Abstract
We tested the hypothesis that loss of substantia nigra neurons in subjects at risk of schizophrenia (1), as reflected by midbrain hyperechogenicity (2) and parkinsonian motor impairment (3), is asymmetric and influenced by sex. We evaluated 62 subjects with never-treated chronic schizophrenia, 80 of their adult, unaffected first degree relatives and 62 healthy controls (matched by sex and age to the cases), part of an Andean population of Northern Argentina. Parkinsonism was scored blindly using UPDRS-3 (Unified Parkinson's Disease Rating Scale) on videotaped exams by 2 independent raters. Trancranial ultrasound was performed by an expert sonographist blind to subject condition with a 2.5 MHz transducer through a temporal bone window. Quantification of echogenic area was carried out on saved images by a different evaluator. We found a significant difference in parkinsonian motor impairment between patients, their relatives as well as controls. All three groups showed worse parkinsonism on the left side than the right, corresponding with increased echogenicity on the right substantia nigra compared with the left. Females had significantly more right echogenicity than males, and patients and unaffected relatives were significantly more echogenic than controls on that side. On the left, only female patients had significant echogenicity. Our data supports the notion that unaffected relatives of schizophrenic subjects have increased parkinsonism and concomitant brainstem abnormalities which may represent a vulnerability to the disease. Both motor and brainstem abnormalities are asymmetric and influenced by sex.
Keywords: transcranial ultrasound, parkinsonism, schizophrenia, laterality, dimorphism
1. Introduction
Schizophrenia is devastating condition that is normally diagnosed upon the onset of psychosis and leads to a functional deterioration in early adult life. It affects nearly 50 million people worldwide. Its global economic burden is estimated between 1.5 and 3% of total health expenditures (Knapp et al., 2004) and has increased by 50% over the past ten years (Murray et al., 2012). Available medications reduce the hallucinations and delusions commonly seen in schizophrenia, but result in very limited functional outcomes.
The prevalence of parkinsonism in schizophrenia has been well documented. All clinically effective antipsychotic medications mediate their therapeutic actions, at least partially, through inhibition of dopaminergic neurotransmission (Yilmaz et al., 2012), often leading to occurrence of parkinsonian features (Leucht et al., 2013). On the other hand, parkinsonsim has been found repeatedly in first-episode untreated patients (Caligiuri et al., 1993;Chakos et al., 1992;Chatterjee et al., 1995;Honer et al., 2005;McCreadie et al., 2005), and in at-risk offspring of schizophrenics (Marcus et al., 1985a;Marcus et al., 1985b). In each of these cases parkinsonism predated exposure to antipsychotic medications. Indeed, dysregulation of brain dopamine function has been shown to correlate with genetic risk of schizophrenia (Egan et al., 2001). Based on these findings we proposed that risk of schizophrenia may correlate with severity of parkinsonian impairment, such that motor disturbances would reflect a mechanism of genetic liability to the disease, expressed as prenatal injury to a subpopulation of dopaminergic neurons projecting from the midbrain to the prefrontal cortex (Finlay, 2001;Weinberger and Berman, 1988;Masciotra et al., 2005). Over time these deficits would lead to an inadequate compensatory response that ultimately manifest as psychotic phenomena. This model is supported by imaging studies in patients with schizophrenia showing that impairment in executive function and working memory correlate with lower dopamine availability in the prefrontal cortex (Egan et al., 2001), whereas the acute psychotic symptoms correlate with excessive release of endogenous dopamine in basal ganglia (Breier et al., 1997).
Structural abnormalities in schizophrenia brains have been replicated to variable degrees and are influenced by gender, laterality, exposure to medications, and duration of illness (Chiapponi et al., 2013). Men, compared to women, have higher incidence, earlier age at onset, greater expression of negative symptoms and neurologic deficits, worse premorbid history and course of illness, and differential treatment outcomes, and sex-specific brain abnormalities (Abel et al., 2010).
