SUMMARY
Arc is a cellular immediate early gene (IEG) that functions at excitatory synapses and is required for learning and memory. We report crystal structures of Arc subdomains that form a bi-lobar architecture remarkably similar to the capsid domain of human immunodeficiency virus (HIV) gag protein. Analysis indicates Arc originated from the Ty3/Gypsy retrotransposon family and was “domesticated” in higher vertebrates for synaptic functions. The Arc N-terminal lobe evolved a unique hydrophobic pocket that mediates intermolecular binding with synaptic proteins as resolved in complexes with TARPγ2 (Stargazin) and CaMKII peptides, and is essential for Arc’s synaptic function. A consensus sequence for Arc binding identifies several additional partners that include genes implicated in schizophrenia. Arc N-lobe binding is inhibited by small chemicals suggesting Arc’s synaptic action may be druggable. These studies reveal the remarkable evolutionary origin of Arc and provide a structural basis for understanding Arc’s contribution to neural plasticity and disease.
INTRODUCTION
Information storage in the brain is mediated by changes in synaptic strength that require rapid de novo synthesis of mRNA and protein (Goelet et al., 1986). Arc provides an exemplary molecule for studies of how the de novo response contributes to memory. Arc was identified based on its rapid transcriptional up-regulation in models of learning (Link et al., 1995; Lyford et al., 1995), and its transcription is tightly linked to neural activity that underlies information processing and storage (Guzowski et al., 1999). Genetic deletion of Arc results in deficits of memory without altering behaviors essential for learning (Plath et al., 2006). Arc is a postsynaptic protein that associates with endocytic vesicular proteins to modulate the trafficking of AMPA type glutamate receptors (Chowdhury et al., 2006; Shepherd et al., 2006). Arc also binds CaMKII in the kinase inactive state, and this interaction targets Arc to synapses in accordance (inverse) with their history of activity, and underlies Arc’s contribution to homeostatic maintenance of distributed synaptic weights (Okuno et al., 2012). Arc contributes importantly to cell-wide homeostatic scaling (non-Hebbian) (Shepherd et al., 2006), and synapse-specific (Hebbian) plasticity (Beique et al., 2011; Jakkamsetti et al., 2013; Park et al., 2008; Waung et al., 2008). Arc has been implicated in diseases of cognition where its action is thought to contribute to reduced synaptic strength. Inhibitory control of Arc translation is disrupted in Fragile X mental retardation syndrome and unregulated expression of Arc contributes to enhanced mGluR-LTD (Niere et al., 2012; Park et al., 2008; Waung et al., 2008). In Angelman syndrome, reduced ubiquitination of Arc may result in an increase of Arc-dependent synaptic down-regulation (Greer et al., 2010). Arc enhances the association of γ-secretase with trafficking endosomes that process amyloid precursor protein (APP) to Aβ peptide. This action of Arc creates activity-dependent increases of Aβ that contribute to amyloid deposition (Wu et al., 2011). Mutations of genes whose protein products can physically associate with Arc were identified in studies of sporadic and inherited schizophrenia (Fromer et al., 2014; Purcell et al., 2014).
Arc’s functions have been challenging to understand in molecular detail because Arc is a single copy gene without identifiable family members or biochemically defined domains. For example, Arc is known to down-regulate synaptic AMPA type glutamate receptors, but the binding partners and regulatory mechanisms that couple Arc to AMPA receptor trafficking remain unknown. Arc’s association with cognitive diseases suggests that knowledge of physical associations that define its binding properties could provide insight into pathogenesis. To address this challenge, we evaluated known interactions of Arc and assessed if they could be reconstituted with recombinant proteins. We discovered that both full length Arc, as well as a specific subdomain of Arc, bound CaMKII. This same subdomain of Arc was found to bind to TARPγ2 (Stargazin), which is known to associate with AMPA receptors and mediate critical aspects of AMPA receptor trafficking (Jackson and Nicoll, 2011). Co-crystal structures of an Arc subdomain bound to CaMKII and TARPγ2 were defined and reveal the structural basis of these interactions. Physiological studies demonstrate that synaptic AMPA receptor down regulation during homeostatic scaling requires Arc N-lobe binding. Remarkably, the subdomain that mediates Arc binding is structurally similar to the capsid domain of human immunodeficiency virus (HIV). A structure of a second subdomain of Arc further substantiates a bi-lobar configuration that is similar to HIV GAG protein and indicates Arc originated from the Ty3/Gypsy retrotransposon family. Analysis across species reveals the evolutionary origin of its unique binding domain. An Arc N-lobe consensus binding sequence was established that identifies several novel binding partners that include proteins of the “Arc complex” linked to schizophrenia (Fromer et al., 2014; Purcell et al., 2014). Arc N-lobe binding is dependent on buried hydrophobic interactions that are notably distinct from many protein-protein interactions and this prompted us to screen for and identify small molecules that can inhibit Arc N-lobe binding. These observations provide a structural basis for understanding critical functions of Arc in synaptic plasticity and cognitive diseases, and support the possibility that Arc functions may pharmacologically targetable.
RESULTS
Evolutionary origin of Arc from Ty3/Gypsy retrotransposon and Phyla-specific domestication
The Arc-CaMKII interaction was previously characterized in co-immunoprecipitation assays (Okuno et al., 2012). We discovered the Arc-TARPγ2 interaction in a survey of synaptic proteins that co-immunoprecipitate with Arc (Figure S1A). TARPγ2 associates with AMPA receptors and modulates AMPA receptor trafficking and localization to the synapse (Jackson and Nicoll, 2011). Rat Arc (Rn Arc) bound TARPγ2 peptide (see Figure 2) and CaMKIIα peptide (Figure S1B) in vitro. Full length RnArc did not crystallize, but RnArc (207-278) crystallized in complex with TARPγ2 and CaMKII peptides (Table S1). Crystal structures revealed RnArc (207-278) forms a four helical bundle (Figure 1A) that is structurally similar to capsid domains of retroviral gag proteins in the PDB database, including HIV and Rous Sarcoma Virus (RSV) (Figure 1A, 1C and Figure S2A, S2B). No other structures were significantly similar. HIV capsid is typical of retroviruses and includes two globular domains termed NTD and CTD (Pornillos et al., 2009; Pornillos et al., 2011). While sequence identity is only 11%, RnArc (207-278) superposes on the HIV CTD with an rmsd of 2.6Å for 61 Cα’s. RnArc (207-278) includes an additional β1-strand at its N-terminus that contacts the α1-helix and surrounds the bound TARPγ2 peptide (Figure 1A). Similarity between Arc and HIV capsid protein was further borne out with the structure of RnArc (278-370) apoprotein (Table S1), which is also superimposable with HIV capsid protein CTD. RnArc (278-370) possesses a more extensive α1′ helix at the N-terminus of the major homology region (MHR) than Retroviruses (Figure 1B and 1C). We term RnArc (207-278) Arc N-lobe, and RnArc (278-370) Arc C-lobe; together they form the Arc Gag domain.
