Abstract
Systemic hypertension is one of the most prevalent cardiovascular diseases. Sleep disordered breathing (SDB) with recurrent apnea is a major risk factor for developing essential hypertension. Chronic intermittent hypoxia (CIH) is a hallmark manifestation of recurrent apnea. Rodent models patterned after the O2 profiles seen with SDB patients showed that CIH is the major stimulus for causing systemic hypertension. This article reviews the physiological and molecular basis of CIH-induced hypertension. Physiological studies have identified that augmented carotid body chemosensory reflex and the resulting increase in sympathetic nerve activity is a major contributor to CIH-induced hypertension. Analysis of molecular mechanisms revealed that CIH activates hypoxia-inducible factor (HIF)-1 and suppresses HIF-2- mediated transcription. Dysregulation of HIF-1- and HIF-2- mediated transcription leads to imbalance of pro-oxidant and anti-oxidant enzyme gene expression resulting in increased reactive species (ROS) generation in the chemosensory reflex which is central for developing hypertension.
Keywords: Carotid body, Sensory long-term facilitation, NADPH oxidase, Superoxide dismutase, Oxidative stress
Systemic hypertension is one of the most prevalent cardiovascular diseases affecting an estimated 26% of the population [1]. It can be either primary (essential), with no known underlying cause, or secondary, caused by known conditions that affect either kidneys or endocrine system [2]. Of these two forms, essential hypertension, which is characterized by enhanced sympathetic nerve activity, is more prevalent, affecting 90–95% of hypertensive cases. Epidemiological studies have identified sleep disordered breathing (SDB) with recurrent apnea as a major risk factor for developing essential hypertension [3–6]. Recurrent apneas are characterized by transient, repetitive cessations of breathing, which can be either due to obstruction of the upper airway (obstructive sleep apnea, OSA) or defective generation of respiratory rhythm by the central nervous system (central apnea) [4, 6]. SDB is highly prevalent, afflicting 9% of women and 24% of adult men in the United States [7]. Peppard et al [8] demonstrated a clear correlation between the severity of apnea and subsequent development of hypertension, independent of other confounding factors.
In severely affected SDB patients, arterial blood O2 saturation during apnea can be reduced to as low as 50%. Thus, chronic intermittent hypoxia (CIH) is hallmark manifestation of recurrent apnea. While apneas also produce chronic intermittent hypercarbia (i.e., elevated arterial blood CO2), rodent models patterned after the O2 profiles encountered in SDB patients, showed that CIH is the major stimulus for causing systemic hypertension [9–11]. The discovery of transcriptional activators HIF-1 and HIF-2 have provided important molecular insights into systemic responses to hypoxia [12, 13]. This article reviews emerging evidence for dysregulated HIF-mediated transcription as a major molecular mechanism underlying hypertension caused by CIH.
PHYSIOLOGICAL BASIS OF CIH-INDUCED HYPERTENSION
Augmented sympathetic nerve activity: A hallmark of CIH-induced hypertension
SDB patients exhibit elevated muscle sympathetic nerve activity (MSNA), which is a measure of systemic vascular resistance [14] as well as increased circulating and urinary norepinephrine and epinephrine levels [5, 15–18]. The increased sympathetic nerve activity in SDB subjects is independent of obesity, which is a common co-morbidity in these patients [14]. Continuous positive airway pressure (CPAP) treatment normalizes sympathetic nerve activity and blood pressure in some, but not in all, OSA patients [18–20]. Rodents exposed to CIH also exhibit augmented sympathetic nerve activity and hypertension [21–25]. Chemical sympathectomy with 6-hydroxy dopamine prevents CIH-induced hypertension in CIH exposed rodents [26]. Taken together, these studies suggest that heightened sympathetic nerve activity is a major contributor to CIH-induced hypertension.