Gestational risk factors for schizophrenia occurring during sexual differentiation of the brain may result in sex-specific abnormalities in the brain in schizophrenia (Abel, 2004). In fact, nigrostriatal, mesolimbic, and mesocortical projection neurons have an early role in the development of sexual dimorphism in the brain (Reisert et al., 1990;Harrison and Tunbridge, 2008). Specifically, there are more dopaminergic neurons in the mesocortical projection in females than in males, and dopaminergic neurons are more resistant to toxic insults in females than in males (Johnson et al., 2010). Thus, if the development of psychosis is a failed compensatory attempt by the mesolimbic projection neurons for the prenatal or perinatal loss of mesocortical projection neurons as mentioned, the relative excess of mesocortical neurons and their greater resistance in females could protect them from environmental insults and explain some gender differences in course of illness. We therefore hypothesized that the dopaminergic neurons giving rise to the nigrostriatal projections are more affected in males than in females with (or at risk for) schizophrenia.
Transcranial ultrasound (TUS) studies have shown a direct relationship between the degree of echogenicity and motor and functional impairment in patients with Parkinson disease (Berg, 2011a;Bouwmans et al., 2013). In schizophrenia, substantia nigra hyperechogenicity predicts susceptibility to drug-induced parkinsonism (Berg et al., 2001), but no previous studies have investigated unaffected first-degree relatives. Given the considerable genetic predisposition to the disease, substantia nigra hyperechogenicity and parkinsonism, if present in unaffected relatives, could serve as an intermediate phenotype and predictor of psychotic derangement in at-risk individuals.
We studied echogenicity and motor function in subjects with chronic schizophrenia never exposed to antipsychotic medications, their unaffected siblings, and healthy controls from a population of the Central Andes in Argentina. Due to its geographical isolation, patients were unlikely to receive antipsychotic treatment and allowed us to ideally address this question at the clinical and imaging level before offering them treatment. In addition, this sample contains approximately equal number of female and male patients, thus allowing for direct comparisons. Here, we tested the hypotheses that parkinsonism:
is a manifestation of risk of schizophrenia as reflected by its presence in at-risk unaffected relatives of patients with schizophrenia and,
is asymmetric and influenced by sex.
2. Experimental/Materials and Methods
2.1. Sample and ascertainment
We evaluated 62 subjects with never-treated chronic schizophrenia, 80 of their adult, unaffected first degree relatives and 62 healthy controls (matched by sex, age and educational level to the cases) of the same Andean population of Northern Argentina. Therefore, all subjects (including controls) come from the same population and from the same ethnic background as the patients and their relatives. Average duration of untreated illness was 68.9 ± 97.5 months. Table 1 shows the demographic characteristics of the sample. Educational level represents completed elementary (1), completed High School (2), or additional education at trade school or college level (3). Diagnostic ascertainment was carried out with the Schedules of Clinical Assessment in Neuropsychiatry scored blindly by 2 independent raters (EP, GG, MC, BMR, GdE, SCAN 2.1, World Health Organization, Geneva). SCAN is a set of instruments and manuals aimed at assessing, measuring and classifying psychopathology and behaviour associated with the major psychiatric disorders in adult life. It can be used for clinical, research- and training purposes and was developed within the framework of the World Health Organization (http://whoscan.org). SCAN has a bottom-up approach where no diagnosis-driven frames are applied in grouping the symptoms. Each symptom is assessed in its own right. It has a proven stability and robustness to differentialy assess psychotic states. Interview data are entered into the program and fed into algorithms for ICD-10 and DSM-IV diagnoses. These algorithms produce a diagnostic classification for both systems. To meet selection criteria all subjects met criteria for DSM-IV schizophrenia when scored by two independent raters. Treatment was offered to all participants and provided free of charge by the local government. All procedures and human protections were approved by the local government Bioethics Committee and by the University of South Florida Internal Review Board.
Table 1.
Demographic and clinical characteristics of the sample.
| Risk status | age | educational level | % male | UPDRS-3 |
|---|---|---|---|---|
| schizophrenia | 28.9 ± 11.7 | 1.9 ± 0.7 | 65 | 17.7 ± 4.6 |
| unaffected relatives | 34.7 ± 15.2 | 2.1 ± 1.1 | 50 | 8.3± 5.5 |
| healthy controls | 31.3 ± 8.9 | 2.7 ± 1.0 | 65 | 2.8 ± 0.4 |
2.2. Motor Assessment
Parkinsonism was scored blindly using UPDRS-3 (Unified Parkinson's Disease Rating Scale) on videotaped exams by 2 independent raters certified on its use (GdE and GG). Thus, the diagnosis of parkinsonism was not based on the UK Brain Bank criteria but on an arbitrary cut-off of UPDRS-3. Rigidity could not be directly assessed on the videotapes and was not scored (thus, the maximum possible score is 88 instead of 108). Video examples of movement impairment are provided as supplementary material. The Asymmetry Index was calculated as the absolute difference between the sides divided by the sum of their scores (L−R/[L+R]) (Espay et al., 2006). A larger Asymmetry Index indicates greater difference in disease burden between sides and therefore more asymmetry.