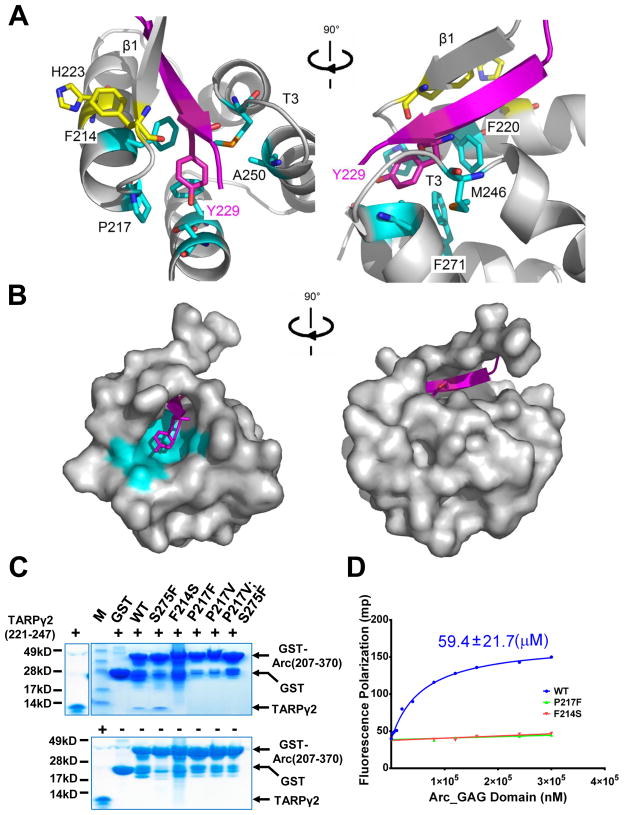
Figure 2. Complex Structure of the Arc N-lobe with TARPγ2 fragment.
(A) Stick representation of hydrophobic pocket at the binding site in two views rotated by ~ 90°. TARPγ2 peptide is colored purple. Residues anchoring β1-strand of Arc N-lobe are shown in yellow. Residues forming pocket wall are show in cyan. (B) Molecular surface representation of Arc N-lobe complexed with TARPγ2 peptide (in purple) showing the hydrophobic pocket and binding tunnel in two views. (C) Binding of WT Arc GAG domain (207-370) and point mutants to TARPγ2 fragment (221-247) determined by in vitro pull-down experiments using GST-fusion constructs (bottom panel without peptide). The mutation to one aromatic A.A. at residue S275 on the bottom of the hydrophobic pocket had no effect on Arc binding to TARPγ2 fragment. (D) Measurement of Kd and relative affinity for Arc GAG domain (207-370) and point mutants using fluorescence polarization assay with 5-Carboxyfluorescein tagged TARPγ2 peptide (225-RIPSYR-230).
Figure 1. Evolutionary Origin of Arc from Retrotransposon.
(A, B) Superposition of Arc N-lobe or C-lobe (blue and orange, respectively) with HIV capsid C-lobe (PDB ID, 1A43; yellow). The r.m.s.d. in Cα positions for superposition of the N-lobe (excluding residues 207–214) and C-lobe are 2.6 and 2.3 Å, respectively. The TARPγ2 peptide complexed with Arc N-lobe is colored purple. Helix labels in parentheses followed the reference for HIV capsid domain (Pornillos et al., 2009; Pornillos et al., 2011). (C) The sequence alignment for typical species based on their 3D structural superposition with retrotransposon or retrovirus (PDB ID, 1A43, HIV capsid C-lobe; 3G29, Rous Sarcoma Virus). Hs, Homo sapiens, NP_056008.1; Rn, Rattus norvegicus, NP_062234.1; Gga, Gallus gallus, NP_989763.1; Aca, Anolis carolinensis, XP_003223977.1; Xtr, Xenopus (Silurana) tropicalis, XP_002934511.1; Dme, Drosophila melanogaster, Arc1, Q7K1U0; Dr2, Danio rerio, XP_001919627; _N and _C refer to N-lobe and C-lobe, respectively. Secondary structures of Arc N-lobe and HIV C-lobe (PDB 1A43) are shown on the top in red and blue, respectively. Arc C-lobe secondary structure is on the bottom. Residues forming the hydrophobic pocket in Arc N-lobe are marked with a red star above the sequence alignment. The pair of aromatic residues anchoring N-terminal beta strand on alpha helix bundle core is highlighted with yellow solid circles. The numbers in the box indicate three categories (1 = N-lobes, 2 = Virus, 3 = C-lobes). The conservative residues across different species are marked in grey. (D) Domain organization of Arc homologues and several retrotransposons from typical species. Arc N-lobe and C-lobe region are colored blue and orange, respectively. CCHC labels zinc knuckle. Other domains of retrotransposon namely RT (Reverse transcriptase), RH (RNase HI) and INT (Integrase) are shown in pink. (E) The phylogenetic tree inferred based on the concatenated analysis of capsid domains from typical species. Oan, Ornithorhynchus anatinus, XP_001512750.1; Dr1, Danio rerio, XP_005157614; DR3, Danio rerio, XP_005156176.1; Tca1, Tribolium castaneum, XP_001812244; Tca2, Tribolium castaneum, XP_001807351.1; Sbi, Sorghum bicolor, XP_002457951.1; Fr, Fragaria vesca subsp.Vesca, XP_004301673.1.
Sequence analysis reveals bi-lobar Arc domains in all metazoans (Figure 1C and 1D). Arc is highly conserved in mammals, birds and reptiles. The bi-lobar Arc domain in the fish Danio (Dr) appears in three genes that are members of the Ty3/Gypsy family of retrotransposons. Ty3/Gypsy are ancient forms of RNA-based self-replicating elements that are present in animal, plant and fungal kingdoms and are considered ancestral to modern retroviruses, including HIV (Llorens et al., 2008), as well as certain cellular genes (Campillos et al., 2006; Volff, 2009). The fish Danio Arc genes Dr1 and Dr3 encode domains typical of Retrotansposons including zinc knuckles, which bind RNA during virion assembly, as well as reverse transcriptase (RT) (Figure 1D). These observations confirm computational predictions of Arc’s retrotransposon origin (Campillos et al., 2006). Danio gene Dr2 is different from Dr1 and Dr3 in that it possesses an N-terminal coiled-coil domain, which is typical of all higher vertebrate Arc genes, and lacks the zinc knuckle domain. This suggests that vertebrate Arc arose through the “domestication” (repurposing of retrotransposon genes in eukaryotic cells; see Campillos et al., 2006) of Ty3/Gypsy in stages that include acquiring the N-terminal coil-coil and loss of the zinc knuckle and RT.