Carotid body chemosensory reflex mediates sympathetic activation by CIH
The chemosensory reflex initiated by the carotid body, the principal O2 sensing organ, is a major regulator of sympathetic tone [27]. Even a modest decrease in arterial blood O2 (hypoxemia) is enough to stimulate the carotid body sensory nerve activity and the response occurs within few seconds after the onset of hypoxia. The exquisite sensitivity of the carotid body to changes in O2 levels and the ensuing activation of the chemosensory reflex are uniquely suited to translate the IH stimulus occurring during recurrent apnea to changes in sympathetic nerve activity.
Several studies showed heightened carotid body chemosensory reflex in SDB patients’ and CIH exposed rodents. Hypoxia-induced sympathetic excitation, stimulation of breathing, and blood pressure elevation, the hallmarks of the chemosensory reflex are more pronounced in SDB patients than in control subjects [28–30]. Brief hyperoxia, which decreases carotid body sensory nerve activity, results in a more pronounced ventilatory depression in OSA patients than in control subjects [29, 31] and reduces blood pressure in OSA subjects [30]. CIH exposed cats [32] and mice [33] also exhibit enhanced ventilatory response to hypoxia, which is a hallmark of the carotid body chemosensory reflex. Rats exposed to CIH show exaggerated renal nerve [22] and thoracic sympathetic nerve responses to hypoxia [25, 34]. Critical evidence for the role of chemosensory reflex in mediating sympathetic activation and hypertension by CIH comes from studies with ablation of carotid bodies. In early 1960’s, surgical ablation of carotid bodies was performed in patients with asthma [35]. Some patients with carotid body resection developed SDB. Remarkably, SDB patients with resected carotid bodies did not develop hypertension [36]. Chronic sectioning of carotid sinus nerves, as in the earlier study by Fletcher and co-workers [37], and selective ablation of carotid bodies, while preserving the carotid baroreceptors, as in a recent study by Peng et al [11] prevented CIH induced sympathetic activation, elevated plasma catecholamine levels and hypertension in rats. Taken together these studies suggest that augmented carotid body chemosensory reflex mediates sympathetic activation and hypertension caused by CIH.
CIH results in remodeling of the carotid body chemosensory reflex pathway
Carotid body
An increase in the carotid body sensory nerve activity is an essential prerequisite for triggering the chemosensory reflex. Several studies showed that CIH leads to augmented hypoxic sensitivity of the carotid body [32, 33, 38]. In addition to affecting the hypoxic response, CIH also induces functional plasticity of the carotid body manifested as sensory long-term facilitation (LTF), which is characterized by long-lasting increase in baseline sensory nerve activity following repetitive hypoxia [39]. In contrast, CIH had no effect on the hypercapnic response of the carotid body [38] suggesting that CIH selectively affects O2 sensing by the carotid body. The CIH-induced functional changes were not associated with any noticeable alterations in carotid body morphology [39, 40].
Brooks et al reported that hypertension develops over time in a canine model of OSA [41]. Similarly, the effects of CIH on the carotid body are also time-dependent in that they appeared after 3 days of IH exposure and magnitude of the responses (i.e., sensitization of the hypoxic response and induction of sensory LTF) further increased after 10 days of IH [39]. SDB patients exhibit pronounced elevations in blood pressure during apnea and increased sympathetic nerve activity and hypertension persists during daytime even in the absence of apneas [8, 42]. It has been proposed that the enhanced hypoxic sensitivity contributes to acute elevations in blood pressure during apnea and the sensory LTF contributes to daytime hypertension and increased sympathetic tone in the absence of apneas [43].
Brainstem neurons
Processing of sensory information from the carotid body at the central nervous system (CNS) is essential for translating the increased carotid body activity to activation of the sympathetic nervous system. Nerve fibers from the carotid body course through the carotid sinus nerve to the brainstem, where they synapse with neurons in the nucleus tractus solitarius (nTS) and rostral ventrolateral medulla (RVLM), from which the efferent signal is transmitted to the sympathetic nervous system. Kline et al. [44] reported that CIH increases postsynaptic neuronal activity in the nTS. CIH also increases RVLM neuronal activity [45] and this effect requires increased glutamatergic transmission [46]. Collectively these studies suggest that altered carotid body function and increased excitability of brainstem neurons underlie the heightened carotid body chemosensory reflex by CIH.