2.3. Transcranial Ultrasound
For transcranial ultrasound examination, we employed a color-coded, phased array ultrasound system, equipped with a 2.5 MHz transducer (Micromaxx, Sonosite Inc, Bothell, Washington). Examinations were performed through a preauricular acoustic bone window (penetration depth= 16 cm, dynamic range= 45 dB) by an expert sonographist (NF) with more than 20 years experience on the technique blind to subject condition. Less than 3% of cases were non-insonnable because of skull thickness (a much lower % of that reported in the Parkinson disease population, most likely because our patients were much younger). The substantia nigra was identified within the butterfly-shaped structure of the brainstem, scanning from each temporal bone window. Since the signal brightness (echogenicity) is not quantifiable by ultrasound, the area of hyperechogenic signals in the substantia nigra (SN) region was measured (in sqare cm) from each ipsilateral side separately by a highly trained radiologist with >15 years experience on the procedure (NF). Unbiased quantification of echogenic area was carried out post hoc on saved images by two different evaluators (DK and LS) blind to subject's condition. Sonographic measurements proved adequately reproducible.
2.4. Statistics
A preliminary general linearl model (GLM) of global UPDRS-3 score using age and sex as factors was carried out (table 1). Following calculation of an Asymmetry Index (Espay et al., 2006), ANOVA was performed with the Asymmetry Index as independent variable and sex or status (affected subject, unaffected relative or control) as factors. Correlation analysis was performed to establish the relationship between transcranial ultrasound abnormalities (hyperechogenicity) and contralateral parkinsonism (measured by lateralized UPDRS-3 scores). Multivariate analysis of variance (MANOVA) was performed to test the effect of sex and group on both parkinsonism (UPDRS-3) and echogenicity of SN for risk group (schizophrenia, unaffected relatives or healthy controls), laterality, and sex (male vs female). All calculations were performed with IBM SPSS statistical package (Armonk, New York).
3. Results
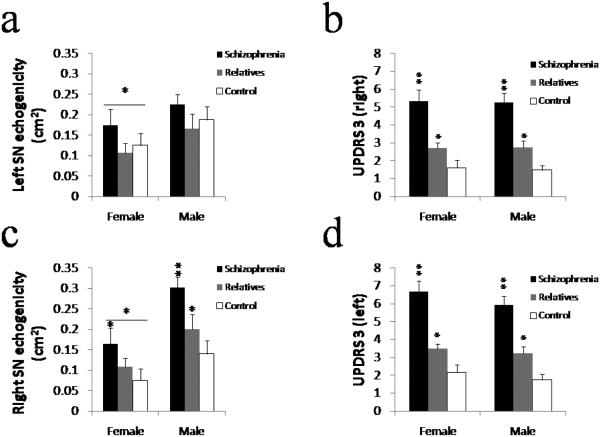
We found a significant difference in parkinsonian motor impairment between patients and their unaffected relatives, as well as between the latter and healthy controls. One patient (n=62) and 23 relatives (n=80) did not have any sign of parkinsonism (using as cut-off a UPDRS-3 score < 3). Overall UPDRS-3 scores differed by risk group but not by sex (there was a trend towards higher values in males) or age (table 2). Differences in motor impairment were asymmetric. When using an asymmetry index, the degree of asymmetry was found to differ significantly by sex but not by risk group (table 2). Motor impairment on the right side in both male and female patients was found to be significantly greater than in their respective unaffected relatives and healthy controls, as well as significantly higher in relatives than in controls (F=3.852, p=0.051). Male patients, but not relatives or controls or females regardless of risk status, showed worse parkinsonism on the left side than the right (Fig #1b,d) ; unaffected relatives of patients with schizophrenia had more motor impairment than healthy controls.
Table 2.