The Arc bi-lobar domain is also present in insects. Arc in beetle Tribolium castaneum (Tca) includes zinc knuckles and reverse transcriptase, while Drosophila Arc1 protein includes zinc knuckles but lacks reverse transcriptase (Figure 1D). Insect Arc lacks the N-terminal CC domain typical of vertebrate Arc. This suggests that Arc domestication in insects followed a different functional path, and is consistent with studies of Dme Arc that define a role in stress-induced behavior but not learning and memory (Mattaliano et al., 2007).
A retrotransposon is present in plant Fragaria vesca that possesses ~14% identity to RnArc within the GAG domain. However, this plant gene appears to be a different family of retrotransposon based on sequence of the nucleotide binding domain encoding CHHC zinc knuckle motif, not CCHC motif (Figure 1E).
Determinants of Arc N-lobe structure important for binding to TARPγ2
We examined determinants of Arc binding to TARPγ2. TARPγ2 passes through the hydrophobic core of the Arc N-lobe four helix bundle (Figure 2A and 2B). The small A250 side chain, together with P217 creates space for interactions with TARPγ2 Y229 aromatic ring. RnArc (207-370) mutations P217F and P217V that fill the pocket and mimic Dme Arc or retroviral Gag (Figure 1 C) abolish binding to TARPγ2 peptide (Figure 2C). Thus, TARPγ2 Y229 appears to replace an aromatic stack that assembles the 4 helices of retroviral/HIV Gag proteins. Arc strand β1, loop T3 and the N terminus of helix α3 create a groove that surrounds the peptide (Figure 2A and 2B). This groove also appears important for binding since RnArc (207-370) F214S, which mimics Xenopus Arc and encodes an aromatic pocket but not the aromatic pair F214-H223 required to anchor β1 strand to helix α1, fails to bind (Figure 2C). As predicted, Xenopus Arc does not bind TARPγ2 (Figure S3A). The evolutionary origin and progression suggest Arc N-lobe binding is unique to the vertebrate genome.
To further assess determinants of peptide binding, we developed a binding assay that monitors the polarization of light emitted from a 5-Carboxyfluorescein tagged TARPγ2 peptide (Fluorescence polarization assay; FP)(Moerke, 2009; Roehrl et al., 2004) and performed binding assays with labeled peptide. TARPγ2 peptide (RIPSYR) binding affinity is ~60μM (Figure 2D). Competition binding assays demonstrated similar binding to the entire C-terminus of TARPγ2 (aa 202-323). This affinity is similar to that of the PDZ domain of PSD95 binding with several of its ligands (Moller et al., 2013), and is consistent with a role for Arc N-lobe binding in protein-protein interactions at the synapse. RnArc (207-370) mutations F214S and P217F abolish binding to TARPγ2 peptide (Figure 2C and 2D). These observations confirm that Arc binding requires both the β′ tunnel and the aromatic pocket.
Arc N-lobe binding site is required for trafficking of TARPγ2 and AMPA receptor during homeostatic scaling
We assessed the physiological role of Arc N-lobe binding by expressing full-length WT Arc or ArcP217F in DIV 14 neuronal cultures by Sindbis virus. Transgene expression was monitored by Western blot and confirmed similar expression of WT Arc or ArcP217F (Figure 3A). Transgene expression was also monitored by co-expression of GFP, which was typically detected in ~80% of neurons (not shown). Neurons were treated with TTX to suppress synaptic activity, and thereby reduce expression of native Arc (Shepherd et al., 2006), during viral gene expression (12–16 hrs). Consistent with previous studies, WT Arc transgene reduced expression of AMPA receptor GluA1 on the membrane surface as assayed by biotinylation of surface proteins (Figure 3A and 3B). TARPγ2 was also prominently downregulated by WT Arc, consistent with its role as a binding partner of GluA1 and regulator of trafficking. By contrast, ArcP217F did not down regulate surface GluA1 or TARPγ2. Since Arc does not bind GluA1 directly (Chowdhury et al., 2006), this supports the hypothesis that Arc binding to TARPγ2 mediates GluA1 downregulation. Recordings from viral transfected neurons confirmed that the mEPSC amplitude and frequency were reduced in neurons expressing WT Arc compared to ArcP217F (Figure 3C). These data establish that Arc-N lobe binding is essential for Arc-dependent downregulation of synaptic strength. It should be noted that the larger amplitude of mEPSCs in ArcP217F increases detection and leaves open the question of whether there are truly more synapses or just more detected.
Figure 3. Arc N-lobe Binding is required for Synaptic Function.
(A) Surface biotinylation and Western blotting assay showing Arc WT expression decreases surface GluA1 and TARPγ2 levels, while Arc P217F mutant does not. (B) Quantification of surface to total GluA1 and TARPγ2. Arc WT expression results in significant decrease of surface GluA1 and TARPγ2. Arc P217F mutant expression has no effect on surface GluA1 or TARPγ2. n=9; **, P<0.01; ***, P<0.0001. (C) Representative traces from whole-cell recordings of WT Arc or Arc P217F overexpression. (Scale = 20 pA, 200 ms). Histogram represents the average mEPSC amplitude of each population. mEPSCs are decreased in neurons expressing WT Arc relative to ArcP217F-expressing neurons. **p < 0.01.
Ligand determinants reveal regulatory mechanisms and consensus sequence for Arc binding
An electron density map of the bound TARPγ2 peptide reveals features of the ligand that are important for binding (Figure 4A). Hydrogen bonds are present to both main chain and side chain positions (Figure 4B). TARPγ2 is a member of type I TARPs, which include TARPγ3, TARPγ4 and TARPγ8 (Tomita et al., 2003). The Arc binding motif is conserved in TARPγ4 and TARPγ8, but TARPγ3 encodes proline rather than serine at position 228 in higher vertebrates (Figure S3B). This substitution interferes with β-strand formation. RnArc (207-370) bound GST-TARPγ2 but not GST-TARPγ3 (Figure 4C), consistent with a requirement for β-strand. The non-binding form of TARPγ3 appears to be an evolutionary adaptation in higher vertebrates since Xenopus and Danio TARPγ3 encode S228 (Figure S3B). Another regulatory mechanism is suggested by the observation that TARPγ2 is dynamically phosphorylated and dephosphorylated in association with synaptic potentiation or depression, respectively (Opazo et al., 2010; Sumioka et al., 2010; Tomita et al., 2005). One of these phosphorylation sites TARPγ2(S228) (Figure S3B) is predicted to disrupt hydrogen bonds made by the side chain hydroxyl and introduce steric clashes with I213 and N247 (Figure 4B). RnArc (207-370) bound GST-TARPγ2 but not a TARPγ2 peptide with phosphomimetic substitutions (Sumioka et al., 2010) (Figure 4D and Figure S3B).