Adrenal medulla: Sympathetic effector organ
The adrenal medulla is one of the major end organs of the sympathetic nervous system and medullary chromaffin cells secrete catecholamines (epinephrine and norepinephrine) during hypoxia via activation of the carotid body chemosensory reflex. Recent studies showed that CIH markedly augments hypoxia-evoked catecholamine secretion from the adrenal medulla [10] and this effect is mediated by activation of sympathetic nerves by the carotid body chemosensory reflex [11]. Either adrenal demedullation [47] or ablation of sympathetic innervation to the adrenals [11] prevents CIH-induced hypertension and elevated plasma catecholamine levels.
Taken together these studies demonstrate that the augmented carotid body chemosensory reflex is associated with functional remodeling of the carotid body, enhanced excitability of brain stem neurons and augmented catecholamine secretion from adrenal medulla, the major end organ of the chemosensory reflex pathway (Fig. 1).
Figure 1.
Schematic illustration of carotid body chemosensory reflex to hypertension caused by obstructive sleep apnea (OSA). O2 sat, arterial blood O2 saturation, PaO2, partial pressure of O2 in arterial blood, nTS, nucleus tractus solitarius, RVLM, rostral ventrolateral medulla, CA, catecholamines.
MOLECULAR BASIS OF CIH-INDUCED HYPERTENSION: ROLE OF HYPOXIA-INDUCIBLE FACTORS (HIFs)
As described above, the effects of CIH on the carotid body chemosensory reflex and the development of hypertension are time-dependent. Such time-associated changes in physiological variables are attributed to transcription factor-mediated gene regulation and the resulting de novo protein synthesis [12]. Recent studies on rodent and cell culture models identified HIFs as the major molecular mechanisms underlying the effects of CIH.
HIF-1 and HIF-2 are well-studied members of the HIF family of transcriptional activators [13]. They are heterodimers comprised of an O2-regulated α subunit and a constitutively expressed β subunit. The transcriptional activation of HIFs by continuous hypoxia requires increased accumulation of α subunit, and dimerization with β subunit along with interaction with co-activators p300 (adenovirus EIA-associated 300-kDa protein) and CBP (cyclic AMP-responsive element-binding protein) [12, 13]. The following section summarizes how CIH affects HIF-α isoforms in the chemosensory reflex pathway and its impact on blood pressure.
Effects of CIH on HIF-α isoform expression in the chemosensory reflex pathway
Carotid body
Glomus cells, which are the primary O2 sensing cells of the carotid body, express both HIF-1α and HIF-2α [48, 49]. Whilst continuous hypoxia increases both HIF-1α and HIF-2α [48, 49], CIH increases HIF-1α [50] and decreases HIF-2α [51] protein levels in the carotid body. The carotid body receives the highest blood flow per tissue weight of any organ in the body [52–54]. Given the high blood flow, changes in HIF-α expression are likely due to direct effects of CIH on the glomus cells. Such a possibility is supported by the finding that rat pheochromocytoma-12 (PC12) cells, which are O2 sensitive like glomus cells, when exposed to IH also exhibit increased HIF-1α and decreased HIF-2α proteins [51].
