) and passing 1 mA current through a lesion probe for 5 sec. The lesion probe was tungsten wire (0.008 in diameter, Everett, WA) triple insulated by Epoxylite® 6001 baking leaving only the cross-sectional tip exposed to allow passage of current. Lesions were made by lowering the lesion probe stereotaxically aimed at the dmpARC (level skull; dorsal-ventral: −6.7 mm below the skull, medial-lateral: +/−0.1 mm from midline, anterior-posterior: −2.1 mm from bregma). The same surgical protocol was used on animals receiving sham lesion except no current was passed through the lesion probe. Sliced apple to facilitate food and fluid intake, and i.p. buprenorphine (0.2 mg/kg body mass) for post-surgical discomfort were given for 2 d post operatively. Siberian hamsters were allowed one wk to recover from surgery.
Groups and Experimental Procedures
After the one wk recovery period, half of each dmpARC treatment group was moved to SDs (8L:16D photoperiod; light offset 1400) and the remainder of the animals was maintained in LDs (light offset 1400). The groups were as follows: 1) dmpARC lesion and housed in LDs (dmpARCx/LD), 2) dmpARC sham lesion and housed in LDs (dmpARCsh/LD), 3) dmpARC lesion and housed in SDs (dmpARCx/SD), and 4) dmpARC sham lesion and housed in SDs (dmpARCsh/SD). Animals remained in their photoperiod treatment for 12 wks. Body mass, food intake, ETV and pelage color (see description below) were assessed weekly. After the 12 wks of daylength treatment, all animals were lightly anesthetized with isoflurane vapors and had blood (~300 μl) taken via the retro-orbital sinus with heparinized glass capillary tubes and stored at 4 °C in culture tubes overnight. All blood samples were spun at 2000 rpm for 20 min at 4 °C and the serum collected and stored at −80 °C until testosterone measurement (testosterone RIA kit, Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer’s instructions. The correlation coefficient and intra-assay reliability of the testosterone assay were 99.6 and 5.6 %, respectively.
After blood collection, animals were deeply anesthetized with pentobarbital sodium (300 mg/kg i.p.) and had the epididymal WAT (EWAT), left inguinal WAT (IWAT), bilateral retroperitoneal WAT (RWAT), interscapular BAT (IBAT) depots and testes excised and weighed. IBAT was snap frozen in liquid nitrogen and stored at −80 °C until western blot analysis for UCP-1 expression (see below). The animals were then perfused transcardially with 100 ml of heparinized saline followed by 125 ml of 4.0 % paraformaldehyde. Brains were removed and stored in 4.0 % paraformaldehyde overnight at 4 °C, transferred to fresh 4.0 % paraformaldehyde the next day and then placed into 30.0 % sucrose and 0.1 % azide solution the following day, remaining there until histological lesion verification.
Western Blot Analysis for UCP-1 Protein
Western blot analysis was performed as previously described (Shrestha, et al., 2010). Briefly, the frozen IBAT were homogenized using hand-held glass homogenizers (Dounce homogenizer) in lipid-associated protein extraction buffer modified from (Greenberg, et al., 1991; Greenberg, et al., 1993) containing 50 mM HEPES, 100 mM NaCl, 10.0 % SDS, 2.0 mM EDTA, 0.5 mM dithiothreitol, 1.0 mM benzamidine, and Protease Inhibitor Cocktail (Calbiochem, EMD Chemicals Inc., Gibbstown, NJ, USA) at 50 μl/g of tissue and Phosphatase Inhibitor Cocktail (Halt Phosphatase Inhibitors cocktail, Peirce, Thermo Fischer Scientific, Waltham, MA, USA) according to manufacturer’s recommendation. The tubes were then vigorously vortexed every 5 min during 60 min incubation at 37 °C and centrifuged at 13,000 rpm for 10 min at room temperature. The infranatant was carefully removed, aliquoted and stored at −80 °C. Protein content was determined using the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Samples of the tissue containing 10 μg of protein were mixed with loading buffer at 2x final concentration and heated at 95 °C for 8 min, loaded onto SDS-PAGE low-Bis gel (10 %:0.07 % acrylamide:Bis) and transferred to polyvinylidene difluoride membranes. We quantified protein load with Sypro Ruby (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions before immunoblotting and adjusted UCP-1 protein band intensity accordingly (Aldridge, et al., 2008; Hagiwara, et al., 2009). Membranes were blocked with 4.0 % non-fat dry milk in Tris-buffered saline (TBS) for 2 h at room temperature and incubated with the primary antibody (rabbit polyclonal UCP-1 antibody, ab23841, Abcam, Cambridge, MA) at 4 °C overnight in 4.0 % non-fat dry milk in TBS buffer. Immunoblots were rinsed in Tween-Tris-buffered saline (TTBS) for 5 min (x 4) and incubated with goat anti-rabbit AP detection kit (Immunostar AP Chemiluminescent Kits, BioRad Laboratories, Hercules, CA, USA) at room temperature for 2 h. Finally, the membranes were washed in TTBS 10 min (x 3) and incubated with Immunostar AP substrate for 5 min. The chemiluminescent bands were visualized using an Innotech Bioimager (Alpha Innotech Corporation, San Leandro, CA, USA) and their band intensities were quantified using the associated densitometric software.
Lesion Verification Histology
Brains were sectioned on a freezing microtome at 35 μm, for cresyl echt violet staining used largely to assess lesion location/histological landmarks (not shown). To assess the completeness of the lesion, alternate sections were prepared for immunohistochemical detection of the H3R used as a marker for the boundaries and central core of the dmpARC (Barrett et al., 2005).
In brief for immunohistochemistry, free-floating brain sections were subsequently rinsed in 0.1 M phosphate buffered saline (PBS; 2 × 15 min) followed by 30 min incubation in a blocking and permiablization solution consisting of 10.0 % normal goat serum (NGS) and 0.3 % Triton X-100 in 0.1 M PBS. Sections were incubated overnight with rabbit anti-H3R antiserum (1:1000; Novus Biologicals, Littleton, CO, USA) in 0.1 M PBS with 1.0 % NGS. For immunohistological controls, the primary antibody was either omitted or preadsorbed with the immunizing peptide overnight at 4 °C resulting in abolished immunostaining. Subsequently, sections were washed in 0.1 M PBS (2 × 15 min) and incubated with biotinylated goat anti-rabbit antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 2 h. Bound secondary antibody was then amplified with the Vector Elite ABC kit (1:800; Vector Laboratories, Burlingame, CA, USA) and antibody complexes were visualized by 0.05 % diaminobenzidine reaction. After mounting, sections were dehydrated and cleared through a series of ethanol, isopropanol and xylene solutions. Cleared slides were immediately mounted with Permount (Thermo Fisher Scientific, Waltham, MA, USA) and cover slipped.