Figure 4. Arc selectively interacts with TARPs.
(A) The 3.5 Å Fo-Fc electron density, contoured at 2.5 σ, of the Arc N-lobe/TARPγ2 peptide complex. TARPγ2 peptide is shown as sticks in purple. (B) The hydrogen bond contacts between TARPγ2 peptide and Arc N-lobe. (C) Pulldown of TARPγ2 fragment (203-247) or TARP γ-3 cytoplasmic fragment (202-249) against Arc (207-370) using GST-fusion construct. (D) Pulldown of TARPγ2 cytoplasmic tail (203-323) or its phosphomimic mutant S9D against Arc (207-370) using GST-fusion construct. Selective pulldowns of Arc (207-370) is confirmed by Western blot (bottom). TARPγ2 phospho mutant S9D does not bind Arc (207-370). (E) Competitive inhibition binding curves of mutant TARPγ2 peptide (225- RIPSYR -230) to the Arc GAG domain detected by FP. pS228 and pY229 are phosphorylated peptides. Tri is tripeptide PSY. P227R is a variant peptide.
We used the FP competition binding assay to assess determinants of binding affinity. We began by examining effects of mutations of the TARPγ2 peptide on Arc binding. Phosphorylation of TARPγ2(S228) or TARPγ2(Y229) reduced binding affinity at least 20 fold (Figure 4E). A minimal tripeptide corresponding to the central PSY sequence failed to bind, suggesting that a basic and long β-strand conformation is important for interaction. CamKII peptide (ATRNFS) is notably distinct from TARPγ2 in that R substitutes for P. While this peptide occupies the same position in the co-crystal (Figure S3C), the affinity of binding is ~ 760μM (Figure S3C and S3D), consistent with TARPγ2(P227R) (Figure 4E). These observations predict a consensus sequence P[STVILMKR][FYH] in the basic context of a β-strand secondary structure. The consensus is similar, but distinct from binding sites for phosphotyrosine binding domain (PTB) (Zhou et al., 1996) or FERM domain (Brahme and Calderwood, 2012) proteins.
A search of the Swiss-Prot protein database for Arc N-lobe consensus identified several synaptic proteins. We focused on proteins that were recently implicated in genetic studies of schizophrenia (Fromer et al., 2014; Purcell et al., 2014) since several reportedly co-precipitate with tandem affinity tagged Arc or NMDA receptor (including CaMKIIα and β)(Fernandez et al., 2009; Husi et al., 2000), or play a role in actin dynamics (Fromer et al., 2014) (Figure 5A and Figure S5D). To assess if these are natural binding partners of Arc N-lobe, we expressed representative proteins IQSEC2 (aa1329-1385) and WAVE1 (aa301-343) as GST-fusion proteins and determined that they bind Arc N-lobe (Figure S4). FP assays confirmed Arc binding to WAVE1, GKAP, IQSEC2 and GluN2A, and estimated their affinity of binding from 11 μM to 160 μM (Figure 5B). GST pull down assays using Arc N-lobe versus ArcP217F N-lobe with native proteins from brain detergent lysates confirmed specific binding (Figure 5C and 5D). Natural association was confirmed in co-immunoprecipitation assays from brain comparing lysates from WT versus Arc−/− forebrain (Figure S4).
Figure 5. Arc N-lobe binding partners and chemical inhibition.
(A) Arc N-lobe binding sequences from GKAP, WAVE1, NR2A and IQSEC2 aligned with TARPγ2. (B) Binding affinity of various binding partners to Arc(207-370) measured by FP competition assay. These peptides are GKAP (aa436-441), WAVE1(aa315-320), NR2A(aa1169-1174) and IQSEC2(aa1329-1385). (C) GST-Arc N(WT) or GST-Arc N(P217F) were used to pull down native TARPγ2, WAVE1, GKAP, GluN2A and GluN2B from mouse brains, and the eluted proteins were blotted with corresponding antibodies. Arc P217F mutant shows reduced binding to TARPγ2, WAVE1, GKAP, GluN2A or GluN2B. (D) Quantification of relative intensity from GST-Arc N(WT) pull-down versus GST-Arc N(P217F) pull-down. n=3~4; **, P<0.01; ***, P<0.001. (E) FP competition binding assay demonstrating Thioridazine inhibition of Arc(207-278) binding to TARPγ2 peptide.
Arc N-lobe binding is inhibited by chemical pharmaceuticals
The robust FP assay afforded an opportunity to screen for chemical agents that might inhibit Arc-N-lobe binding. Typical protein-protein interactions that are mediated by relatively large, flat surfaces are difficult to inhibit, however the peptide binding groove and hydrophobic pocket required for Arc N-lobe binding suggested that chemical agents might inhibit binding. We screened a library of FDA approved compounds (~2700) (Chong et al., 2007) and initial positives (20) were rescreened for inhibition of GST-Arc(207-370) binding to TARPγ2(221-247) (Figure S5A and S5B). Thioridazine inhibited binding with an IC50 of 194 μM; ~80% of binding inhibited over 1 log [Thioridazine] (Figure 5E). The structurally related phenothiazine Trifluoperazine also inhibited binding, but with lower affinity (Figure S5C), while Chlorpromazine which has no hexatomic ring to mimic the aromatic group of side chain in the Arc binding motif did not (data not shown). This SAR together with the low IC50, which for Thioridazine is ~104 lower than for D2 dopamine receptors (Cohen et al., 1979), indicates that inhibition of Arc N-lobe binding is not related to antipsychotic action. These observations support the feasibility of pharmacological interruption of Arc binding, but also highlight the challenge of specificity.