Mechanisms underlying CIH-induced dysregulation of HIF-α isoform proteins were examined in PC12 cell cultures. These studies showed that CIH increases reactive oxygen species (ROS) by activating xanthine oxidase [55]. ROS in turn elevates cytosolic Ca2+, which by activating protein kinase C-dependent NADPH oxidase increases HIF-1α protein via mammalian target of rapamycin (mTOR)-dependent protein synthesis and decreased proline hydroxylation [55–57]. The decreased HIF-2α protein by CIH, on the other hand, is due to increased protein degradation by Ca2+-dependent calpain proteases [51, 56]. The complex signaling pathways mediating the dysregulated HIF-α isoforms by CIH are schematically illustrated in Figure 1. Cell culture studies also revealed that CIH-induced changes in HIF-α isoform proteins are reflected in increased HIF-1 and decreased HIF-2 mediated transcriptional activity [51, 58].
Brainstem and adrenal medulla
Similar to the carotid body, CIH exposure also results in an imbalance of HIF-α isoform protein expression in the neurons of nTS and RVLM as well as in the adrenal medulla [11, 33, 51, 59]. The CIH-evoked HIF-α imbalance in the brainstem and in the adrenal medulla was prevented by selective ablation of the carotid body [11]. Unlike carotid body, isolated brainstem neurons or adrenal medulla are relatively insensitive to hypoxia [60]. Consistent with those findings, a recent study by Peng et al [11] demonstrated that CIH-evoked carotid body neural activity triggers HIF-α and ROS imbalance in the nTS and RVLM of the brainstem and in the sympathetic end-organ, the adrenal medulla.
Relevance of HIF-α dysregulation by CIH to hypertension
Complete deficiency of either HIF-1α or HIF-2α is lethal, whereas heterozygous (HET) mice, which are partially deficient in either HIF-1α or HIF-2α expression, develop normally and are indistinguishable from wild-type (WT) littermates under normoxic conditions [61–63]. Hif1a+/− mice showed remarkable absence of CIH-induced hypertension as compared with gender-matched wild-type littermates [33]. The lack of hypertension was associated with absence of all responses to CIH, including: sensitization of carotid body response to hypoxia, induction of sensory LTF, increased HIF-1α expression and elevated plasma norepinephrine levels. These findings suggest that activation of HIF-1α is required for activation of the carotid body chemosensory reflex that leads to hypertension in response to CIH.
In contrast, Hif2a+/− mice exhibit phenotypic changes under normoxic conditions that are similar to CIH-exposed wild-type mice, including: hypertension, elevated plasma catecholamines, increased incidence of apnea, enhanced carotid body response to acute hypoxia, and augmented catecholamine secretion from adrenal chromaffin cells [64]. These phenotypic changes were associated with increased HIF-1α expression in the chemosensory reflex pathway. Blockade of HIF-1α expression either by systemic administration of digoxin prevented the development of hypertension, respiratory abnormalities, and chemosensory reflex responses in Hif2a+/− mice [65]. Similarly, restoring HIF-2α levels by administration of a calpain inhibitor also prevented hypertension in CIH exposed rats [51]. Taken together these findings provide evidence for dysregulated HIF-α isoforms as a critical molecular mechanism underlying CIH-induced hypertension.
HOW DO HIFs CONTRIBUTE TO CIH-EVOKED HYPERTENSION?
Oxidative stress
Since IH is characterized by periods of hypoxia interspersed with normoxia, it was proposed that reactive oxygen species (ROS) generated during the re-oxygenation phase of IH mediate hypertension by CIH [66]. Consistent with this possibility, Dyugobskaya et al. [67] were one of the first to report increased ROS generation in CD11C-positive monocytes isolated from OSA patients, and they further showed that ROS contribute to increased expression of adhesion molecules (CD15 and CD11C) in monocytes and increased adhesion to endothelial cells. Subsequently, a number of studies reported elevated levels of several biomarkers of oxidative stress in plasma, urine, exhaled breath, and cells derived from SDB patients [68]. A study by Grebe et al [69] reported decreased vasodilation of the brachial artery in OSA patients and this response was restored by anti-oxidant treatment, suggesting that elevated oxidative stress contributes to increased vascular tone in these patients. Nasal CPAP treatment reversed the oxidative stress in SDB patients [68].