Univariate analysis of variance (ANOVA) and linear regressions. The first three rows display the results of ANOVA of global UPDRS-3 scores by sex, age or risk status (i.e., subjects with schizophrenia, unaffected first degree relatives or healthy controls) respectively. Rows four and five show the results of univariate analysis of the UPDRS-3 Asymmetry Index (Espay et al., 2006) by sex and risk status.
| GLM Model | F | p |
|---|---|---|
| Total UPDRS-3 by Age | 0.909 | 0.621 |
| Total UPDRS-3 by Sex | 2.818 | 0.095 |
| Total UPDRS-3 by Status | 62.453 | 0.000 |
| Asymmetry Index (UPDRS-3) by Sex | 3.967 | 0.048 |
| Asymmetry Index (UPDRS-3 ) by Status | 0.577 | 0.563 |
Figure 1.
Parkinsonism and Echogenicity of SN in Subjects with Neuroleptic Naïve Schizophrenia, their Unaffected Relatives and Healthy Controls.
Panel a. Displays average echogenicity values (+/− SEM) for male and female left SN. Only the female patients had significant hyperechogenicity while the three male groups had similar levels of echogenicity. Panel b. Displays average severity of parkinsonism (UPDRS-3 scores +/− SEM) on the right side of the body. Patients performed significantly worse compared to their relatives and healthy controls, and unaffected relatives performed significantly worse than controls. Panel c. Displays average echogenicity values (+/− SEM) for male and female, right and left SN. Both male and female patients had significantly more SN echogenicity than their relatives. Male relatives' right SN were significantly more echogenic than controls'. Overall, male right SN was significantly more echogenic than female. Panel d. Displays average severity of parkinsonism (UPDRS-3 scores +/− SEM) on the left side of the body. Patients performed significantly worse compared to their relatives and healthy controls, and unaffected relatives performed significantly worse than controls.
The transcranial ultrasound results were consistent with the motor findings, and indeed severity of hyperechogenicity was significantly correlated with severity of contralateral parkinsonism (table 3) regardless of sex or risk group. Males had significantly more right SN echogenicity than females, and patients and unaffected relatives were more echogenic than controls on that side (Fig #1c). On the left, only female patients had significant echogenicity of SN compared to their relatives and control whereas males did not show any group differences in echogenicity of SN in the left midbrain (Fig #1c).
Table 3.
Linear regression of lateralized parkinsonism over contralateral transcranial ultrasound hyperechogenicity area of SN.
| Regression Model | F | p | r |
|---|---|---|---|
| right UPDRS-3 over left SN area | 1.435 | 0.047 | 0.409 |
| left UPDRS-3 over right SN area | 2.466 | 0.000 | 0.616 |
A multivariate analysis of variance using a generalized linear model with interactions was performed to test the effect of sex, laterality and risk group (i.e., patients, unaffected relatives or healthy controls) on both parkinsonism and echogenicity (table 4). There were significant main effects for sex and risk group on UPDRS and echogencity (table 4), confirming the results of individual ANOVAs. There were also highly significant interactions between sex and risk group on the echogenicity of SN (which was more symmetric but less severe in females patients; F=4.511, p=0.012), and between laterality and risk group on parkinsonism (that was significantly worse on the left for patients but not for unaffected relatives or controls; F=4.244, p=0.040).
Table 4.
MANOVA of Parkinsonism (UPDRS-3) and transcranial ultrasound echogenicity of SN for group (schizophrenia, unaffected relatives or healthy controls) and sex (male vs female). Mean values and significant main effects are shown.
|
Dependent
Variable |
Control | Relatives | Schizophrenia | Main effects (p) | interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | sex | risk | sex*risk | |
| UPDRS-3 right | 1.64 | 1.31 | 2.20 | 3.18 | 6.25 | 5.18 | 0.853 | 0.000 | ns |
| UPDRS-3 left | 2.12 | 1.50 | 3.09 | 3.68 | 7.38 | 5.68 | 0.228 | 0.000 | ns |
| left SN (cm 2) | 0.08 | 0.16 | 0.10 | 0.21 | 0.21 | 0.29 | 0.035 | 0.161 | ns |
| right SN (cm2) | 0.13 | 0.20 | 0.12 | 0.19 | 0.22 | 0.21 | 0.000 | 0.001 | ns |
4. Discussion
It has been well established that antipsychotic drugs can lead to parkinsonism, (Klemp et al., 2011;Leucht et al., 1999). However, it is worth pointing out that the presence of parkinsonism in untreated schizophrenia is not surprising or a new finding (Caligiuri et al., 1993;Chakos et al., 1992;Chatterjee et al., 1995;Honer et al., 2005;McCreadie et al., 2005).