DISCUSSION
The present study provides the first atomic structure for Arc, and reveals that Arc N- and C-lobes evolved from the capsid domain of Ty3/Gypsy retrotransposon. Ty3/Gypsy sequence homologs are recognized by computational methods in nearly 100 mammalian proteins, and many of these derive from the Ty3/Gypsy Gag domain (Campillos et al., 2006). Arc is the only synaptic protein of this class and its structure provides the first structural information beyond HIV for this group of genes. In addition to revealing evolutionary adaptations that underlie synaptic functions of Arc, comparison of Arc N-lobe with HIV NTD reveals evolutionary adaptations that likely contribute to flexibility between HIV NTD and CTD (Figure S2), which is important for its unique capsid structure (Pornillos et al., 2009; Pornillos et al., 2011). Arc mRNA and protein expression exhibit several unusual and dynamic regulatory mechanisms that may be related to Arc’s retrotransposon origin (Volff, 2009). Like viral genes, Arc mRNA possesses an internal ribosome entry sequence (IRES) (Pinkstaff et al., 2001), and Arc is rapidly translated in dendrites in response to signals that inhibit general mRNA translation (Park et al., 2008). Trafficking of Arc mRNAs to distal dendrites (Steward et al., 1998) shares cell biological challenges of retroviral RNA trafficking from the nucleus to the cell cortex where Gag protein must assemble at the plasma membrane (Cochrane et al., 2006; Shida, 2012). Host defenses against viruses include incorporation of introns in the 3′UTR to engage non-sense mediated decay (NMD) (Isken and Maquat, 2007) and microRNAs (Umbach and Cullen, 2009). Arc mRNA is regulated by both mechanisms (Giorgi et al., 2007; Huang et al., 2012). Emergence of Arc as a cellular IEG provides a compelling example of retrotransposon-host co-evolution.
Arc N-lobe binding function emerges via amino acid substitutions that interrupt an intramolecular aromatic stack at the center of the four helix bundle and consequently create a unique hydrophobic pocket that together with a β-sheet binding groove mediates intermolecular binding. Arc binding to TARPγ2 defines a protein assembly that presumably includes AMPA receptors and can mediate Arc-dependent down regulation of AMPA receptors during homeostatic scaling. Structural features of Arc-TARPγ2 binding and peptide binding experiments predict that the interaction is regulated by phosphorylation of TARPγ2, which is consistent with physiological effects of TARPγ2 phosphorylation to increase synaptic strength (Opazo et al., 2010; Sumioka et al., 2010; Tomita et al., 2005). AMPA receptor sensitivity to Arc may also be controlled at the level of TARP gene expression since TARPγ3 evolves to escape Arc binding in higher vertebrates. Arc-N lobe binding to CaMKII is relatively low affinity, but consistent with the high concentration of CaMKII at synapses, and with a model in which Arc binding to CaMKII targets Arc to inactive synapses (Okuno et al., 2012). Identification of an Arc N-lobe consensus binding motif expands the protein interaction network for Arc. The functional role for each of these interactions remains to be defined, but a model of binding and down-regulation similar to action with TARPγ2-AMPA receptor is consistent with activity-dependent down-regulation of synaptic NMDA receptors (Watt et al., 2004), GKAP (Shin et al., 2012), and Arc-dependent changes in spine structure (Greer et al., 2010; Peebles et al., 2010).
The present study provides structural information about the C-terminal half of Arc that includes a region previously suggested to possess homology with spectrin repeats (Lyford et al., 1995). We were not able to crystalize full length Arc, and consequently the structural basis of several critical functions of Arc remain unknown. For example, Arc interactions with endophilin and dynamin are required for its synaptic function, however these interactions are not defined in the present study as they require a region of the N-terminal half of Arc (Chowdhury et al., 2006). Based on sequence analysis, we hypothesize the Arc N-terminal half includes alpha-helical regions and mediates associations with phospholipids and endocytic proteins. In this model, Arc N-lobe defines the target specificity in Arc-dependent trafficking of specific proteins from the plasma membrane and cytosol in endocytic vesicle pathways.
The Arc N-lobe binding consensus is similar to the NPXY sequence that is present in the cytosolic domains of several transmembrane proteins important in synaptic function including integrins, growth factor receptors, LDL receptor family, and amyloid precursor protein (APP) (Chen et al., 1990) (Pandey, 2010). The NPXY motif is bound by proteins that encode phosphotyrosine binding domains (PTB) and FERM domains, which function in cargo recognition to regulate endocytosis, trafficking and function of the transmembrane proteins (Traub and Bonifacino, 2013). In some (but not all) cases PTB binding is regulated by tyrosine phosphorylation or receptor ubiquitination (Goh and Sorkin, 2013), and instances of dynamic competition between multiple binding partners are described (Brahme and Calderwood, 2012). Crystal structures of NPXY bound to PTB/FERM domains reveals distinct binding mode and conformation of the ligand compared to Arc N-lobe (Dvir et al., 2012) (Ghai et al., 2013). Nevertheless, Arc might represent a competitive mechanism for binding and alternative endocytic trafficking for these membrane proteins.
Arc N-lobe binding partners include protein products of genes linked to schizophrenia (Fromer et al., 2014; Purcell et al., 2014). In genetic studies of schizophrenia, certain genes were grouped as part of an Arc complex based on their co-precipitation with tagged Arc, while other genes were grouped as part of a separate NMDA receptor complex, or of an actin dynamics complex. Since mutations are rare in schizophrenia patients, grouping is important to reach a statistical association with disease. Arc N-lobe binding provides an alternative criterion for clustering genes linked to schizophrenia that merges components of each of these three groupings. Nearly half of the synaptic genes with large deletions associated with schizophrenia (see extended data Table 3 in Purcell et al., 2014) possess an Arc N-lobe consensus binding sequence (Figure S5D). To date, mutations reported in schizophrenia do not implicate Arc itself, which together with the present data, suggests the shared property of rare mutations linked to schizophrenia may be that they result in accentuation of processes mediated by Arc that down-regulate neuronal excitability at individual synapses (Beique et al., 2011; Jakkamsetti et al., 2013; Park et al., 2008; Waung et al., 2008) or in cell wide adaptations to altered cellular or network activity (Shepherd et al., 2006). The observation that Arc-N lobe binding can be inhibited by chemical pharmaceuticals suggests that the action of Arc to down regulate synaptic proteins may be a feasible target for therapeutics although it remains to be seen if agents with sufficient specificity can be discovered. Our studies anticipate the development of genetic and pharmacological tools to test the hypothesized role of Arc in physiology and disease.
EXPERIMENTAL PROCEDURES
Expression constructs and antibodies
All the expression constructs were made by PCR and the PCR products were cloned into expression vectors pGEX-6P-1 (GE Healthcare) or pSinRep5 (Invitrogen). Point mutants were made using Quick Change Site-Directed Mutagenesis Kit (Stratagene). The sequence of the primers used to generate each mutant will be supplied upon request. All constructs were verified by DNA sequencing.
GluA1 antibody was kindly provided by Dr. Richard Huganir (Johns Hopkins University). Monoclonal Arc antibody was generated in Monoclonal Antibody Core at Johns Hopkins University using Arc C-terminal (155-396) as antigen. The other antibodies were purchased from commercial sources: Stargazin (Millipore), β-actin (Sigma).