Rodents exposed to CIH showed increased ROS levels in carotid bodies [39], nTS and RVLM [11] and in adrenal medulla [10, 11] as evidenced by decreased aconitase enzyme activity, a robust biochemical marker of ROS [70], or increased malondialdehyde levels, an index of oxidized lipid [71]. ROS has been shown to augment the carotid body chemosensory reflex in CIH exposed rodents by affecting neurotransmitters [71, 72] and ion channels in the carotid body [73], neuronal excitability in brainstem neurons [11, 44], as well as catecholamine secretion from adrenal medullary chromaffin cells by affecting calcium signaling [10, 74]. Remarkably, treating CIH exposed rats with ROS scavengers prevented the sensitization of the carotid body response to hypoxia, sensory LTF [39, 71, 72, 75], augmented catecholamine secretion from the adrenal medulla and hypertension [10]. These studies suggest that increased generation of ROS is a major cellular mechanism, which by augmenting the carotid body chemosensory reflex contributes to CIH-induced hypertension.
How does CIH lead to increased ROS generation? Cellular ROS levels depend on the balance between their generation by pro-oxidant enzymes and metabolism by anti-oxidant enzymes. The NADPH oxidase (Nox) family of enzymes including Nox1, 2, 3, and 4 isoforms are one of the major sources of ROS [76]. Of these four isoforms, Nox2 is expressed in all components of the chemosensory reflex pathway including the carotid body, nTS as well as RVLM neurons, and the adrenal medulla [11, 72]. CIH increases Nox2 mRNA levels, protein expression., and enzyme activity in all of these tissues [11]. On other hand, CIH decreases the mRNA, protein and enzyme activity of superoxide dismutase 2 (Sod2), a major anti-oxidant enzyme in the mitochondria [51]. Inhibition of the mitochondrial electron transport chain (ETC) at complexes I and III also generate ROS [77]. It was shown that ROS generated by Nox2 inhibit complex I activity resulting in long-lasting ROS generation (i.e., ROS-induced ROS) [78]. These studies suggest that CIH-induced transcriptional imbalance between Nox2, representing the pro-oxidant enzymes, and Sod2, an anti-oxidant enzyme, is a key mechanism contributing to the increased generation of ROS in response to CIH.
HIFs mediate the transcriptional imbalance of redox regulating enzymes by CIH
HIF-1 mediates increased Nox2 gene expression by CIH
The effects of CIH on ROS generation was examined in wild-type and Hif1a+/− littermate mice [33]. CIH increased ROS levels in wild-type but not in Hif1a+/− mice. IH increased Nox2 mRNA, protein, and enzyme activity in PC12 cells as well as in wild-type mouse embryonic fibroblasts (MEFs), and in brain cortex, brainstem, and carotid body but not in cerebellum of wild-type mice and this effect was abolished or attenuated by blocking HIF-1 activity through RNA interference or pharmacologic inhibition (digoxin or YC-1) or by genetic knockout of HIF-1α in MEFs and in Hif1a+/− mice [59]. In contrast, increasing HIF-1α expression by treating PC12 cells with the iron chelator deferoxamine or by transfecting them with HIF-1α expression vector increased Nox2 expression and enzyme activity. These findings suggest that HIF-1 contributes to increased transcription of Nox2 by CIH.
HIF-2 contributes to decreased Sod2 gene transcription by CIH
Scortegagna et al [63] reported that HIF-2 is potent activator of genes encoding anti-oxidant enzymes. The following findings suggest that decreased HIF-2α protein contributes to down-regulation of Sod2 mRNA by CIH: a) CIH-evoked HIF-2α degradation led to inhibition of SOD2 transcription, resulting in oxidative stress, b) over-expression of transcriptionally active HIF-2α prevented CIH-evoked oxidative stress and restored SOD2 activity, and c) systemic treatment of IH-exposed rats with ALLM, a calpain inhibitor, rescued HIF-2α degradation and restored SOD2 activity, thereby preventing oxidative stress and hypertension [51].