In a sample of never-treated patients with chronic schizophrenia, their unaffected relatives, and healthy controls from an Andean population in Northern Argentina, we found that parkinsonism was present in all but one of the subjects with schizophrenia. Remarkably, the same motor deficits (though of lesser severity) were detected in more than two thirds of unaffected first degree relatives of patients but were completely absent in healthy controls. The fact that we identified parkinsonism in all but one of the subjects may be a consequence of either the fact that (unlike most published series) we recruited subjects with several years of symptoms, and/or a factor of a lower cut-off point on the UPDRS-3. Furthermore, we found a corresponding hyperechogenicity of SN which was linearly correlated with contralateral parkinsonism (table 3) as reported in patients with Parkinson disease (Berg et al., 2001;Berg, 2011a) and in patients with schizophrenia treated with antipsychotic medications (Berg et al., 2001). In chronically treated patients with schizophrenia, hyperechogenicity correlates with age and severity of parkinsonian symptoms but not with dose or type of antipsychotics (Jabs et al., 2003). This is consistent with the notion that hyperechogenicity of SN represents a risk factor, rather than a consequence of the use of medications. It is worth pointing out, however, that our findings extend to unaffected first degree relatives of subjects with schizophrenia, a fact not yet reported in the literature. On the other hand, hyperechogenicity of substantia nigra correlates inversely with uptake of the dopamine precursor fluoroDOPA in striatum (Schweitzer et al., 2006), and directly with severity of motor involvement in patients with Parkinson disease, their unaffected relatives and in undiagnosed elderly subjects (Berg, 2011b), which is consistent with our results.
Motor deficits were found bilaterally but to varying degrees on either side. The asymmetry index (Espay et al., 2006) was similar across risk groups (patients, relatives or healthy controls), but was significantly affected by sex (table 2) such that more asymmetry was observed in male patients and relatives than in females. On the other hand, no significant differences were found between males and females in global UPDRS-3 scores regardless of risk category (table 2). Furthermore, left sided motor impairment and hyperchogenicity of the right substantia nigra are significantly more severe in male subjects with chronic untreated schizophrenia than in their first-degree relatives or in controls, and these abnormalities are significantly more prominent in unaffected relatives from schizophrenics than in controls. A similar asymmetry of motor dysfunction has been noted in both drug-induced parkinsonism (Jerussi and Taylor, 1982) and in Parkinson Disease (Scherfler et al., 2012) as well.
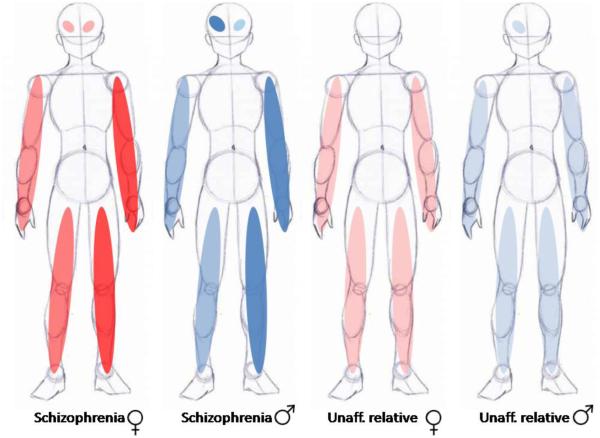
Our results should not be entirely unexpected, since the presence of abnormalities in the dopaminergic system is affected by sex. Female patients have less severe, but more symmetric hyperechogenicity of SN compared to males. On the other hand, male patients and unaffected relatives have much more apparent motor impairment on the left side and corresponding contralateral hyperechogenicity of SN (Figure 2). Goldstein has argued previously that gestational risk factors for schizophrenia occurring during the sexual differentiation of the brain will result in sex-specific abnormalities (Seidman et al., 2003). Interestingly, obstetric complications associated with parkinsonism in schizophrenia (Peralta and Cuesta, 2011) may be influenced by sex (Lane et al., 1996). Midbrain dopamine neurons are known to play a role in the development of sexual dimorphism in the brain and have been shown to be particularly susceptible to environmental insults (de Erausquin et al., 2003;Dorsey et al., 2006;Landreau et al., 2012). Specifically, dopaminergic neurons in the mesocortical projections are more numerous (Kritzer and Creutz, 2008), and have been shown to be more resistant to cytotoxic insults in females than in males (Bourque et al., 2009).
Figure 2.
