Abstract
Myocardial infarction (MI) is the leading cause of death in developed countries. Though timely revascularization of the ischemic myocardium and current standard therapy reduce acute mortality after MI, long-term morbidity and mortality remain high. During the first 1 to 2 weeks after MI, tissues in the infarcted myocardium undergo rapid turnover, including digestion of extracellular matrix and fibrosis. Post-MI repair is crucial to survival. Monocytes recruited to the infarcted myocardium remove debris and facilitate the repair process. However, exaggerated inflammation may also impede healing, as demonstrated by the association between elevated white blood cell count and in-hospital mortality after MI. Monocytes produced in the bone marrow and spleen enter the blood after MI and are recruited to the injured myocardium in 2 phases. The first phase is dominated by Ly-6chigh monocytes and the second phase by Ly-6clow monocytes. Yet the number of Ly6Clow monocytes recruited to the infarct is much lower, and Ly6Chigh monocytes can differentiate to Ly6Clow macrophages in later healing stages. Understanding the signals regulating monocytosis after MI will help design new therapies to facilitate cardiac healing and limit heart failure.
Keywords: monocytes, macrophages, hematopoiesis, myocardial infarction
Monocytes
The innate immune system initiates defense against microorganisms quickly and efficiently, and monocytes are innate immunity’s major players. Monocytes comprise 10% and 4% of human and mouse blood leukocytes, respectively. The main subset of CD115+ monocytes in mice express high levels of Ly-6c, CCR2 and CD62L and low level of CX3CR11. Ly-6chigh monocytes are recruited to inflamed sites and produce high levels of pro-inflammatory cytokines, such as TNF-α and IL-1β. Hence, Ly-6chigh monocytes are named inflammatory monocytes. The second subset of mouse monocytes express a high level of CX3CR1 and a low level of Ly-6c. They reside in blood vessels in steady state and may play important roles in scavenging oxidized lipids, dead cells and pathogens2. Both monocyte subsets circulate in the blood and survey steady-state tissue by transporting self-antigens to lymph nodes with minimal differentiation to macrophages, but they can differentiate into macrophages and dendritic cells at sites of inflammation3. At least 3 subsets of monocytes exist in humans. CD14++ CD16− monocytes resemble mouse Ly-6chigh monocytes, CD14++ CD16+ monocytes have pro-inflammatory roles, and CD14+ CD16++ monocytes exhibit patrolling behavior similar to mouse Ly-6clow monocytes.
Macrophage origins
During embryonic development, various organs are seeded by macrophages derived from yolk sac or liver progenitors. Most of these macrophages can self-maintain by homeostatic proliferation4,5. Such self-maintenance was first investigated in microglia, which respond to various injuries, including CNS damage, and can self-renew without blood monocyte contribution6. Steffen Jung and his colleagues used constitutive and conditional CX3CR1 reporter mice to demonstrate that tissue-resident macrophages, including Kupffer cells and lung, splenic and peritoneal macrophages, are established before birth and can replenish themselves in adulthood by local proliferation7. These data were consistent with the findings by Geissmann and his colleagues8, who described that the origin of yolk sac-derived tissue resident macrophages is independent of Myb, a transcription factor required for HSC and monocyte development. Taken together, these studies, in addition to others, indicate that many tissue resident macrophages are not derived from monocytes in steady state, at least in young mice. Two notable exceptions are dermal and intestinal macrophages. The dermis is populated by various myeloid cells, including macrophages and dendritic cells. Dermal macrophages are highly phagocytic but do not efficiently activate T cells, whereas dermal dendritic cells have strong T cell stimulatory capacity. In a recent study9, Sandrine Henri and her colleagues found that, after 8 weeks of parabiosis, about 20% of dermal macrophages were parabiont-derived, indicating their monocytic origin. Intestinal macrophage maintenance also depends on blood monocytes10,11. A recent study12 showed that although yolk sac and fetal macrophages seed the lamina propria, they begin to wane right after birth and are replaced by blood monocytes. This process depends on CCR2 and commensal gut microbiota; mice maintained in germ-free conditions have fewer colon macrophages than those in regular housing.