Feed-forward regulation of ROS by dysregulation of HIF-α isoforms
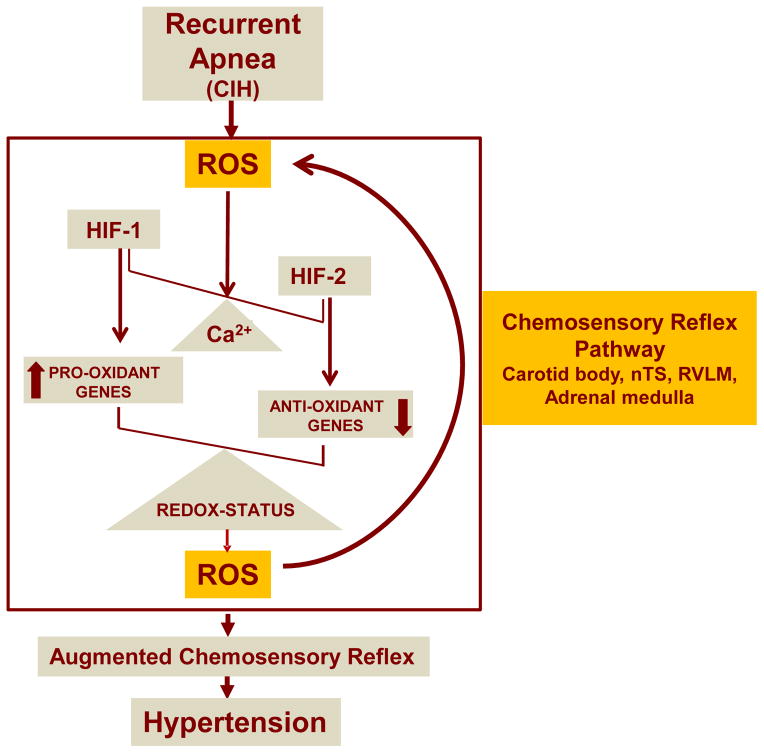
The studies described thus far suggest that imbalance between HIF-1α and HIF-2α, which contributes to CIH-induced oxidative stress in the carotid body chemosensory reflex pahway via transcriptional up regulation of pro-oxidants (Nox2) by HIF-1 and down regulation of anti-oxidant enzymes (Sod2) by HIF-2, resulting in increased ROS levels emerges as a major molecular mechanism underlying hypertension caused by CIH. Intriguingly, as described in the preceeding section, CIH-induced ROS triggers dysregulation of HIF-α isoforms via Ca2+-dependent mechanisms. This dysregulation of HIF-α isoforms by creating transcriptional imbalance of pro-oxidant and anti-oxidant enzyme genes leads to further ROS generation, thereby creating a feed-forward mechanism that is central for developing hypertension (Fig. 2).
Figure 2.
Schematic illustration of dysregulation of HIF-1 and HIF-2 by chronic intermittent hypoxia (CIH) resulting from recurrent apnea and feed forward regulation of reactive oxygen species (ROS) in the carotid body chemosensory reflex pathway. Ca2+, calcium signaling, nTS, nucleus tractus solitarius, RVLM, rostral ventrolateral medulla.
PERSPECTIVE
HIF’s regulate hundreds of genes associated with O2 homeostasis [12]. Regulation of sympathetic tone by carotid body chemosensory reflex requires proper expression of ion channels and various enzymes involved in the synthesis or degradation of neurotransmitters. Given that HIF-α isoforms also regulate genes encoding various ion channels [79] as well as enzymes involved in the synthesis of various neurotransmitters/modulators [12], further studies are needed to identify other genes that are HIF-1α– or HIF-2α-dependent (in addition to those associated with redox regulation) that may contribute to hypertension caused by altered carotid body chemosensory reflex function by CIH. It would be of interest to investigate whether HIF mediated feed-forward regulation of ROS contributes to other forms of hypertension such as those seen in spontaneous hypertensive rats and in rats with compromised kidney function.