Protein expression and purification
The cDNAs of Rn Arc (GenBank ID NP_062234) N-lobe and C-lobe domains (residues 207–277 and 278–370, respectively) were subcloned into vector pGEX-6P-1 (GE Healthcare) between the EcoRI and XhoI restriction sites for bacterial expression as a GST-fusion protein. The transfected E. coli BL21(DE3) cell culture was grown at 37°C until OD600 nm reached 0.6. The temperature was reduced to 25°C, and cells were induced with 0.2 mM isopropyl-β-D-thiogalactopyranoside (IPTG) and grown for 16 h. The cells were harvested and lysed by sonicating in PBS (phosphate buffered saline, pH 7.3) supplemented with 1% Triton X-100. The recombinant protein in the supernatant of the cell lysate was mixed with Glutathione Sepharose resin (GE Healthcare), and the mixture was washed extensively in PBS with 1% Triton X-100. After cleaving with Precision protease overnight to remove the GST tag, the protein was purified by using Q Sepharose Fast Flow and Superdex 75 size-exclusion chromatography, and was exchanged into a buffer of 20 mM Tris-HCl (pH 8.0), 100 mM NaCl and 1 mM DTT (dithiothreitol) and was concentrated to 15 mg/ml immediately before crystallization. Selenomethionyl-substituted protein was expressed and purified as WT. All point mutants of RnArc (207-370) were expressed and purified similarly to the WT.
The cDNAs of mouse TARPγ2 (GenBank ID NP_031609) cytoplasmic region (residues 203–323, 203-247 and 221–247, respectively), TARP γ-3 (NP_062303) cytoplasmic region (residues 202-249), IQSEC2 fragment (aa1329-1385, NP_001108136) and WAVE1 (aa301-343, NP_114083) and CAMK2A peptide (NP_803126, residues 278-329) were cloned from a mouse brain cDNA library. The cDNA clone of Xenopus tropicalis Arc (XP_004918984, residues 168-266) was obtained from Dr. Sabrina S. Burmeister of University of North Carolina Department of Neurobiology (Mangiamele et al., 2010). These constructs were subcloned into vector pGEX-6P-1 (GE Healthcare) between the EcoRI and XhoI restriction sites for expression in E. coli. The construct of the GST-fusion S9D was obtained from Dr. Susumu Tomita of the Yale University School of Medicine (Sumioka et al., 2010). All recombinant proteins contained an N-terminal GST-tag and were purified with GSH-affinity chromatography. (221-247) and CaMKIIα peptide (278-329) were further purified by SP Sepharose Fast Flow after removing the GST tag.
Crystallography studies
Recombinant protein Arc C-lobe (278-370) was crystallized using the hanging drop vapor diffusion method. The protein sample (~15 mg/ml) was mixed with an equal volume (2+2 μL) of the precipitant solution containing 1 M sodium citrate and 0.1 M imidazole (pH 8.0). The best-diffracting crystals grew as plates to their maximum size of 0.2× 0.8× 0.8 mm in about 10 d at 20°C. Co-crystallization of Arc N-lobe (207-277) and (221-247) with molar ratio ~1:2 was performed under a crystallization condition 0.2M Potassium sulfate, 20% PEG 3350 and 0.1 M MES (pH 6.5). The best-diffracting crystals grew as rods to their maximum size of 0.2× 0.2× 1.2 mm in about two weeks at 4°C. Co-crystallization of Arc N-lobe (207-277) and CAMK2A peptide (278-329) with molar ratio ~1:2 was grown under a crystallization condition 2M ammonium sulfate and 0.1 M Tris (pH 8.0). The best-diffracting crystals grew as blocks to their maximum size of 0.2× 0.2× 0.4 mm in about one and half a month at 20°C.
For the crystal of Arc C-lobe and co-crystal of Arc N-lobe with CaMKIIα peptide, X-ray diffraction data were collected at beamline 23-ID at the Argonne National Laboratory (Argonne, IL) from a flash-cooled crystal after soaking the crystal in a cryo-protectant solution containing 15% (v/v) glycerol. For the complexed crystal of Arc N-lobe with fragment, X-ray diffraction data were collected on Rigaku FR-E x-ray generator with the detector of a Saturn 944+ CCD. Data were processed, scaled, and merged using the HKL2000 program package (Otwinowski and Minor, 1997).
Phases of the Arc C-lobe crystal structure and the co-crystal of Arc N-lobe with CaMKIIα peptide were solved by the Se-based single-wavelength anomalous dispersion method (Hendrickson and Ogata, 1997)39393938 using the program PHENIX (Adams et al., 2010) and the data at the Se-absorption edge. The electron density map calculated at 2.0-Å resolution was readily interpretable. Model building was performed using the program COOT (Emsley and Cowtan, 2004) guided by 2Fo–Fc and Fo–Fc difference Fourier electron density maps. Atomic coordinates were refined with iterative cycles of manual building using COOT (Emsley and Cowtan, 2004), REFMAC (Vagin et al., 2004), and PHENIX (Adams et al., 2010). Phases of the crystal structure of the Arc N-lobe- complex were determined by the molecular replacement method using the program MOLREP (Vagin and Teplyakov, 2010) and the Arc N-lobe complex with CaMKIIα peptide as the search template, and refined similarly as above. Final X-ray data collection and refinement statistics are presented in Table S1. All structure figures were prepared with program Pymol (Schrödinger, LLC.).
Fluorescence polarization assay
5-Carboxyfluorescein (FAM) labeled peptide (RIPSYR) (GenScript, Piscataway, NJ) was dissolved in 10 mM Tris-Cl, pH 8.0, 0.5% NP-40 and 100 mM NaCl. Purified wild type or mutant Arc (202-370) protein samples were diluted with the above buffer and mixed with 5 nM FAM labeled peptides. The mixtures were assayed in black 384 well plates with the Tecan Safire2™ Microplate Reader (Tecan Group Ltd., Männedorf, Switzerland). Data were analyzed and plotted using program GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Fluorescence polarization competition assay followed protocol (Moerke, 2009; Roehrl et al., 2004) using synthesis peptides of various TARPγ2 mutant, GKAP (aa436-441, NP_808307), WAVE1(aa315-320, NP_114083), NR2A(aa1169-1174, NP_032196) and purified fragment of IQSEC2(aa1329-1385, NP_001108136).