Schematic representation of sex and laterality differences in parkinsonian impairment and SN echogenicity. Shades of color reflect severity.
If, as proposed here, schizophrenia is associated with a fixed developmental lesion in mesocortical projection neurons, interacting with normal brain maturational events, then it is conceivable that the greater abundance of mesocortical projections in females and their greater resistance to environmental injuries could provide a protective buffer from environmental insults and could explain the variable expression of certain clinical phenotypes in schizophrenia. Indeed, we found more extensive midbrain hyperechogenicity in males, a proxy for dopaminergic loss of function, but in females mild deficits were present bilaterally. Motor impairment was consistently also worse (and more asymmetric) in males. A number of additional observations argue in favor of a loss of dopaminergic neuronal function in schizophrenia (Finlay, 2001;Masciotra et al., 2005):
eurons in the ventral tegmental area appear dystrophic and may be reduced in number (Bogerts et al., 1983;Kolomeets and Uranova, 1999).
Axonal dopaminergic terminals, and expression of dopamine-regulated proteins are decreased in the dorsolateral prefrontal cortex (DLPFC) of schizophrenic brains (Akil et al., 1999)
Reduced availability of dopamine in the DLPFC is associated with increased risk of schizophrenia, and with poor performance on a working memory task and cortical metabolic inefficiency (Callicott et al., 2000;Egan et al., 2001).
These findings resemble those observed in adult rats after lesioning of dopaminergic projections to cortex during development, namely cortical dysfunction and a compensatory increase in subcortical dopamine release (Carter and Pycock, 1980). A similar developmental lesion in humans could cause prefrontal deficits and psychosis (Masciotra et al., 2005;Weinberger and Berman, 1988).
Our findings are consistent with data suggesting that male schizophrenics are more likely than females to experience motor dysfunction if they experience perinatal insults, supporting a greater susceptibility to environmental insults (Lane et al., 1996). Our results also indicate that sexual dimorphism at the level of midbrain may serve as a protective mechanism in the development of parkinsonian motor impairment for females. The degree of echogenicity may alternatively reflect gender differences in the course of illness.
This unique sample of subjects with chronic untreated schizophrenia is ideally suited to address the question of the role of sex influences on the pathogenesis and course of schizophrenia because it contains a larger proportion of females than first episode samples, thus allowing for direct comparisons. In any case, our data strongly support the use of midbrain hyperechogenicity as intermediate phenotype of risk for major psychoses that, in principle, could serve as clinically useful biomarker in at risk populations.
Supplementary Material
Acknowledgements
This work was funded in part by grants NIMH K08MH077220, NIH FIC 7R21TW007882-05, NIH FIC 3R21TW007882-04S1, by the Roskamp Endowment fund and by two NARSAD (Brain and Behavior Foundation) grants to GdeE.
GdE is Constance and Stephen Lieber Investigator, Sidney R. Baer Junior Investigator and Roskamp Chair of Biological Psychiatry. The authors wish to thank the Agentes Sanitarios of the Province of Jujuy for their generous and untiring collaboration with the study.
Role of the Funding Source. The funding agencies had no participation whatsoever in the design, analysis, interpretation, or communication of the data and in no way influenced the contents of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest. None of the authors has any conflict of interests to declare.