Acknowledgments
Research from authors’ laboratory is supported by grants from National Institutes of Health, Heart, Lung and Blood Institute PO1-HL-90554 and UH2-HL-123610.
Footnotes
AUTHORS STATEMENT
The authors hereby state that the contents of this review article have not been submitted for publication to any other journal.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. joc91797 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Rio F, Racionero MA, Pino JM, Martinez I, Ortuno F, Villasante C, Villamor J. Sleep apnea and hypertension. Chest. 2000;117:1417–1425. doi: 10.1378/chest.117.5.1417. [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. MJBA-421901 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Fletcher EC. An animal model of the relationship between systemic hypertension and repetitive episodic hypoxia as seen in sleep apnoea. J Sleep Res. 1995;4:71–77. doi: 10.1111/j.1365-2869.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. jphysiol.2006.112524 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol. 2014;592:3841–3858. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. 92/3/967 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 14.Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 1997;15:1613–1619. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- 15.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10:35–44. doi: 10.1093/sleep/10.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103:722–727. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 20.Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–1413. doi: 10.1378/chest.06-2580. 131/5/1406 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y, Weiss JW. Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol. 2009;166:102–106. doi: 10.1016/j.resp.2009.02.010. S1569-9048(09)00035-4 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. expphysiol.2006.035758 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. S1569-9048(10)00049-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. jphysiol.2008.154187 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992;20:612–619. doi: 10.1161/01.hyp.20.5.612. [DOI] [PubMed] [Google Scholar]
- 27.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Comprehensive Physiology. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE. A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis. 1992;146:1240–1245. doi: 10.1164/ajrccm/146.5_Pt_1.1240. [DOI] [PubMed] [Google Scholar]
- 29.Kara T, Narkiewicz K, Somers VK. Chemoreflexes--physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. 1083[pii] [DOI] [PubMed] [Google Scholar]
- 30.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 31.Tafil-Klawe M, Thiele AE, Raschke F, Mayer J, Peter JH, von Wichert W. Peripheral chemoreceptor reflex in obstructive sleep apnea patients; a relationship between ventilatory response to hypoxia and nocturnal bradycardia during apnea events. Pneumologie. 1991;45(Suppl 1):309–311. [PubMed] [Google Scholar]
- 32.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. jphysiol.2004.072033 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. jphysiol.2006.114033 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braga VA, Soriano RN, Machado BH. Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp Physiol. 2006;91:1025–1031. doi: 10.1113/expphysiol.2006.034868. expphysiol.2006.034868 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Honda Y. Role of carotid chemoreceptors in control of breathing at rest and in exercise: studies on human subjects with bilateral carotid body resection. Jpn J Physiol. 1985;35:535–544. doi: 10.2170/jjphysiol.35.535. [DOI] [PubMed] [Google Scholar]
- 36.Somers VK, Abboud FM. Chemoreflexes--responses, interactions and implications for sleep apnea. Sleep. 1993;16:S30–33. discussion S33–34. [PubMed] [Google Scholar]
- 37.Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 38.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. discussion 1196. 00820.2003 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. 1734109100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Rio R, Munoz C, Arias P, Court FA, Moya EA, Iturriaga R. Chronic intermittent hypoxia-induced vascular enlargement and VEGF upregulation in the rat carotid body is not prevented by antioxidant treatment. Am J Physiol Lung Cell Mol Physiol. 2011;301:L702–711. doi: 10.1152/ajplung.00128.2011. ajplung.00128.2011 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209. 09031936.00204209 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. 27/17/4663 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol. 2000;121:173–184. doi: 10.1016/s0034-5687(00)00126-2. S0034568700001262 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol. 2011;589:1463–1476. doi: 10.1113/jphysiol.2010.200691. jphysiol.2010.200691 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 48.Roux JC, Brismar H, Aperia A, Lagercrantz H. Developmental changes in HIF transcription factor in carotid body: relevance for O2 sensing by chemoreceptors. Pediatr Res. 2005;58:53–57. doi: 10.1203/01.PDR.0000163390.78239.EA. 01.PDR.0000163390.78239.EA [pii] [DOI] [PubMed] [Google Scholar]
