Abstract
Celiac disease is an autoimmune disorder induced by dietary gluten in genetically predisposed individuals. It has a prevalence of ∼1% in many populations worldwide. New diagnoses have increased substantially, due to increased awareness, better diagnostic tools, and probable, real increases in incidence. The breadth of recognized clinical presentations continues to expand, making the disorder highly relevant to all physicians. Newer diagnostic tools, including serologic tests for antibodies against tissue transglutaminase (tTG) and deamidated gliadin peptide, greatly facilitate diagnosis. Tests for celiac-permissive HLA DQ2 and DQ8 molecules are useful in defined clinical situations. Celiac disease is diagnosed by histopathologic examination of duodenal biopsies. However, according to recent controversial guidelines, a diagnosis can be made without biopsy in certain circumstances, especially for children. Symptoms, mortality, and risk for malignancy can each be reduced by adherence to a gluten-free diet. This treatment is a challenge, however, as the diet is expensive, socially isolating, and not always effective in controlling symptoms or intestinal damage. Hence, there is increasing interest in developing non-dietary therapies.
Keywords: Gluten, malabsorption, auto-immune, wheat, cereal, serology, villous atrophy, enteritis, enteropathy, diet, nutritional deficiency, refractory, lymphoma, gluten free diet
Celiac disease is defined as a chronic small-intestinal, immune-mediated enteropathy precipitated by exposure to dietary gluten in genetically predisposed individuals.1 It is a common autoimmune disorder, affecting ∼1% of the population in many parts of the world.2, 3 The only treatment is a strict, lifelong, gluten-free diet (GFD). Although some symptoms are overt and easy to recognize, others may be subtle or only become manifest as long-term complications of untreated disease. Many new diagnoses are now made through screening individuals considered to be at risk because of a family history of celiac disease, type 1 diabetes mellitus, autoimmune thyroid or liver disease, or Down Syndrome. Many of these people are asymptomatic (or have subclinical symptoms). The common feature among these at-risk groups is that they carry the alleles encoding HLA-DQ2 or DQ8. Risk for childhood celiac disease does not appear to be influenced by breast feeding or the timing of dietary gluten introduction.4, 5
We review the clinical features of celiac disease, discussing who to test and how to establish a diagnosis. We also cover treatment and monitoring of patients who have been diagnosed with celiac disease, the challenges of a GFD, and management of non-responsive or refractory celiac disease.
Clinical Features
The clinical manifestations of celiac disease are classical (signs and symptoms of malabsorption including diarrhea, steatorrhea, weight loss, or growth failure) or non-classical and symptomatic (with evident gastrointestinal and/or extra-intestinal symptoms) or asymptomatic.1 Celiac disease has diverse manifestations and associations, so it is important for all physicians to be aware of its many potential clinical presentations. With greater awareness more patients are being diagnosed—particularly with non-classical or asymptomatic disease.6, 7 As is the case for many other autoimmune disorders the true population incidence also appears to have increased (in one study up from 0.2% fifty years ago to 0.9% currently).6-8 Table 1 presents information about individuals who may be at an increased risk for celiac disease and for whom the threshold for testing is, accordingly, lower.
Table 1. Patients Who Might Require Testing for Celiac Disease.
| Symptoms and Signs | Associated Conditions | |
|---|---|---|
| Gastrointestinal | Extra-intestinal | |
| Chronic diarrhea Chronic abdominal pain Malabsorption Bloating Erratic bowel habit (similar to IBS) Constipation (more commonly in children) Failure to thrive/weight loss Anorexia Vomiting GERD |
Iron-deficiency anemia Other deficiency states (Vitamin B12, Vitamin D, folate, zinc, Vitamin B6. Fatigue Recurrent aphthous stomatitis Elevated hepatic transaminases Short stature Delayed puberty / menarche Amenorrhea Early menopause Dermatitis herpetiformis Osteopenia/osteoporosis Dental enamel hypoplasia Peripheral neuropathy Hyposplenism |
Family history of celiac disease Type 1 diabetes Autoimmune thyroid disease Autoimmune liver disease Selective IgA deficiency Sjögren syndrome Down Syndrome Turner Syndrome Williams Syndrome |
Gastrointestinal features
The classic presentation of celiac disease is more common in young children, consisting primarily of gastrointestinal symptoms with malabsorption (chronic diarrhea, abdominal pain, distension, and failure to thrive or weight loss). Some patients also present with constipation. In older teenagers and adults, the presentation of celiac disease is often more subtle and can be mistaken for irritable bowel syndrome. Some patients lack any evident gastrointestinal symptoms and instead present with nutritional deficiencies (most commonly iron deficiency) or extra-intestinal symptoms, or are asymptomatic.