To be included in the lesion group, bilateral damage to the dmpARC was at least ~ 85 % (i.e., destruction of > 90 % of the anterior-to-caudal extent, > 80 % of the dorsal-to-ventral extent and > 80 % of the medial-to-lateral extent) of each dmpARC based on H3R-immunoreactivity (ir). Siberian hamsters with insufficient damage or damage beyond the targeted nuclei were excluded from further analysis.
Pelage Color Determination
Pelage color was determined by the four stage 1–4 integer scale criteria of Duncan and Goldman (Duncan and Goldman, 1984a), where 1= “LD pelage” (dark gray-brown summer coat) and 4 = “SD pelage” (snow-white winter coat).
Statistical Analysis
Body mass, ETV, food intake and pelage color were analyzed using a three-way ANOVA for repeated measures (dmpARC treatment × photoperiod × time; NCSS version 2000, Kaysville, UT). Terminal measures (fat pad and testis masses, serum testosterone concentration, IBAT UCP-1 expression) were analyzed using a two-way ANOVA (dmpARC treatment × photoperiod; NCSS version 2000, Kaysville, UT). Tukey-Kramer Multiple Comparison post hoc tests were used when appropriate. Differences were considered significant when Ps< 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation.
RESULTS
Lesion Verification
Complete bilateral ablation of the dmpARC resulted in general abolishment of H3R-ir or separation of the dmpARC from the rest of the brain albeit with these severely disrupted fragments showing some H3R-ir. Some third ventricle enlargement also was seen (Fig 1). Complete lesions also caused some damage to the tuberal magnocellular nucleus and more ventrally to the posterior ARC, but this damage was minimal. Fourteen hamsters with incomplete bilateral lesions, or lesions beyond damage described above, were excluded from subsequent analyses, resulting in 36 animals used for statistical analysis (final group sizes: dmpARCx/LD n = 5, dmpARCsh/LD n = 11, dmpARCx/SD n = 7, dmpARCsh/SD n = 13).
Figure 1. dmpARC Lesion Verification.
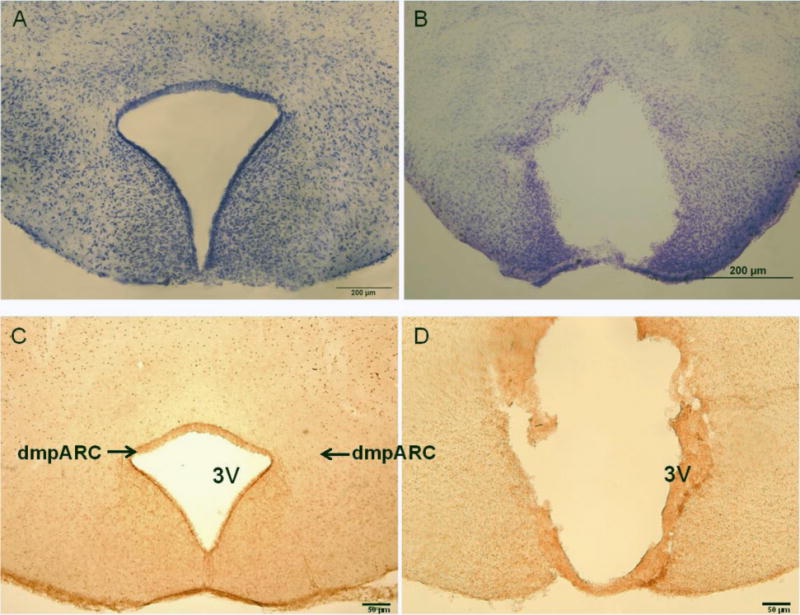
(Left) Representative microphotograph of the intact dorsomedial posterior arcuate nucleus (dmpARC) seen in the upper lateral extending corners of the ARC shown here by histamine-3 receptor immunoreactivity. (Right) A representative bilateral lesion of the dmpARC (dmpARCx) in a Siberian hamster. dmpARCsh/LD, n = 11; dmpARCx/LD, n = 5; dmpARCsh/SD, n = 13; dmpARCx/SD, n = 7. Bar: 50 μm.
Repeated Measures: Body Mass, Food Intake, Testicular Response and Pelage Color
SD exposure significantly decreased body mass (F(12,384) = 18.06, η2= 0.223, Fig 2A), food intake (F(12,384) = 3.21, η2 = 0.026, Fig 2B), ETV (F(12,384) = 33.92, η2 = 0.321, Fig 3A) and caused a whitening of pelage color typical for 12 SD wk exposure (e.g., (Bartness, et al., 1987; Duncan et al., 1984a; Duncan and Goldman, 1984b; Duncan and Goldman, 1985); data not shown; F(12,384) = 17.04, η2 = 0.149,). Lesions of the dmpARC did not block or attenuate any of the typical SD responses nor did they alter any of these measures in LD-housed hamsters bearing complete dmpARC lesions. More specifically, SD hamsters with an intact or ablated dmpARC showed a decreased body mass compared with their LD counterparts beginning at wk 3 (Ps< 0.05; Fig 2A). In addition, regardless of the dmpARC status, SDs decreased food intake from wk 8 to the end of the experiment compared with LD-housed animals (Ps< 0.05; Fig 2B). ETV was significantly decreased in SD hamsters compared with their LD controls beginning at wk 2 until the termination of the experiment and was seen in animals bearing dmpARC lesions and intact controls. (Ps< 0.05; Fig 3A). Pelage color of SD-housed hamsters began shifting to the winter-white phenotype and was significantly different from LD controls beginning at wk 8 in SDs and this response also was not blocked by dmpARC lesions (data not shown).
Figure 2. Body Mass and Food Intake.