GST pull-down assay
E. coli expressed GST or GST fusion protein that is immobilized on glutathione-Sepharose beads was used to incubate with E. coli expressed protein or brain lysate at 4°C for 4 hr to overnight. After incubation, glutathione-Sepharose beads were washed with 0.5~1.0% Triton X-100 buffer (in PBS) 3 times. Elutes from beads were used for either SDS-PAGE gel electrophoresis and Coomassie blue staining or Western blotting with specific antibody.
Co-immunoprecipitation
C57BL/6 mouse brain was sonicated in 20 volumes of lysis buffer (PBS, pH 7.4, 1% Triton X-100, 5 mM EDTA, 5 mM EGTA) containing Complete EDTA-Free protease inhibitor (Roche) and PhosSTOP phosphatase inhibitor (Roche). The brain lysate was centrifuged at 161,000 × g for 15 min and the supernatant was mixed with 1 μg Arc monoclonal antibody for 4 hr at 4 °C. Then 60 μl of 50% Protein G Sepharose slurry (Amersham-Pharmacia Biotech) was added for an additional 2 hr. The protein beads were washed three times with lysis buffer containing 1% Triton X-100. The protein samples were eluted with SDS loading buffer and analyzed by gel electrophoresis and western blotting.
Recombinant Sindbis virus production and infection
Arc WT or Arc P217F was subcloned into pIRES2-EGFP vector and then transferred into pSinRep5 (Invitrogen). All constructs were verified by sequencing. To generate pseudovirions, recombinant RNA and helper RNA were first generated using mMESSAGE mMACHINE SP6 Transcription kit (Ambion). Sindbis pseudovirions were produced following the manufacture protocol for Sindbis Expression System (Invitrogen). At 11-14DIV, cultured neurons were infected with virus. Experiments were performed 12–16 hr after infection.
Primary cortical neuronal culture
High-density cortical cultures from embryonic day18 (E18) C57/Bl6 mouse pups were prepared as reported previously (Wu et al., 2011) with minor alterations. For surface biotinylation experiments, 1 × 106 neurons were added to each well of a 6-well plate (Corning) coated with poly-L-lysine. For recording experiments, 2 × 106 neurons were added to each 60mm dish (Corning) with cover slips coated with poly-L-lysine. Growth medium consisted of NeuroBasal (Invitrogen) supplemented with 1% fetal bovine serum (Hyclone), 2% B27, 1% Glutamax (Invitrogen), 100U/mL penicillin, and 100U/mL streptomycin (Invitrogen). Neurons were fed twice per week with glia conditioned growth medium.
Surface biotinylation assay
For surface biotinylation, infected cortical neurons were cooled on ice, washed twice with ice-cold PBS containing 1mM CaCl2 and 0.5mM MgCl2, and then incubated with PBS containing 1mM CaCl2, 0.5mM MgCl2, and 1mg/ml Sulfo-NHS-SS-Biotin (Pierce) for 30min at 4°C. Unreacted biotin was quenched by washing cells three times with ice-cold 100mM Glycine (pH7.4). Cultures were harvested in RIPA buffer. Cell lysate was sonicated and centrifuged at 132,000 rpm for 20min at 4°C. 15% of the resulting supernatant was saved as the “total” protein samples. The remaining 85% of the lysate was rotated overnight at 4°C with NeutrAvidin beads (Pierce). Precipitates were washed with RIPA buffer and analyzed by Western blotting with indicated antibodies.
Electrophysiology and mEPSC Analysis
Whole-cell patch-clamp recordings were performed from cortical cultures. To isolate AMPAR-mediated mEPSCs, neurons were continuously perfused with artificial cerebral-spinal fluid (aCSF) including 124 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2, 26.2 mM NaHCO3, 10 mM Glucose, 0.01 mM gabazine, and 0.001 mM tetrodotoxin. Intracellular saline consisted of the following: 135 mM Cs-MeSO4, 10 mM CsCl, 10 mM HEPES, 5 mM EGTA, 2 mM MgCl2, 4 mM Na-ATP, and 0.1 mM Na-GTP adjusted to the osmolarity of 305–310 and 7.3–7.4 pH. Transfected neurons were selected based on fluorescent (eGFP) signal. Once the whole-cell recording configuration was achieved, neurons were voltage clamped and passive properties were monitored throughout. In the event of a change in series resistance (Rs) or input resistance (Ri) >15% during the course of a recording, the data were excluded from the set. mEPSCs were acquired through a MultiClamp 700B amplifier (Axon Instruments), filtered at 2 kHz, and digitized at 5 kHz. Sweeps of 20 s with zero latency were acquired until a sufficient number of events were recorded (a minimum of 5 and no longer than 30 min). Data were recorded continuously only after a period of 5 minutes, during which the cell was allowed to stabilize. mEPSCs were detected manually with miniAnalysis software (Synaptosoft Inc) by setting the amplitude threshold to O RMS 3 3 (usually 4 pA). Once a minimum of 100 events had been collected from a neuron, the amplitude and frequency were measured. In all electrophysiological experiments, a similar amount of data was acquired from both Arc WT and Arc P217F overexpressing neurons on the same day. Data from each group were then averaged, and statistical significance was determined by Student’s t test.
Supplementary Material
Acknowledgments
Diffraction data for this study were measured at beamline X29 of the National Synchrotron Light Source with the generous assistance of Annie Heroux. Financial support comes principally from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy, and from the National Center for Research Resources (P41RR012408) and the National Institute of General Medical Sciences (P41GM103473) of the National Institutes of Health. We thank Alan Long for fluorescence polarization assay, and Chris Ross, Daniel Weinberger, and Jeremy Nathans for helpful comments. Thanks to Susumu Tomita (Yale University School of Medicine) and Sabrina S. Burmeister (University of North Carolina) for Stargazin S9D construct and Xenopus tropicalis Arc cDNA clone, respectively. This work was supported by NIMH grant RO1 MH053608 (P.F.W.), NARSAD Young Investigator Grant (J.W.) and Biogen Idec. Atomic coordinates and X-ray structure factors have been deposited in the Protein Data Bank with accession numbers 4X3X (Arc C-lobe), 4X3H (Arc N-lobe with TARPγ2 peptide) and 4X3I (Arc N-lobe with CaMKIIα peptide).