Extra-intestinal features
Celiac disease has many extra-intestinal manifestations, including delayed puberty and short stature. Fatigue and iron deficiency anemia are common. Dermatitis herpetiformis, characterized by an often symmetrical, intensely itchy blistering rash, is likely under-recognized. Frequent oral aphthous ulcers and dental enamel hypoplasia can occur, as well as low bone mineral density and osteoporosis. Celiac hepatitis develops in as many as 9% of patients evaluated for cryptogenic elevated transaminases, and can take 6-12 months to resolve when patients are placed on GFDs. Celiac hepatitis usually follows a benign course, but there have been reports that liver failure was reversed with a GFD. Patients may report fibromyalgia or arthralgia, which might not always respond to a GFD.9 Young adults with celiac disease might also be at increased risk of early atherosclerosis.10 In addition, microvascular complications can accelerate in patients who also have type 1 diabetes.11 There have been reports of cardiomyopathy and carditis in patients with celiac disease, but the evidence for this association is weak.
Infertility and miscarriages have been reported to be a complication of untreated celiac disease; a recent meta-analyses of observational studies supported this association.12, 13. However, another report found that women with celiac disease did not have an overall higher risk of fertility problems than the general population.14
Peripheral neuropathy, seizure disorders, ataxia, and impaired cognitive function have most often been described in adults with celiac disease, at a lower incidence than in children.15, 16 Peripheral neuropathy may precede the diagnosis of celiac disease and was reported by 39% of patients, based on responses to a validated questionnaire.17 Seizures (sometimes associated with bilateral occipital calcifications), headaches, learning disorders, developmental delays, hypotonia, and attention deficit hyperactivity disorder are also observed more frequently in children with celiac disease, compared to controls.18 Up to one third of adult patients were found to have a history of psychiatric disorders such as depression or personality changes, and less commonly, psychosis.19
Little is understood about the mechanisms by which celiac disease might lead to neurologic disorders. Chronic neurological changes do not seem to disappear on a GFD. However, a patient's response to a GFD could be more substantial if they are diagnosed early—especially patients with gluten-associated ataxia or those who test positive for anti-gliadin antibodies, with neurologic symptoms or abnormal findings from brain imaging analyses.20 It is important to note that many of these patients with neurologic symptoms have not met the diagnostic criteria for celiac disease.
Nutritional deficiencies
Patients with celiac disease frequently have nutritional deficiencies—most commonly in iron, vitamin D folate, vitamin B12, vitamin B6, and zinc.21 Iron deficiency has been reported in up to half of newly diagnosed adults, and in itself is an indication for screening.22 A GFD usually leads to recovery from iron deficiency anemia within 6 to 12 months, whereas zinc deficiency improves within weeks.23, 24 Some patients with folate or vitamin B12 deficiency develop macrocytic anemia, which can hard to detect in patients who also have iron deficiency. Neurologic disorders have been reported in association with malabsorption of vitamin B12, folate, copper, and vitamin D.25
Associated Autoimmune and Other Conditions
Autoimmune thyroid disease and type 1 diabetes mellitus are the most common autoimmune diseases that occur with celiac disease. Celiac disease is observed in about 10% of patients with type 1 diabetes. On the other hand, people with celiac disease have a 2.4-fold increase in risk for type 1 diabetes before they are 20 ys old.26 Celiac disease is observed in about 7% of patients with autoimmune thyroid disorders. Autoimmune hypothyroidism, the thyroid disorder most frequently associated with celiac disease, is over 4-fold more common in people with celiac disease than without.27
Celiac disease, type 1 diabetes mellitus, and autoimmune thyroid disease are all associated with HLA risk alleles (namely HLA-DQ2 and/or DQ8). Associations with Sjögren syndrome, Addison's disease, parathyroid disorders, and growth hormone deficiency have also been reported. Celiac disease also has higher prevalence among patients with autoimmune hepatitis or primary biliary cirrhosis, with weaker data for primary sclerosing cholangitis.28 There is only weak evidence that a GFD affects risk for other autoimmune conditions in patients with celiac disease.