- 49.Lam SY, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxia-inducible factor-1alpha, -2alpha and -3alpha in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23:271–280. doi: 10.14670/HH-23.271. [DOI] [PubMed] [Google Scholar]
- 50.Lam SY, Tipoe GL, Liong EC, Fung ML. Hypoxia-inducible factor (HIF)-1alpha and endothelin-1 expression in the rat carotid body during intermittent hypoxia. Adv Exp Med Biol. 2006;580:21–27. doi: 10.1007/0-387-31311-7_4. discussion 351–359. [DOI] [PubMed] [Google Scholar]
- 51.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. 0811018106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnett S, Mulligan E, Wagerle LC, Lahiri S. Measurement of carotid body blood flow in cats by use of radioactive microspheres. J Appl Physiol (1985) 1988;65:2484–2489. doi: 10.1152/jappl.1988.65.6.2484. [DOI] [PubMed] [Google Scholar]
- 53.Clarke JA, de Burgh Daly M, Ead HW. Dimensions and volume of the carotid body in the adult cat, and their relation to the specific blood flow through the organ. A histological and morphometric study. Acta Anat (Basel) 1986;126:84–86. doi: 10.1159/000146193. [DOI] [PubMed] [Google Scholar]
- 54.DE BURGH DALYM, LAMBERTSEN CJ, SCHWEITZER A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol. 1954;125:67–89. doi: 10.1113/jphysiol.1954.sp005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR. Xanthine oxidase mediates hypoxia-inducible factor-2alpha degradation by intermittent hypoxia. PLoS One. 2013;8:e75838. doi: 10.1371/journal.pone.0075838. PONE-D-13-28149 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanduri J, Vaddi D, Khan S, Wang N, Makarenko V, Semenza G, Prabhakar NR. HIF-1α activation by Intermittent Hypoxia Requires NADPH Oxidase Stimulation by Xanthine Oxidase. PLos One. 2015 doi: 10.1371/journal.pone.0119762. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. M407706200 [pii] [DOI] [PubMed] [Google Scholar]
- 59.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226:2925–2933. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol. 1997;498 (Pt 2):503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. ng1266 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci U S A. 2011;108:3065–3070. doi: 10.1073/pnas.1100064108. 1100064108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci U S A. 2013;110:E1788–1796. doi: 10.1073/pnas.1305961110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 67.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 68.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 69.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. 200508-1223OC [pii] [DOI] [PubMed] [Google Scholar]
- 70.Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 71.Peng YJ, Nanduri J, Raghuraman G, Wang N, Kumar GK, Prabhakar NR. Role of oxidative stress-induced endothelin-converting enzyme activity in the alteration of carotid body function by chronic intermittent hypoxia. Exp Physiol. 2013;98:1620–1630. doi: 10.1113/expphysiol.2013.073700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. 29/15/4903 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ortiz FC, Del Rio R, Varas R, Iturriaga R. Contribution of TASK-like potassium channels to the enhanced rat carotid body responsiveness to hypoxia. Adv Exp Med Biol. 2012;758:365–371. doi: 10.1007/978-94-007-4584-1_49. [DOI] [PubMed] [Google Scholar]
- 74.Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. 30/32/10763 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R735–742. doi: 10.1152/ajpregu.90490.2008. 90490.2008 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. 87/1/245 [pii] [DOI] [PubMed] [Google Scholar]
- 77.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 78.Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal. 2011;14:533–542. doi: 10.1089/ars.2010.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol. 2014;116:867–874. doi: 10.1152/japplphysiol.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]