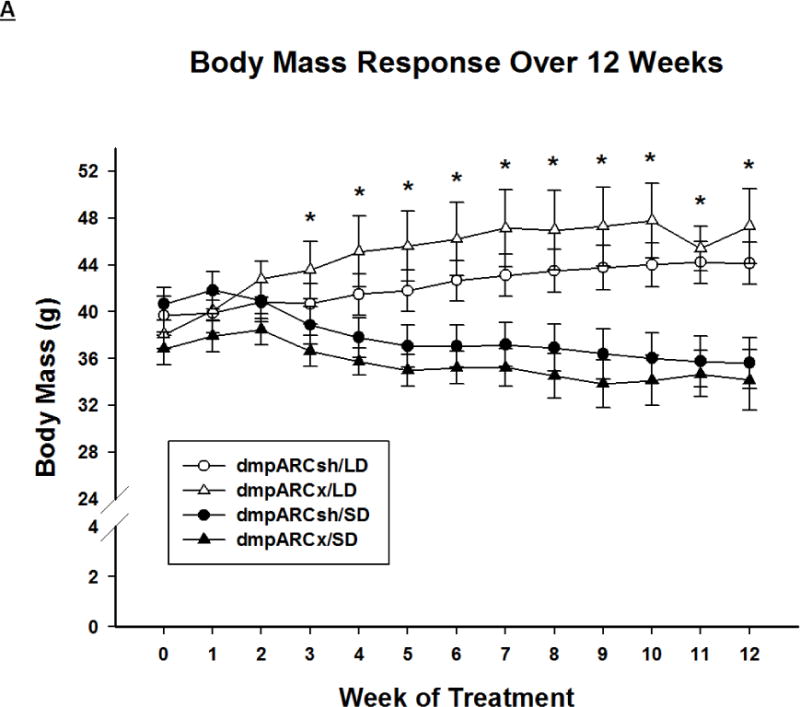
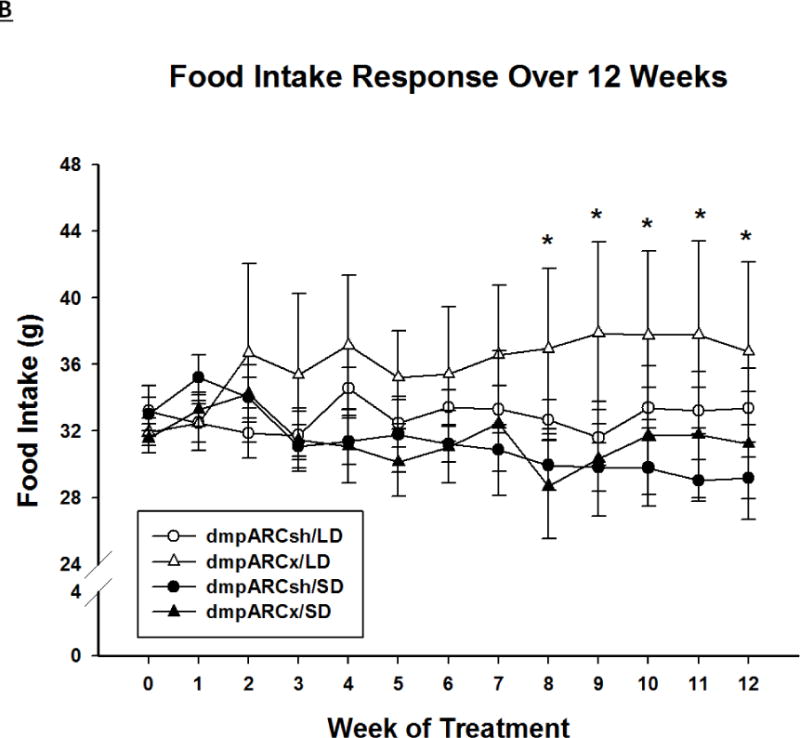
(A) Weekly mean body mass ± SEM (g) and (B) weekly mean food intake ± SEM (g) for 12 wks in Siberian hamsters bearing either sham (dmpARCsh, circles) or lesions of the dorsomedial posterior arcuate nucleus (dmpARCx, triangles) placed in either long-days (LD, empty symbols) or short-days (SD, filled symbols). * = P< 0.05 between LDs and SDs.
Figure 3. Testicular Response.
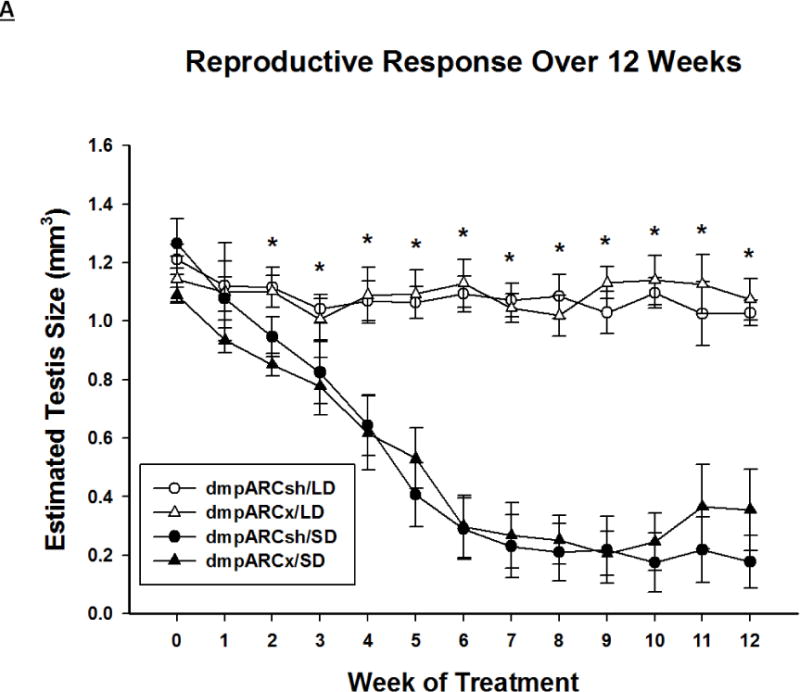
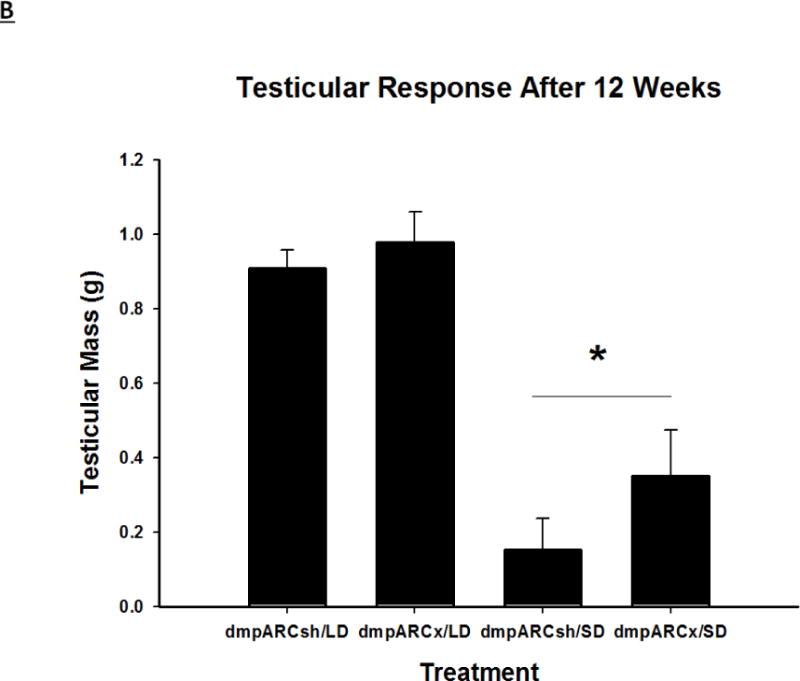
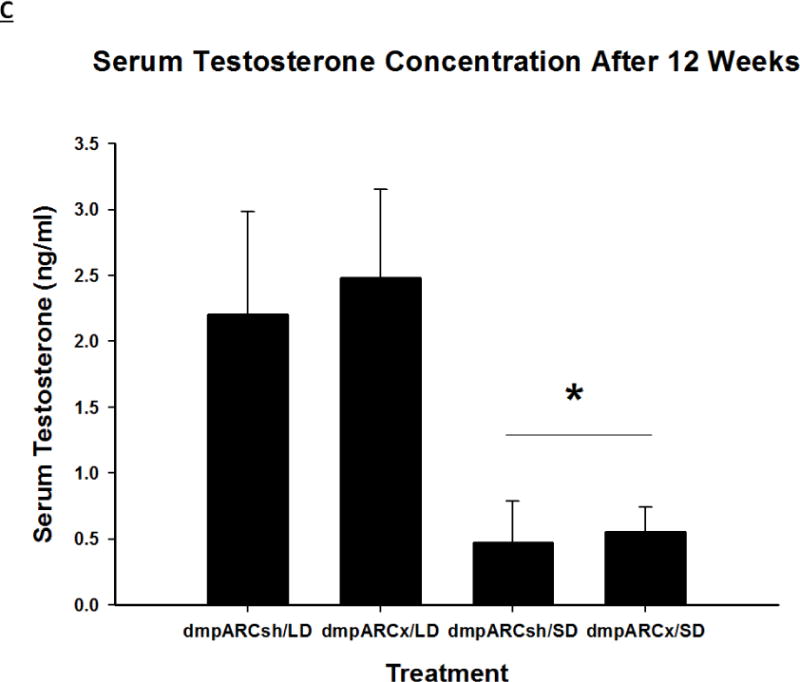
(A) Weekly mean estimated testis volume ± SEM (mm3), (B) terminal mean paired testis mass ± SEM (g) and (C) terminal mean serum testosterone concentrations ± SEM (ng/ml) after 12 wks in Siberian hamsters bearing either sham (dmpARCsh, circles) or lesions of the dorsomedial posterior arcuate nucleus (dmpARCx, triangles) placed in either long-days (LD, empty symbols) or short-days (SD, filled symbols). * = P< 0.05 between LDs and SDs.
Terminal Measures: Testis Mass, Serum Testosterone Concentration, Fat Pad Masses and UCP-1 Protein