Like other organs, the heart contains macrophages in steady state13,14. A recently published study15 characterized cardiac macrophage subsets and investigated their origins. The authors reported four cardiac macrophage subsets expressing varying levels of Ly-6c and MHC class II. Cardiac macrophages are derived from yolk sac and fetal monocyte progenitors and are replenished by local proliferation in steady state. Yet in injury, such as myocardial infarction, cardiac macrophages are replaced by blood monocytes13. Contrary to the notion that cardiac macrophages self-maintain by proliferation, a very recent study16 demonstrated that embryonic-derived cardiac macrophages are continuously replenished by blood monocytes in adulthood. Almost all cardiac macrophages are CX3CR1+ and MHC class II− at birth; however, with age they diversify into four subpopulations with progressive increases in MHC class II+ and decreases in CX3CR1+ subpopulations.
Monocyte production in steady state
Monocytes develop from bone marrow hematopoietic stem cells (HSC) after going through several progenitor stages, including common myeloid progenitor (CMP), granulocyte/macrophage progenitor (GMP) and macrophage/dendritic cells progenitor (MDP). Blood monocyte development depends on M-CSF1,17. M-CSF-deficient op/op mice have drastic reductions in blood monocyte numbers18 and atherosclerotic plaque burden if crossbred with LDLR−/− mice19. M-CSF is also involved in tissue resident macrophage proliferation. Erlebacher and his colleagues20 found that macrophage proliferation in the uterus during pregnancy was driven by M-CSF. Moreover, the proliferating macrophages produced higher levels of mcp-1, a CCR2 ligand in the myometrium, leading to extravasation of Ly-6chigh monocytes. Another example of M-CSF-dependent macrophage proliferation is that the cytokine induces Gata6-dependent peritoneal macrophage proliferation21. Additionally, M-CSF enhances tissue macrophage survival by reducing apoptosis22.
Several transcription factors, such as PU.1, determine HSC differentiation into CMP rather than common lymphoid progenitors (CLP)23. PU.1 binds to GATA-1 and inhibits commitment towards a megakaryocyte-erythroid progenitor, which facilitates myeloid differentiation24. Moreover, PU.1 represses mast cell development. Other transcription factors, such as CCAAT/enhancer binding protein (Cebpa), early growth response gene and IFN consensus sequence binding protein1,25–27, also determine myeloid vs. lymphoid lineage fate. Surprisingly, Cebpa expression in B and T lymphocytes can transdifferentiate them into macrophages28,29. Ikaros, a transcription factor that encodes a family of hematopoietic-specific zinc finger proteins, is a central regulator of lymphocyte differentiation.
Transcription factors involved in Ly-6chigh vs. Ly-6clow monocyte generation are not well understood, with the exception of the orphan nuclear hormone receptor Nr4a1 (also known as Nur77), which is involved in Ly-6clow monocyte production and survival30. However, some reports indicate that the transcription factor is dispensable for Ly-6clow macrophage production31.
Monocyte production after myocardial infarction
Myocardial infarction activates adrenergic signaling that alerts bone marrow niche cells, which reduce production of HSC retention factors32 (Figure 1). Consequently, HSC egress from the bone marrow and seed in the spleen. This triggers extramedullary hematopoiesis and monocyte production. Within 24 hours after myocardial infarction, the spleen’s monocyte reservoir is released33. Splenectomy experiments indicated that the organ may contribute as much as half of the monocyte population recruited to the infarct. Within four days after MI, the splenic monocyte reservoir refills by proliferation and differentiation of HSC and progenitors34. In the spleen, HSC proliferation is stem cell factor (SCF)-dependent. Neutralizing SCF reduces HSC proliferation and monocyte production32. Splenic monocyte production from hematopoietic progenitors also depends on IL-1β34, IL-3 and GM-CSF35. Currently, mechanics of splenic HSC maintenance are mostly unknown. We recently found that macrophages are important players in splenic HSC retention, as depleting splenic macrophages with M-CSFR knockdown or diphtheria toxin in CD169-DTR mice mobilized splenic HSC and reduced monocyte production36. Interestingly, splenic macrophages retain HSC via VCAM-1.