Screening for silent disease
A substantial proportion of patients with autoimmunity and enteropathy are asymptomatic, identified only because of genetic risk or celiac-associated disease. There is controversy over whether patients without symptoms or signs should be screened and treated. The rationale for proactive testing and treatment is to prevent long-term complications of untreated celiac disease. Conversely, diagnosis and subsequent adherence to a GFD carry substantial economic and psychosocial costs. We need more comprehensive and reliable data on the feasibility and cost:benefit ratio of screening at-risk, symptomless populations for celiac disease, to guide rational development of screening programs.29
Diagnosis
The rate of diagnosis of celiac disease is increasing worldwide due, in part, to a greater appreciation of the variability in clinical presentation. Until the 1950s, celiac disease was diagnosed based on clinical observations focused on malabsorptive features. Development of the peroral intestinal biopsy (1955-1956) produced a substantial change in the diagnostic paradigm. Since that time, gluten-dependent enteropathy, based on histologic assessment of intestinal mucosa, has been the standard for diagnosis.30 In the 1980's, sensitive and specific serologic tests were developed for celiac disease. These are now used as the first step when there is a suspicion of celiac disease, to identify patients who should undergo intestinal biopsy analysis (Figure 1)
Figure 1. Approach to celiac disease diagnosis. Serology is usually the first step in diagnosis or exclusion of celiac disease for symptomatic patients or for screening. Biopsy is important for definitive diagnosis. HLA testing is valuable in selected patients.
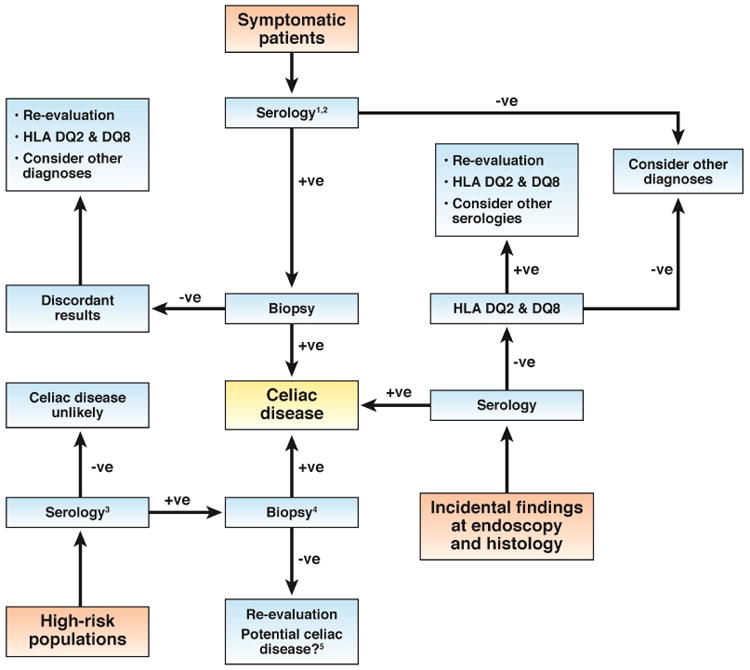
- Serologic markers of celiac disease: IgA against tTG, Endomysial antibody (IgA), IgG against DGD, IgA against deamidated gliadin peptide, IgG against tTG.
- A small number of patients with celiac disease have negative results from serologic tests. Biopsies should therefore be performed if the clinical suspicion for celiac disease is high, regardless of these results.
- Tests for HLA DQ2 and DQ8 can be performed. Negative results mean that celiac disease can be permanently excluded. However, many individuals without celiac disease are carriers of these alleles—especially those with a family history of celiac disease or related autoimmune disorder.
- For symptomless patients, especially children, with mild increases in serologic markers of disease, biopsy analysis can be delayed, pending results from serologic tests performed at intervals of 3–6 months.