SD exposure significantly decreased paired testis mass (F(1,32) = 56.3, η2 = 0.553, Fig 3B), serum testosterone concentration (F(1,32) = 9.07, η2 = 0.216, Fig 3C), and all fat pad masses (EWAT: F(1,32) = 31.86, η2 = 0.453; IWAT: F(1,32) = 9.55, η2 = 0.214; RWAT: F(1,32) = 13.14, η2 = 0.273; Figs 4A–C) and increased IBAT UCP-1 protein content (F(1,32) = 11.8, d = 0.254, Figs 5A and B; Ps< 0.05); these SD responses were not blocked or attenuated by dmpARCx. Furthermore, paired testis mass was significantly correlated with wk 12 ETV (r2 = 0.97, P< 0.05; data not shown). The dmpARC lesions did not block SD-induced testicular regression with both treatment groups (sham and lesion) showing similar paired tested masses; however, they were significantly decreased compared with their respective LD controls (Ps< 0.05; Fig 3B). Serum testosterone concentrations similarly were significantly decreased by ~83 % after 12 wks of SD exposure for both sham dmpARC and dmpARC lesion groups compared with their respective LD-housed counterparts (Ps< 0.05; Fig 3C). The dmpARC lesions did not affect paired testes mass nor serum testosterone concentrations in LDs (Figs 3B and 3C).
Figure 4. Adipose Tissue Response.