Figure 1. Monocytosis after myocardial infarction.
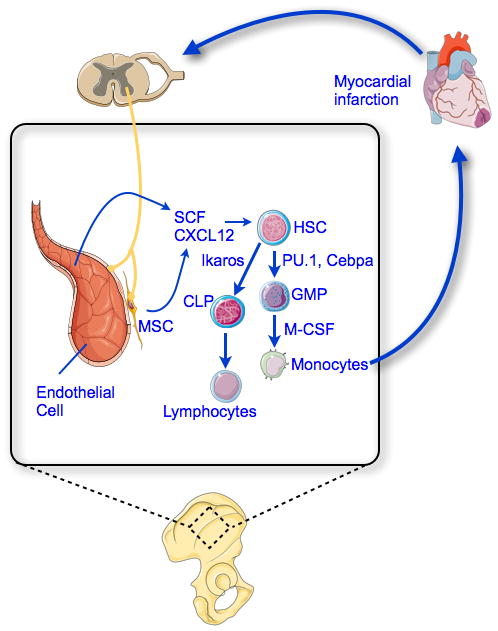
Myocardial infarction activates sympathetic activity in the bone marrow. Consequently, HSC niche cells, such as mesenchymal stem cells (MSC) and endothelial cells, produce lower levels of HSC retention factors, like CXCL12. This leads to increased myeloid progenitor proliferation and differentiation into monocytes. Newly made monocytes are released from the bone marrow and spleen and then recruited to the infarct via blood.
Monocyte release from hematopoietic organs in steady state and after MI
Monocyte release from the bone marrow follows the circadian rhythm, peaking at ZT4 and reaching nadir at ZT 1637. Blood monocytes’ diurnal rhythm is linked to fluctuation of several clock genes, such as Bmal1, Nrld1 and Dbp. Diurnal variation of monocyte egress from the bone marrow and increaed release during inflammaiton are driven by changing Mcp-1 levels in the blood. Listeria monocytogenes infection triggers higher blood monocyte levels and results in higher mortality due to massive ‘cytokine storm’. Consistent with this, TLR9 expression on peritoneal macrophages followed a similar circadian rhythm, and vaccination using a TLR9 ligand when TLR9 expression is high improved the adaptive immune response38.
Like monocyte fluctuation, mortality and morbidity after myocardial infarction also follow circadian rhythms. Disrupted diurnal levels aggravated myocardial remodeling and function following MI39. During the first 5 days after MI, a critical time for scar formation, there are high macrophage levels in the infarct. Homozygous clock mutant mice exhibited similarly aggravated ventricular remodeling after MI, which accords with the idea that blood monocyte levels, regulated by circadian rhythm, may determine myocardial repair post MI. This theory was also supported by a clinical study40 reporting that infarct size peaked at 1:00 a.m. in patients with ST-segment elevation myocardial infarction.
Monocytes leave the bone marrow during diseases, such as infections, atherosclerosis and myocardial infarction. Monocyte release after LPS challenge was accompanied by elevated Mcp-1 production by mesenchymal stem cells and Cxcl12-abundant reticular cells lining bone marrow sinusoids. Conditional deletion of Mcp-1 from these cells significantly reduced monocyte egress after LPS challenge. In addition to mesenchymal stem cells, bone marrow endothelial cells may produce Mcp-1 after MI. Similar to monocyte release after LPS challenge, these cells can also produce Mcp-1, resulting in their mobilization into the blood. However, this hypothesis remains to be investigated. As mentioned above, the spleen functions as monocyte reservoir. After MI, monocyte departure from the splenic red pulp depends on angiotensin II-angiotensin 1 receptor signaling33. Angiotensin II infusion in mice reproduced MI-induced motility of splenic monocytes and their release into the blood41.