Footnotes
Supplemental Information includes five figures, and one table
AUTHOR CONTRIBUTIONS
W.Z. and P.F.W. designed the experiments. J.W. designed and carried out all in vivo experiments. J.W. constructed Arc mutants. M.D.W. collected x-ray data, and D.J.L. supervised analysis. S.Y. recorded and analyzed electrophysiological data. W.Z. performed all other experiments. W.Z. and J.W. made figures. W.Z. and J.W. contributed to the writing of the manuscript. D.J.L. and P.F.W. wrote the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahme NN, Calderwood DA. Cell adhesion: a FERM grasp of the tail sorts out integrins. Current biology: CB. 2012;22:R692–694. doi: 10.1016/j.cub.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campillos M, Doerks T, Shah PK, Bork P. Computational characterization of multiple Gag-like human proteins. Trends Genet. 2006;22:585–589. doi: 10.1016/j.tig.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. The Journal of biological chemistry. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Jr, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS chemical biology. 2007;2:263–270. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane AW, McNally MT, Mouland AJ. The retrovirus RNA trafficking granule: from birth to maturity. Retrovirology. 2006;3:18. doi: 10.1186/1742-4690-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BM, Herschel M, Aoba A. Neuroleptic, antimuscarinic, and antiadrenergic activity of chlorpromazine, thioridazine, and their metabolites. Psychiatry research. 1979;1:199–208. doi: 10.1016/0165-1781(79)90062-3. [DOI] [PubMed] [Google Scholar]
- Dvir H, Shah M, Girardi E, Guo L, Farquhar MG, Zajonc DM. Atomic structure of the autosomal recessive hypercholesterolemia phosphotyrosine-binding domain in complex with the LDL-receptor tail. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6916–6921. doi: 10.1073/pnas.1114128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, Zografos L, Armstrong JD, Choudhary JS, Grant SG. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Molecular systems biology. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014 doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Bugarcic A, Liu H, Norwood SJ, Skeldal S, Coulson EJ, Li SS, Teasdale RD, Collins BM. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E643–652. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory--a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013;5:a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hendrickson WA, Ogata CM. Methods in Enzymology. 1997;276 doi: 10.1016/S0076-6879(97)76074-9. [DOI] [PubMed] [Google Scholar]
- Huang YW, Ruiz CR, Eyler EC, Lin K, Meffert MK. Dual regulation of miRNA biogenesis generates target specificity in neurotrophin-induced protein synthesis. Cell. 2012;148:933–946. doi: 10.1016/j.cell.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nature neuroscience. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes & development. 2007;21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. Stargazing from a new vantage--TARP modulation of AMPA receptor pharmacology. The Journal of physiology. 2011;589:5909–5910. doi: 10.1113/jphysiol.2011.223495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkamsetti V, Tsai NP, Gross C, Molinaro G, Collins KA, Nicoletti F, Wang KH, Osten P, Bassell GJ, Gibson JR, et al. Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron. 2013;80:72–79. doi: 10.1016/j.neuron.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens C, Fares MA, Moya A. Relationships of gag-pol diversity between Ty3/Gypsy and Retroviridae LTR retroelements and the three kings hypothesis. BMC Evol Biol. 2008;8:276. doi: 10.1186/1471-2148-8-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Mattaliano MD, Montana ES, Parisky KM, Littleton JT, Griffith LC. The Drosophila ARC homolog regulates behavioral responses to starvation. Mol Cell Neurosci. 2007;36:211–221. doi: 10.1016/j.mcn.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke NJ. Fluorescence Polarization (FP) Assays for Monitoring Peptide-Protein or Nucleic Acid-Protein Binding. Curr Protoc Chem Biol. 2009;1:1–15. doi: 10.1002/9780470559277.ch090102. [DOI] [PubMed] [Google Scholar]
- Moller TC, Wirth VF, Roberts NI, Bender J, Bach A, Jacky BP, Stromgaard K, Deussing JM, Schwartz TW, Martinez KL. PDZ domain-mediated interactions of G protein-coupled receptors with postsynaptic density protein 95: quantitative characterization of interactions. PLoS One. 2013;8:e63352. doi: 10.1371/journal.pone.0063352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niere F, Wilkerson JR, Huber KM. Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:5924–5936. doi: 10.1523/JNEUROSCI.4650-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell. 2012;149:886–898. doi: 10.1016/j.cell.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffreaction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pandey KN. Small peptide recognition sequence for intracellular sorting. Current opinion in biotechnology. 2010;21:611–620. doi: 10.1016/j.copbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles CL, Yoo J, Thwin MT, Palop JJ, Noebels JL, Finkbeiner S. Arc regulates spine morphology and maintains network stability in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18173–18178. doi: 10.1073/pnas.1006546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkstaff JK, Chappell SA, Mauro VP, Edelman GM, Krushel LA. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2770–2775. doi: 10.1073/pnas.051623398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pornillos O, Ganser-Pornillos BK, Kelly BN, Hua Y, Whitby FG, Stout CD, Sundquist WI, Hill CP, Yeager M. X-ray structures of the hexameric building block of the HIV capsid. Cell. 2009;137:1282–1292. doi: 10.1016/j.cell.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos O, Ganser-Pornillos BK, Yeager M. Atomic-level modelling of the HIV capsid. Nature. 2011;469:424–427. doi: 10.1038/nature09640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kahler A, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014 doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrl MH, Wang JY, Wagner G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein-protein interactions by fluorescence polarization. Biochemistry. 2004;43:16056–16066. doi: 10.1021/bi048233g. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H. Role of Nucleocytoplasmic RNA Transport during the Life Cycle of Retroviruses. Front Microbiol. 2012;3:179. doi: 10.3389/fmicb.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SM, Zhang N, Hansen J, Gerges NZ, Pak DT, Sheng M, Lee SH. GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nature neuroscience. 2012;15:1655–1666. doi: 10.1038/nn.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Traub LM, Bonifacino JS. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb Perspect Biol. 2013;5:a016790. doi: 10.1101/cshperspect.a016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Cullen BR. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes & development. 2009;23:1151–1164. doi: 10.1101/gad.1793309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66:22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- Vagin AA, Steiner RA, Lebedev AA, Potterton L, McNicholas S, Long F, Murshudov GN. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D Biol Crystallogr. 2004;60:2184–2195. doi: 10.1107/S0907444904023510. [DOI] [PubMed] [Google Scholar]
- Volff JN. Cellular genes derived from Gypsy/Ty3 retrotransposons in mammalian genomes. Ann N Y Acad Sci. 2009;1178:233–243. doi: 10.1111/j.1749-6632.2009.05005.x. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nature neuroscience. 2004;7:518–524. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Petralia RS, Kurushima H, Patel H, Jung MY, Volk L, Chowdhury S, Shepherd JD, Dehoff M, Li Y, et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent beta-amyloid generation. Cell. 2011;147:615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MM, Huang B, Olejniczak ET, Meadows RP, Shuker SB, Miyazaki M, Trub T, Shoelson SE, Fesik SW. Structural basis for IL-4 receptor phosphopeptide recognition by the IRS-1 PTB domain. Nature structural biology. 1996;3:388–393. doi: 10.1038/nsb0496-388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.