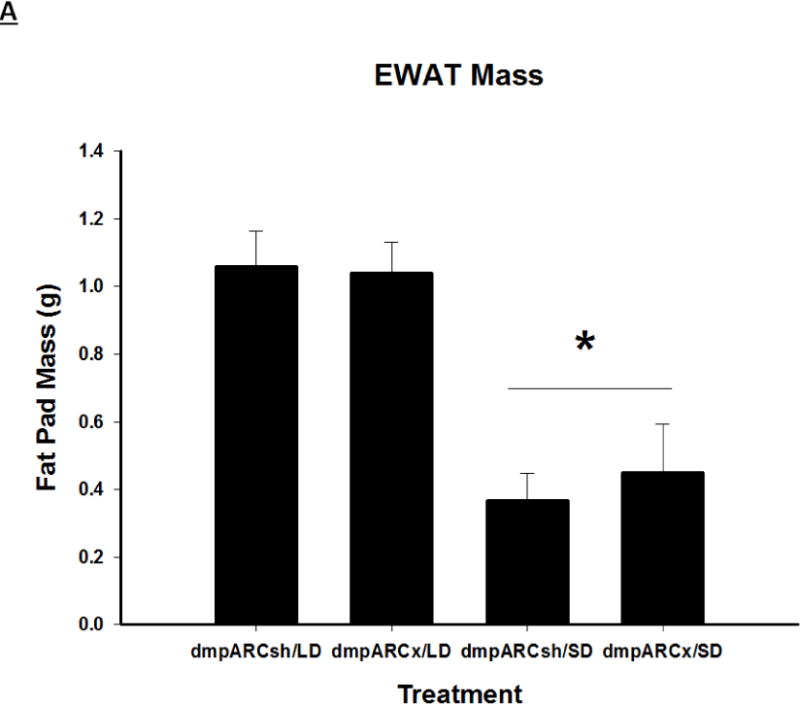
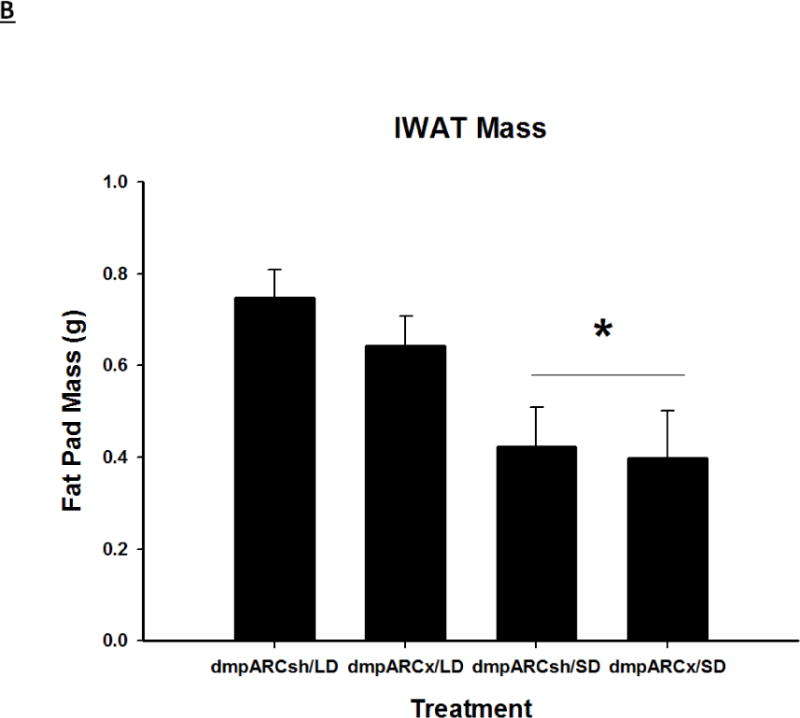
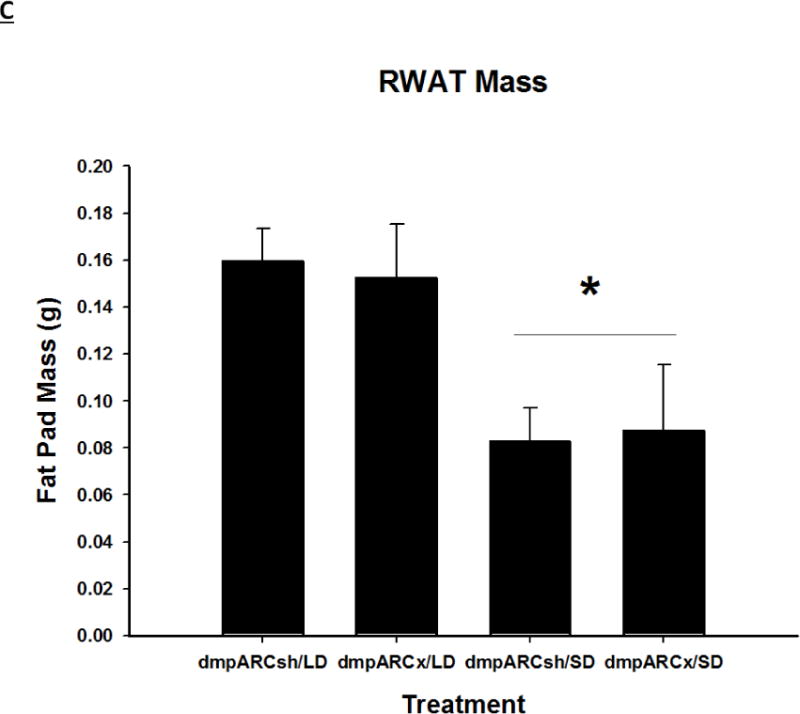
Terminal mean (A) epididymal, (B) inguinal and (C) retroperitoneal white adipose tissue masses ± SEM (g) after 12 wks in Siberian hamsters bearing either sham (dmpARCsh) or lesions of the dorsomedial posterior arcuate nucleus (dmpARCx) placed in either long-days (LD) or short-days (SD). * = P< 0.05 between LDs and SDs.
Figure 5. UCP-1 Protein Content in BAT after 12 wks of SD Exposure.
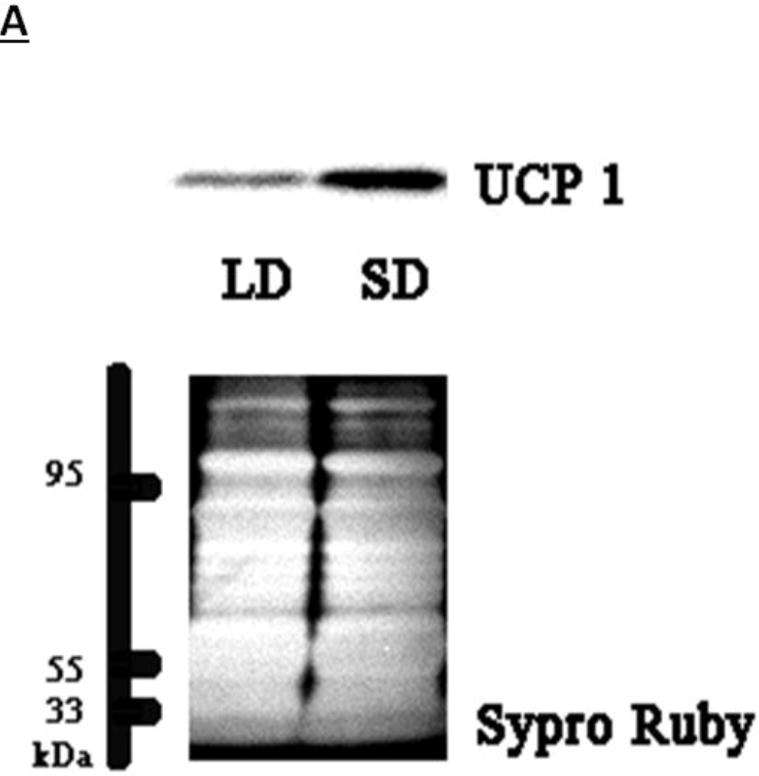
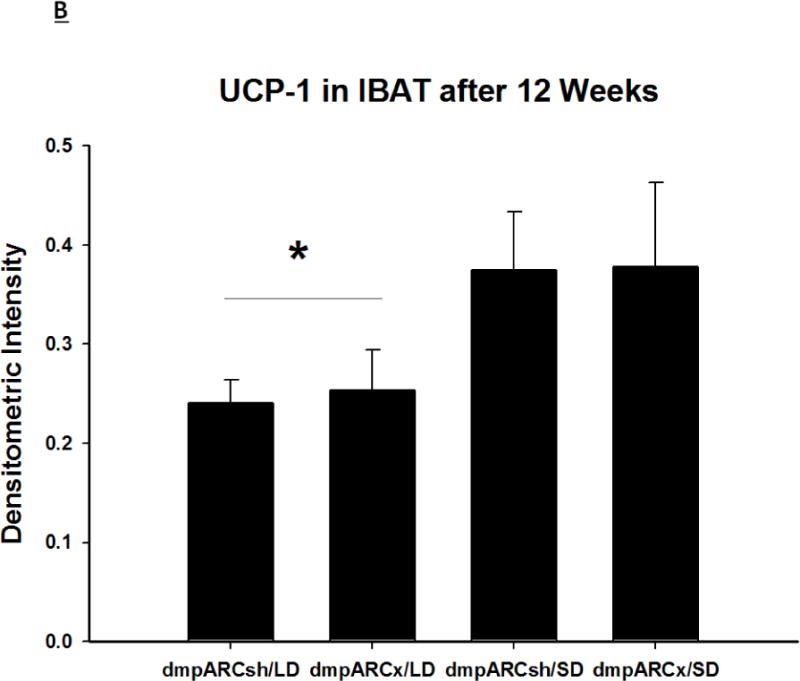
(A) Representative immunoblots of uncoupling protein 1 (UCP-1) in brown adipose tissue (BAT) for Siberian hamsters after 12 wks of either long-day (LD) or short-day (SD) exposure and quantified protein load (Sypro Ruby) under ultraviolet light B exposure. (B) Mean chemiluminescent densitometric intensity (adjusted after protein load adjustment) of UCP-1 in BAT ± SEM in Siberian hamsters bearing either sham (dmpARCsh) or lesions of the dorsomedial posterior arcuate nucleus (dmpARCx) placed in either LD or SD. * = P< 0.05 between LDs and SDs.