Monocyte recruitment to the myocardium after MI
After myocardial infarction, circulating monocytes produced in the bone marrow and spleen are recruited to the infarct in two phases42, with the first phase dominated by Ly-6chigh monocytes. Recruitment of Ly-6chigh monocytes is CCR2-dependent. Ccl2 and Ccl7, both ligands for CCR2, are expressed at high levels in infarcted myocardium43,44. B cells in ischemic myocardium are likely the source of Ccl7 after MI. Depleting B cells resulted in improved ventricular function accompanied by reduced monocyte recruitment44. In the second phase of post-MI monocyte response, Ly-6clow monocyte recruitment depends on Cx3cr142. However, compared to the early recruitment of inflammatory monocytes, far fewer Ly6Clow monocytes are recruited to the infarct, and Ly6Chigh monocytes can give rise to Ly6Clow macrophages in later healing stages45. Other mononuclear chemoattractants, such as Ccl-3 and Ccl4, are also highly expressed in infarcts46, but their role in myocardial injury remains unstudied. Additionally, ELR-containing Cxc chemokines, which are strong neutrophil chemoattractants, are present in the infarct at high levels47.
Monocyte/macrophage functions after myocardial infarction
The two sequential monocyte/macrophage phases are both important for healing after acute MI. Ly-6chigh monocytes give rise to early inflammatory macrophages, and both clear damaged tissue by phagocytosis and secreting proteolytic enzymes. In the second phase, Ly-6clow macrophages facilitate wound healing and regeneration by promoting myofibroblast accumulation, collagen deposition and angiogenesis. Infiltrated monocytes may also interact with extracellular matrix in the damaged myocardium, leading to fibronectin release48. Fibronectin stabilizes the infarct and reduces infarct rupture. Once in the infarct, monocytes differentiate into macrophages in the presence of M-CSF. Macrophages promote angiogenesis, fibroblast proliferation and extracellular matrix deposition. Myofibroblasts, which are modified fibroblasts and α-smooth muscle actin-positive, are the major sources of collagen in the infarct. Myofibroblast differentiation is TGF-β-dependent. Macrophages also play a role in organ regeneration. Though myocardial infarction in adult mammals leads to scarring and diminished ventricular function, neonatal mouse hearts can regenerate after MI without scarring49, but depleting cardiac macrophages impedes this repair process. A recent study50 showed that embryonic-derived cardiac macrophages promote angiogenesis and healing after myocardial damage. Consistent with this, salamander limb regeneration also depends on macrophages51. While inflammation is required for cellular debris removal and new tissue formation after ischemic injury, exaggerated inflammation may impede the healing process, as shown in ApoE−/− mice with coronary ligation52. Accordingly, blood monocyte count after MI positively correlates with left ventricular end-diastolic volume and negatively correlates with ejection fraction in patients53. Mcp-1-deficient mice have markedly less monocyte recruitment to the infarct and, consequently, significantly fewer macrophages therein43. Even though Mcp-1-deficient and wild type mice had similar infarct sizes, the Mcp-1-deficient mice had improved ventricular function, thereby indicating monocytes’ importance in myocardial healing after MI.
Conclusions
Monocytosis ensues during myocardial infarction and is vital to eradicating pathogens in systemic or local infection. However, since exaggerated monocytosis impairs healing, as discussed above, curbing monocyte recruitment to the infarct may improve ventricular function54. Additionally, MI-induced monocytosis exacerbates other cardiovascular complications. In a clinical study by Chen and his colleagues55, patients with ST elevation MI had accelerated non-culprit coronary artery lesion atherosclerosis. Consistent with the finding in mice32, patients with acute myocardial infarction had higher splenic metabolic activity as determined by whole body 18F-FDG PET-CT56. Although the PET imaging agent reports glucose uptake and is not cell-type specific, the data may indicate higher hemaotpoietic progenitor proliferation in the spleen after MI. By blocking the β3 adrenoreceptor, we decreased HSC egress from the bone marrow and thereby reduced monocytosis after MI in ApoE−/− mice32. Additionally, splenic HSC proliferation depends on stem cell factor (SCF), and SCF neutralization reduced extramedullary monocytosis after MI. Monocyte release from hematopoietic organs depends on angiotensin-II receptor signaling and CCR2 and could be a target for drug development. Following this line of inquiry, we found that CCR2 knockdown decreased inflammatory monocyte recruitment to the infarct, thereby facilitating the healing process34,54. Like monocyte production and migration, macrophage polarization may be harnessed to reduce complications of myocardial ischemia, especially heart failure57.