5. References
- Abel KM. Foetal origins of schizophrenia: testable hypotheses of genetic and environmental influences. Br. J. Psychiatry J. Ment. Sci. 2004;184:383–385. doi: 10.1192/bjp.184.5.383. [DOI] [PubMed] [Google Scholar]
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int. Rev. Psychiatry Abingdon Engl. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. doi:10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Berg D. Hyperechogenicity of the substantia nigra: pitfalls in assessment and specificity for Parkinson’s disease. J. Neural Transm. Vienna Austria. 2011a;118:453–461. doi: 10.1007/s00702-010-0469-5. doi:10.1007/s00702-010-0469-5. [DOI] [PubMed] [Google Scholar]
- Berg D. Substantia nigra hyperechogenicity is a risk marker of Parkinson’s disease: yes. J. Neural Transm. Vienna Austria. 2011b;118:613–619. doi: 10.1007/s00702-010-0565-6. doi:10.1007/s00702-010-0565-6. [DOI] [PubMed] [Google Scholar]
- Berg D, Jabs B, Merschdorf U, Beckmann H, Becker G. Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol. Psychiatry. 2001;50:463–467. doi: 10.1016/s0006-3223(01)01190-8. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Häntsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol. Psychiatry. 1983;18:951–969. [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front. Neuroendocrinol. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. doi:10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Bouwmans AEP, Vlaar AMM, Mess WH, Kessels A, Weber WEJ. Specificity and sensitivity of transcranial sonography of the substantia nigra in the diagnosis of Parkinson’s disease: prospective cohort study in 196 patients. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002613. doi:10.1136/bmjopen-2013-002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. Am. J. Psychiatry. 1993;150:1343–1348. doi: 10.1176/ajp.150.9.1343. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex New York. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Pycock CJ. Behavioural and biochemical effects of dopamine and noradrenaline depletion within the medial prefrontal cortex of the rat. Brain Res. 1980;192:163–176. doi: 10.1016/0006-8993(80)91016-1. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Mayerhoff DI, Loebel AD, Alvir JM, Lieberman JA. Incidence and correlates of acute extrapyramidal symptoms in first episode of schizophrenia. Psychopharmacol. Bull. 1992;28:81–86. [PubMed] [Google Scholar]
- Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA. Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am. J. Psychiatry. 1995;152:1724–1729. doi: 10.1176/ajp.152.12.1724. [DOI] [PubMed] [Google Scholar]
- Chiapponi C, Piras Fabrizio, Fagioli S, Piras Federica, Caltagirone C, Spalletta G. Age-related brain trajectories in schizophrenia: A systematic review of structural MRI studies. Psychiatry Res. 2013 doi: 10.1016/j.pscychresns.2013.05.003. doi:10.1016/j.pscychresns.2013.05.003. [DOI] [PubMed] [Google Scholar]
- De Erausquin GA, Hyrc K, Dorsey DA, Mamah D, Dokucu M, Mascó DH, Walton T, Dikranian K, Soriano M, García Verdugo JM, Goldberg MP, Dugan LL. Nuclear translocation of nuclear transcription factor-kappa B by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors leads to transcription of p53 and cell death in dopaminergic neurons. Mol. Pharmacol. 2003;63:784–790. doi: 10.1124/mol.63.4.784. [DOI] [PubMed] [Google Scholar]
- Dorsey DA, Mascó DH, Dikranian K, Hyrc K, Masciotra L, Faddis B, Soriano M, Gru AA, Goldberg MP, de Erausquin GA. Ultrastructural characterization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-induced cell death in embryonic dopaminergic neurons. Apoptosis Int. J. Program. Cell Death. 2006;11:535–544. doi: 10.1007/s10495-006-5268-y. doi:10.1007/s10495-006-5268-y. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. doi:10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Morgante F, Gunraj C, Chen R, Lang AE. Mirror movements in Parkinson’s disease: effect of dopaminergic drugs. J. Neurol. Neurosurg. Psychiatry. 2006;77:1194–1195. doi: 10.1136/jnnp.2005.086892. doi:10.1136/jnnp.2005.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM. Mesoprefrontal dopamine neurons and schizophrenia: role of developmental abnormalities. Schizophr. Bull. 2001;27:431–442. doi: 10.1093/oxfordjournals.schbul.a006885. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. doi:10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Honer WG, Kopala LC, Rabinowitz J. Extrapyramidal symptoms and signs in first-episode, antipsychotic exposed and non-exposed patients with schizophrenia or related psychotic illness. J. Psychopharmacol. Oxf. Engl. 2005;19:277–285. doi: 10.1177/0269881105051539. doi:10.1177/0269881105051539. [DOI] [PubMed] [Google Scholar]
- Jabs BE, Bartsch AJ, Pfuhlmann B. Susceptibility to neuroleptic-induced parkinsonism--age and increased substantia nigra echogenicity as putative risk factors. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2003;18:177–181. doi: 10.1016/s0924-9338(03)00045-2. [DOI] [PubMed] [Google Scholar]