SDs significantly decreased individual (EWAT [Fig 4A], IWAT [Fig 4B], and RWAT [Fig 4C]) fat pad masses, and dmpARC lesions did not block the typical SD-induced reductions in WAT mass for any of the adipose depots compared with LD controls (Ps< 0.05). IBAT UCP-1 protein content significantly increased with SD exposure (Fig 5A and 5B), as expected (Barrett et al., 2005; Kronfeld-Schor, et al., 2000; Zhao and Wang, 2005), compared with LDs and occurred for both sham dmpARC and dmpARC-ablated hamsters (Ps< 0.05; Fig 5A and B).
DISCUSSION
We investigated the necessity of an intact dmpARC for many of the well-documented SD responses in Siberian hamsters, including reproductive quiescence, body and fat mass loss and whitening of the pelage color. We found that dmpARC lesions did not block SD-induced testicular regression, serum testosterone reduction, decreased body and WAT pad masses, decreases in food intake, increases in IBAT UCP-1 protein content or whitening of the pelage color. Therefore, the inability of dmpARC lesions to block the SD responses assayed here indicates that an intact dmpARC is not necessary for the decoding nor the relay of SD signals driving these responses in Siberian hamsters.
The dmpARC is a recently defined sub-region of the ARC that has been suggested to be part of the pathway between central MEL binding sites and responses to photoperiod, as it contains at least two seasonally-modulated constituents that are known to be part of energy homeostasis within the dmpARC and other brain regions, VGF and H3R (Barrett et al., 2005; Jethwa et al., 2009; Jethwa et al., 2007). The up/down regulation of VGF/H3R in SDs, respectively, has been suggested as integral to the eventual SD-induced decrease in food intake (starting ~5–7 wk) and body mass loss (staring at 1 wk (Barrett et al., 2005)). It has been additionally speculated that the dmpARC is critical to the relay of daylength information to other components of the seasonal responses that includes reproductive quiescence in SDs (Nilaweera et al., 2009). The dmpARC also contains a few seasonally-expressed genes that code for proteins in secretory pathways for metabolic enzymes or energy homeostasis. Specifically secretogranin III and VI (SgIII and SgVI, respectively) that belong to the same family as VGF (secretogranin VII; (Nilaweera et al., 2009)), as well as for the melanocortin 3 receptor (MC3-R; (Butler and Cone, 2003; Butler, et al., 2000; Cone, et al., 2001)) and serotonin receptors 5-HT2A and 5-HT7 (Hedlund, et al., 2003; Heisler, et al., 2003; Park, et al., 1999). Changes in these proteins during SDs led to the hypothesis that the dmpARC is an important output pathway for photoperiod information acting as a relay of daylength information (Barrett et al., 2009) even though it does not contain MEL receptors (Song et al., 2001; Song et al., 1999; Song et al., 2000). The dmpARC also exhibits increases in c-Fos-ir in SDs (Barrett et al., 2009), but this marker of neural activation does not seem to be related to the expression of any of the SD responses examined here, most notably in the decreases in food intake and body/fat mass loss (Barrett et al., 2009). The present data clearly demonstrate that an intact dmpARC is not necessary for responses to SDs, despite showing photoperiod changes in receptor, peptide and gene expression. Therefore, the hypothesis positing that the dmpARC is required for seasonal responses is unsupported. The dmpARC does, however, contain sympathetic outflow neurons to WAT (Bamshad, et al., 1998; Barrett et al., 2009; Bowers et al., 2004; Song et al., 2001; Song, et al., 2005) and appears to be involved in the mobilization of lipid (Barrett et al., 2009), but SD-induced lipid mobilization can proceed without an intact dmpARC as shown here.
The central forebrain sites that mediate SD responses of food intake and lipolysis are not fully understood in Siberian hamsters. Previous work tested the necessity of an intact ARC for seasonal responses using MSG administration to destroy the ARC and then examined the response of these animals to SDs as adults (Barrett et al., 2005; Ebling et al., 1998). These animals did not lose seasonal responses to SDs, but the dmpARC appears spared by MSG treatment (Barrett et al., 2005; Ebling et al., 1998), a finding that some contend supports the hypothesis that the dmpARC plays a vital role in the response to photoperiod (Ebling et al., 1998). Here we ablated the dmpARC and these animals responded to SDs similarly to hamsters that were neurologically intact and not unlike animals bearing ARC MSG lesions. Thus, as noted above, the results of the present experiment suggests that a neurologically intact dmpARC is not necessary to trigger SD-induced responses.
To date, there appear to be two brain sites in Siberian hamsters that are necessary for normal SD responses including those of decreased body and lipid mass. The SCN and DMH, (Bartness et al., 1991; Leitner et al., 2011; Song et al., 1996; Song et al., 1998) both contain neurons that express MEL1a-R mRNA (Song et al., 2001). Moreover, both sites also contain SNS outflow neurons to WAT that express MEL1a-R mRNA suggesting their role in SD/MEL-induced decreases in body fat mass (Song et al., 2001). That is, we previously demonstrated that an intact SCN is necessary for the reception/transmission of SD-like MEL signals as bilateral lesions of the SCN block the response to daily SD-like, long duration, subcutaneous infusions of MEL in pinealectomized Siberian hamsters, whereas neurologically intact Siberian hamsters responded by decreasing body and lipid mass and regress their gonads (Bartness et al., 1991; Song et al., 1996; Song et al., 1998). Until recently, the necessity of an intact DMH for expression of SD responses only was demonstrated in pinealectomized Syrian hamsters (Mesocricetus auratus) receiving timed daily SD-like subcutaneous infusions of MEL (Maywood, et al., 1996). Others (Ebling and Barrett, 2008) and we recently (Leitner et al., 2011) found that DMH lesions block SD and SD-like MEL-induced decreases in body mass, fat pad mass and gonadal regression in Siberian hamsters as well.