Significance.
Myocardial infarct is the leading cause of death in developed countries. The myocardium undergoes a rapid turnover after myocardial infarction, which is crucial for proper healing. Cells of myeloid origin, particularly monocytes, play a major role in the healing process.
Acknowledgments
Source of funding: NIH: K99HL121076, R01HL096576, R01HL114477, R01HL117829, R01NS084863.
Abbreviations
- MI
Myocardial infarction
- HSC
Hematopoietic stem cells
- GMP
Granulocyte macrophage progenitors
- CLP
Common lymphoid progenitors
- M-CSF
Macrophage colony stimulating factor
Footnotes
Disclosure: none
References
- 1.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 3.Jakubzick C, Gautier EL, Gibbings SL, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 6.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 7.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 9.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidt T, Courties G, Dutta P, Sager H, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential Contribution of Monocytes to Heart Macrophages in Steady-State and After Myocardial Infarction. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auffray C, Fogg DK, Narni-Mancinelli E, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med. 2011;208:1901–1916. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautier EL, Ivanov S, Williams JW, Huang SC, Marcelin G, Fairfax K, Wang PL, Francis JS, Leone P, Wilson DB, Artyomov MN, Pearce EJ, Randolph GJ. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J Exp Med. 2014;211:1525–1531. doi: 10.1084/jem.20140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaposhnik Z, Wang X, Lusis AJ. Arterial colony stimulating factor-1 influences atherosclerotic lesions by regulating monocyte migration and apoptosis. J Lipid Res. 2010;51:1962–1970. doi: 10.1194/jlr.M005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutta P, Nahrendorf M. Regulation and consequences of monocytosis. Immunol Rev. 2014;262:167–178. doi: 10.1111/imr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 25.Krishnaraju K, Hoffman B, Liebermann DA. Early growth response gene 1 stimulates development of hematopoietic progenitor cells along the macrophage lineage at the expense of the granulocyte and erythroid lineages. Blood. 2001;97:1298–1305. doi: 10.1182/blood.v97.5.1298. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 27.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 28.Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 30.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga T, Mounier R, Gogolak P, Poliska S, Chazaud B, Nagy L. Tissue LyC6- macrophages are generated in the absence of circulating LyC6- monocytes and Nur77 in a model of muscle regeneration. J Immunol. 2013;191:5695–5701. doi: 10.4049/jimmunol.1301445. [DOI] [PubMed] [Google Scholar]
- 32.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutta P, Hoyer FF, Grigoryeva LS, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Ex Med. 2015 doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O’Sullivan ML, Pyle WG, Sole MJ, Martino TA. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res. 2014;114:1713–1722. doi: 10.1161/CIRCRESAHA.114.302995. [DOI] [PubMed] [Google Scholar]
- 40.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res. 2012;110:105–110. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 44.Zouggari Y, Ait-Oufella H, Bonnin P, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh Monocytes Depend on Nr4a1 to Balance Both Inflammatory and Reparative Phases in the Infarcted Myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frangogiannis NG, Entman ML. Chemokines in myocardial ischemia. Trends Cardiovasc Med. 2005;15:163–169. doi: 10.1016/j.tcm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 49.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–246. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 54.Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y, Jing J, Tu S, Tian F, Xue H, Chen W, Chen J, Reiber JH, Chen Y. ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging. 2014;30:253–261. doi: 10.1007/s10554-013-0354-z. [DOI] [PubMed] [Google Scholar]
- 56.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic Activity of the Spleen and Bone Marrow in Patients With Acute Myocardial Infarction Evaluated by 18F-Fluorodeoxyglucose Positron Emission Tomograpic Imaging. Circ Cardiovasc Imaging. 2014;7:454–460. doi: 10.1161/CIRCIMAGING.113.001093. [DOI] [PubMed] [Google Scholar]
- 57.Courties G, Heidt T, Sebas M, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63:1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