- Jerussi TP, Taylor CA. Bilateral asymmetry in striatal dopamine metabolism: implications for pharmacotherapy of schizophrenia. Brain Res. 1982;246:71–75. doi: 10.1016/0006-8993(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J. Neuroendocrinol. 2010;22:226–237. doi: 10.1111/j.1365-2826.2010.01964.x. doi:10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemp M, Tvete IF, Skomedal T, Gaasemyr J, Natvig B, Aursnes I. A review and Bayesian meta-analysis of clinical efficacy and adverse effects of 4 atypical neuroleptic drugs compared with haloperidol and placebo. J. Clin. Psychopharmacol. 2011;31:698–704. doi: 10.1097/JCP.0b013e31823657d9. doi:10.1097/JCP.0b013e31823657d9. [DOI] [PubMed] [Google Scholar]
- Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr. Bull. 2004;30:279–293. doi: 10.1093/oxfordjournals.schbul.a007078. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Uranova NA. Synaptic contacts in schizophrenia: studies using immunocytochemical identification of dopaminergic neurons. Neurosci. Behav. Physiol. 1999;29:217–221. doi: 10.1007/BF02465329. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. doi:10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreau F, Galeano P, Caltana LR, Masciotra L, Chertcoff A, Pontoriero A, Baumeister E, Amoroso M, Brusco HA, Tous MI, Savy VL, Arnaiz Lores, de Erausquin GA. Effects of two commonly found strains of influenza A virus on developing dopaminergic neurons, in relation to the pathophysiology of schizophrenia. PloS One. 2012;7:e51068. doi: 10.1371/journal.pone.0051068. M. del R. doi:10.1371/journal.pone.0051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A, Colgan K, Moynihan F, Burke T, Waddington JL, Larkin C, O’Callaghan E. Schizophrenia and neurological soft signs: gender differences in clinical correlates and antecedent factors. Psychiatry Res. 1996;64:105–114. doi: 10.1016/0165-1781(96)02602-9. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013 doi: 10.1016/S0140-6736(13)60733-3. doi:10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr. Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Marcus J, Hans SL, Lewow E, Wilkinson L, Burack CM. Neurological findings in high-risk children: childhood assessment and 5-year followup. Schizophr. Bull. 1985a;11:85–100. doi: 10.1093/schbul/11.1.85. [DOI] [PubMed] [Google Scholar]
- Marcus J, Hans SL, Mednick SA, Schulsinger F, Michelsen N. Neurological dysfunctioning in offspring of schizophrenics in Israel and Denmark. A replication analysis. Arch. Gen. Psychiatry. 1985b;42:753–761. doi: 10.1001/archpsyc.1985.01790310015002. [DOI] [PubMed] [Google Scholar]
- Masciotra L, Landreau F, Conesa HA, de Erausquin GA. Trends in Schizophrenia Research. Nova Biomedical Books; New York: 2005. Pathophysiology of schizophrenia: a new look at the role of dopamine; pp. 27–44. [Google Scholar]
- McCreadie RG, Srinivasan TN, Padmavati R, Thara R. Extrapyramidal symptoms in unmedicated schizophrenia. J. Psychiatr. Res. 2005;39:261–266. doi: 10.1016/j.jpsychires.2004.08.002. doi:10.1016/j.jpsychires.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. doi:10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr. Psychiatry. 2011;52:139–145. doi: 10.1016/j.comppsych.2010.05.009. doi:10.1016/j.comppsych.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Reisert I, Schuster R, Zienecker R, Pilgrim C. Prenatal development of mesencephalic and diencephalic dopaminergic systems in the male and female rat. Brain Res. Dev. Brain Res. 1990;53:222–229. doi: 10.1016/0165-3806(90)90010-v. [DOI] [PubMed] [Google Scholar]
- Scherfler C, Seppi K, Mair KJ, Donnemiller E, Virgolini I, Wenning GK, Poewe W. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain J. Neurol. 2012;135:3348–3354. doi: 10.1093/brain/aws253. doi:10.1093/brain/aws253. [DOI] [PubMed] [Google Scholar]
- Schweitzer KJ, Hilker R, Walter U, Burghaus L, Berg D. Substantia nigra hyperechogenicity as a marker of predisposition and slower progression in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2006;21:94–98. doi: 10.1002/mds.20669. doi:10.1002/mds.20669. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Pantelis C, Keshavan MS, Faraone SV, Goldstein JM, Horton NJ, Makris N, Falkai P, Caviness VS, Tsuang MT. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr. Bull. 2003;29:803–830. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr. Bull. 1988;14:157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Zai CC, Hwang R, Mann S, Arenovich T, Remington G, Daskalakis ZJ. Antipsychotics, dopamine D2 receptor occupancy and clinical improvement in schizophrenia: a meta-analysis. Schizophr. Res. 2012;140:214–220. doi: 10.1016/j.schres.2012.06.027. doi:10.1016/j.schres.2012.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.