The SCN, DMH and several other sites [ReN, PVT, and subZI] that express MEL1a-R mRNA also have been shown to be sufficient to induce SD responses (Leitner et al., 2010). That is, using daily MEL infusions (Badura et al., 1992; Bartness et al., 1991; Song et al., 1998) or constant release MEL implants (Freeman and Zucker, 2001; Teubner and Freeman, 2007; Teubner, et al., 2008), the SCN, ReN, and PVT have previously been demonstrated to be sufficient to drive photoperiodic seasonal reproductive responses or changes in immune responses (Freeman, et al., 2007). More recently, we found that daily, timed MEL intracerebral microimplants into the SCN, ReN, PVT, DMH, and subZI are able to induce testicular regression, and all but the PVT also triggered SD-induced decreases in body/WAT mass (Leitner et al., 2010). The dmpARC was not tested for the ability to induce SD-like MEL responses by the timed MEL implants because it does not contain MEL1a receptors (Song et al., 2001;Weaver et al., 1989).
One possible caveat for the present experiment would be that the dmpARC lesions were not complete, but given the criteria we used (see Methods above) and using both cresyl violet Nissl staining (not shown) as well as the complete or nearly complete ablation of H3R-ir that clearly demarks not only the extent of the dmpARC but also its interior (Fig 1), it seems unlikely that this explains the lack of the ability of dmpARC lesions to block SD responses. In addition, given the extent of the lesions, it would seem highly unlikely that any remaining H3R possessing neurons in the area [and hence, by definition (Barrett et al., 2009), dmpARC neurons], would have disrupted inputs and outputs and thus would be non-functional.
In summary, the dmpARC of Siberian hamsters contains photoperiod-modulated constituents (Barrett et al., 2009; Nilaweera et al., 2009), the precise nature of how or if they, in turn, control seasonal reproductive and energy metabolism responses is unknown. It was hypothesized that they either do so directly by affecting the peripheral target tissues or by acting as relay to other nuclei that ultimately affect the target tissues (Barrett et al., 2005; Nilaweera et al., 2009). The results of the present study clearly show, however, that an intact dmpARC is not necessary for many of the well-known photoperiodic responses in Siberian hamsters, as its ablation did not alter the decreases in body and fat pad mass, regression of the testes, whitening of the pelage color, or UCP-1 protein content in IBAT with exposure to SD for 12 weeks.
HIGHLIGHTS.
A subregion of the arcuate nucleus (ARC), the dorsomedial (dmpARC)
dmpARC has been implicated as critical for the transmission of short day (SD) cues
SD testes, body/fat pad mass decreases are not blocked by bilateral dmpARC lesions
SD BAT UCP-1, testosterone decreases not blocked by bilateral dmpARC lesions
An intact dmpARC is not necessary for SD responses by contrast to claims by others
Acknowledgments
The authors thank Mary Karom for assistance running the testosterone assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was funded, in part by NIH R37 DK35254 to TJB and NIH F32 DK091984 to BJWT.
References
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semiquantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Barrett P, Ross AW, Balik A, Littlewood PA, Mercer JG, Moar KM, Sallmen T, Kaslin J, Panula P, Schuhler S, Ebling FJ, Ubeaud C, Morgan PJ. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. Endocrinology. 2005;146:1930–1939. doi: 10.1210/en.2004-1452. [DOI] [PubMed] [Google Scholar]
- Barrett P, van den Top M, Wilson D, Mercer JG, Song CK, Bartness TJ, Morgan PJ, Spanswick D. Short photoperiod induced decrease of histamine H3 receptors facilitates activation of hypothalamic neurons in the Siberian hamster. Endocrinology. 2009 doi: 10.1210/en.2008-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones and the sympathetic nervous system. Exp Biol Med. 2002;227:363–376. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN, Goldman BD. Are the short photoperiod decreases in serum prolactin responsible for the seasonal changes in energy balance in Syrian and Siberian hamsters? J Exptl Zool. 1987;244:437–454. doi: 10.1002/jez.1402440310. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Gettys TW, Prpic V, Harris RBS, Bartness TJ. Short photoperiod exposure increases adipocyte sensitivity to noradrenergic stimulation in Siberian hamsters. Am J Physiol. 2005;288:R1354–R1360. doi: 10.1152/ajpregu.00792.2004. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Butler AA, Cone RD. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann N Y Acad Sci. 2003;994:240–245. doi: 10.1111/j.1749-6632.2003.tb03186.x. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. I Role of the gonads and pituitary. J Exp Zool. 1984a;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. II Role of prolactin. J Exp Zool. 1984b;230:97–103. doi: 10.1002/jez.1402300113. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Physiological doses of prolactin stimulate pelage pigmentation in Djungarian hamster. Am J Physiol. 1985;248:R664–R667. doi: 10.1152/ajpregu.1985.248.6.R664. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Takahashi JS, Dubocovich ML. Characteristics and autoradiographic localization of 2-[125I] Iodomelatonin binding sites in Djungarian hamster brain. Endocrinology. 1989;125:1011–1018. doi: 10.1210/endo-125-2-1011. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Arthurs OJ, Turney BW, Cronin AS. Seasonal neuroendocrine rhythms in the male Siberian hamster persist after monosodium glutamate-induced lesions of the arcuate nucleus in the neonatal period. J Neuroendocrinology. 1998;10:701–712. doi: 10.1046/j.1365-2826.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Barrett P. The regulation of seasonal changes in food intake and body weight. J Neuroendocrinol. 2008;20:827–833. doi: 10.1111/j.1365-2826.2008.01721.x. [DOI] [PubMed] [Google Scholar]
- Ellis C, Moar KM, Logie TJ, Ross AW, Morgan PJ, Mercer JG. Diurnal profiles of hypothalamic energy balance gene expression with photoperiod manipulation in the Siberian hamster, Phodopus sungorus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1148–R1153. doi: 10.1152/ajpregu.00825.2007. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Kampf-Lassin A, Galang J, Wen JC, Prendergast BJ. Melatonin acts at the suprachiasmatic nucleus to attenuate behavioral symptoms of infection. Behav Neurosci. 2007;121:689–697. doi: 10.1037/0735-7044.121.4.689. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci U S A. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Moos MC, Jr, Londos C, Kimmel AR. Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci U S A. 1993;90:12035–12039. doi: 10.1073/pnas.90.24.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Kobayashi K, Tadokoro T, Yamamoto Y. Application of SYPRO Ruby- and Flamingo-stained polyacrylamide gels to Western blot analysis. Anal Biochem. 2009;389:171–173. doi: 10.1016/j.ab.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Hancock AA. H3 receptor antagonists/inverse agonists as anti-obesity agents. Curr Opin Investig Drugs. 2003;4:1190–1197. [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, Cone RD, Elmquist JK. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Jethwa PH, Barrett P, Turnbull Y, Enright RA, Warner A, Murphy M, Ebling FJ. The role of histamine 3 receptors in the control of food intake in a seasonal model of obesity: the Siberian hamster. Behav Pharmacol. 2009;20:155–165. doi: 10.1097/FBP.0b013e32832a8099. [DOI] [PubMed] [Google Scholar]
- Jethwa PH, Warner A, Nilaweera KN, Brameld JM, Keyte JW, Carter WG, Bolton N, Bruggraber M, Morgan PJ, Barrett P, Ebling FJ. VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology. 2007;148:4044–4055. doi: 10.1210/en.2007-0038. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Schor N, Haim A, Dayan T, Zisapel N, Klingenspor M, Heldmaier G. Seasonal thermogenic acclimation of diurnally and nocturnally active desert spiny mice. Physiol Biochem Zool. 2000;73:37–44. doi: 10.1086/316718. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Enquist LW, Card JP. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur J Neurosci. 1998;10:128–145. doi: 10.1046/j.1460-9568.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Food deprivation-induced changes in body fat mobilization after neonatal monosodium glutamate treatment. Am J Physiol. 2008;294:R775–R783. doi: 10.1152/ajpregu.00369.2007. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Acute brown adipose tissue temperature response to cold in monosodium glutamate-treated Siberian hamsters. Brain Res. 2009;1292:38–51. doi: 10.1016/j.brainres.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Distributed forebrain sites mediate melatonin-induced short-day responses in Siberian hamsters. Endocrinology. 2010;151:3133–3140. doi: 10.1210/en.2010-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. An intact dorsomedial hypothalamic nucleus, but not the subzona incerta or reuniens nucleus, is necessary for short-day melatonin signal-induced responses in Siberian hamsters. Neuroendocrinology. 2011;93:29–39. doi: 10.1159/000320474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln GA, Short RV. Seasonal breeding: nature’s contraceptive. Rec Prog Horm Res. 1980;3:1–52. doi: 10.1016/b978-0-12-571136-4.50007-3. [DOI] [PubMed] [Google Scholar]
- Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood E, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod. 1996;54:470–477. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Ross AW, Mercer JG, Barrett P. What can we learn from seasonal animals about the regulation of energy balance? Prog Brain Res. 2006;153:325–337. doi: 10.1016/S0079-6123(06)53019-5. [DOI] [PubMed] [Google Scholar]
- Nilaweera KN, Archer ZA, Campbell G, Mayer CD, Balik A, Ross AW, Mercer JG, Ebling FJ, Morgan PJ, Barrett P. Photoperiod regulates genes encoding melanocortin 3 and serotonin receptors and secretogranins in the dorsomedial posterior arcuate of the Siberian hamster. J Neuroendocrinol. 2009;21:123–131. doi: 10.1111/j.1365-2826.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- Nilaweera KN, Wilson D, Bell L, Mercer JG, Morgan PJ, Barrett P. G protein-coupled receptor 101 mRNA expression in supraoptic and paraventricular nuclei in rat hypothalamus is altered by pregnancy and lactation. Brain Res. 2008;1193:76–83. doi: 10.1016/j.brainres.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Park S, Harrold JA, Widdowson PS, Williams G. Increased binding at 5-HT(1A), 5-HT(1B), and 5-HT(2A) receptors and 5-HT transporters in diet-induced obese rats. Brain Res. 1999;847:90–97. doi: 10.1016/s0006-8993(99)02055-7. [DOI] [PubMed] [Google Scholar]
- Pelz KM, Routman D, Driscoll JR, Kriegsfeld LJ, Dark J. Monosodium glutamate-induced arcuate nucleus damage affects both natural torpor and 2DG-induced torpor-like hypothermia in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R255–R265. doi: 10.1152/ajpregu.00387.2007. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 93–156. [Google Scholar]
- Purvis CC, Duncan MJ. Discrete thalamic lesions attenuate winter adaptations and increase body weight. Am J Physiol. 1997;273:R226–R235. doi: 10.1152/ajpregu.1997.273.1.R226. [DOI] [PubMed] [Google Scholar]
- Ross AW, Bell LM, Littlewood PA, Mercer JG, Barrett P, Morgan PJ. Temporal changes in gene expression in the arcuate nucleus precede seasonal responses in adiposity and reproduction. Endocrinology. 2005;146:1940–1947. doi: 10.1210/en.2004-1538. [DOI] [PubMed] [Google Scholar]
- Shrestha YB, Vaughan CH, Smith BJ, Jr, Song CK, Baro DJ, Bartness TJ. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–R149. doi: 10.1152/ajpregu.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Bartness TJ. The effects of anterior hypothalamic lesions on short-day responses in Siberian hamsters given timed melatonin infusions. J Biol Rhythms. 1996;11:14–26. doi: 10.1177/074873049601100102. [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ. Dorsocaudal SCN microknife-cuts do not block short day responses in Siberian hamsters given melatonin infusions. Brain Res Bull. 1998;45:239–246. doi: 10.1016/s0361-9230(97)00234-7. [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol. 2001;281:R666–R672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ, Petersen SL, Bittman EL. SCN cells expressing mt1 receptor mRNA coexpress AVP mRNA in Syrian and Siberian hamsters. Adv Exp Med Biol. 1999;460:229–232. doi: 10.1007/0-306-46814-x_25. [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ, Petersen SL, Bittman EL. Co-expression of melatonin (MEL1a) receptor and arginine vasopressin mRNAs in the Siberian hamster suprachiasmatic nucleus. J Neuroendocrinol. 2000;12:627–634. doi: 10.1046/j.1365-2826.2000.00479.x. [DOI] [PubMed] [Google Scholar]
- Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- Teubner BJ, Freeman DA. Different neural melatonin-target tissues are critical for encoding and retrieving day length information in Siberian hamsters. J Neuroendocrinol. 2007;19:102–108. doi: 10.1111/j.1365-2826.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Teubner BJ, Smith CD, Freeman DA. Multiple melatonin target tissues mediate termination of photorefractoriness by long day lengths in Siberian hamsters. J Biol Rhythms. 2008;23:502–510. doi: 10.1177/0748730408325233. [DOI] [PubMed] [Google Scholar]
- Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, Klingenspor M. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology. 2004;145:1185–1193. doi: 10.1210/en.2003-1382. [DOI] [PubMed] [Google Scholar]
- Turek FW, Campbell CS. Photoperiodic regulation of neuroendocrine-gonadal activity. Biol Reprod. 1979;20:32–50. doi: 10.1093/biolreprod/20.1.32. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Johnston JD, Tournier BB, Ebling FJ, Hazlerigg DG. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus) Eur J Neurosci. 2007;25:485–490. doi: 10.1111/j.1460-9568.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- Watson-Whitmyre M, Stetson MH. A mathematical method for estimating paired testes weight from in situ testicular measurements in three species of hamster. Anat Rec. 1985;213:473–476. doi: 10.1002/ar.1092130313. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J Neurosci. 1989;9:2581–2590. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZJ, Wang DH. Short photoperiod enhances thermogenic capacity in Brandt’s voles. Physiol Behav. 2005;85:143–149. doi: 10.1016/j.physbeh.2005.03.014. [DOI] [PubMed] [Google Scholar]