- Potential celiac disease has been defined as a normal small intestinal mucosa with an increased risk for celiac disease based on results from serologic analysis.1
Histologic features
Endoscopy allows identification of gross mucosal changes that are markers of enteropathy, sometimes even in patients evaluated for reasons other than suspicion of celiac disease. Although cohort studies have suggested that observation of endoscopic markers such as scalloping is a reliable predictor of enteropathy, others have shown less satisfactory results.31 Chromoendoscopy using indigo carmine or methylene blue and water immersion have been shown to enhance endoscopic markers, allowing for visualization of villi and identification of patchy atrophic areas.32
Diagnostic intestinal biopsies should be performed in patients who consume gluten. Mucosal injury is generally more pronounced in the proximal intestine, and mild or absent distally. It is important to note that the location, number, and quality (size and orientation) of biopsies can affect diagnostic yield. As many as 70% of cases have patchy mucosal damage—this should be considered by endoscopists and pathologists.33 Biopsy samples taken from the duodenum proximal to the ampulla of Vater's can have artifacts that can be interpreted falsely as flat mucosa. However, recent studies have estimated that as many as 13% of patients have the characteristic enteropathy, only localized to the duodenal bulb.34 To maximize diagnostic accuracy, ≥5 duodenal biopsies should be collected, with duodenal bulb samples labelled and submitted separately.35
Under light microscopy, the most characteristic histology findings are: blunted or atrophic villi, crypt hyperplasia, an increase in number of intra-epithelial lymphocytes (IELs), especially at the villus tip, infiltration of the lamina propria by mononuclear cells, and structural abnormalities in epithelial cells. Since 2000, studies from Europe and North and South America reported that 13%-46% of cases are misdiagnosed by histology analysis (over- and under-diagnosis). For this reason, in equivocal cases, especially when there is a discrepancy between histology and serology results, re-evaluation by a gastrointestinal pathologist with expertise in celiac disease is recommended.
Histologic changes, including increased numbers of IELs and villous atrophy, are not specific for celiac disease; they are also associated with disorders such as giardia infection, common variable immune deficiency, Crohn's disease, and Helicobacter pylori infection. Patients with increases in only IELs and positive results from serologic tests are considered as potential candidates for celiac disease. However, most patients with only increases in intraepithelial lymphocytes do not have celiac disease.36, 37
Serologic features
In the 1980's, a new era in celiac disease research began with the identification of specific antibodies circulating in plasma of untreated patients. Immunoglobulin A (IgA) and IgG against gliadin (AGA), which bind native gliadin, were associated with the disease but identified patients with celiac disease with low levels of sensitivity and specificity, making them obsolete.3 Subsequently, IgA against the endomysium (EmA) of monkey esophagus was found to be highly sensitive and specific marker of celiac disease.38 Although a test for anti-EmA detects celiac disease with lower levels of sensitivity than other modern serologic assays, the antibody is an extremely specific marker of mucosal damage in untreated patients. Further research identified the ubiquitous enzyme tTG as the autoantigen that reacts with EmA, leading to the development of ELISAs that detect antibodies against tTG.39
A new generation of IgA- and/or IgG-based AGA assays, which use synthetic deamidated gliadin peptides (DGP) as substrates, perform almost as well as the anti-tTG test.40 Specifically, IgG-DGP tests are the most accurate available assays for patients with selective IgA-deficiency. A study in infants showed that high concentrations of DGP antibodies correlated with the severity of intestinal damage. Tests for DGP antibodies more accurately detect celiac disease in children than tests for anti-tTG, and might be used to evaluate dietary adherence.41
Recently, easy-to-use, on site test for anti-tTG have been introduced for rapid identification of disease candidates, using blood samples collected from a finger tip.42 These tests appear to be reasonably reliable and well accepted by patients. However, results do not obviate the need for subsequent testing by conventional serology and duodenal biopsy.
Thus, a number of valuable serological markers are now available, and used routinely for diagnosis and monitoring. However, it is important to note that 2%–3% of people with celiac disease have negative results in serologic tests, have low antibody titers, or titers that fluctuate between positive and negative levels with time. Serologic tests also vary in quality and some have not been well standarized—obstacles in clinical practice. A recent multi-national study evaluated the diagnostic performance of IgA-tTG tests in 150 serum samples, blindly assessed in 15 different clinical labs, and found a disappointing range of sensitivities (from 62% to 92%).43 Notwithstanding these limitations, the simultaneous or consecutive determination of IgA-tTG and/or IgG–DGP may be used as strong predictors of celiac disease in most settings.
Capsule endoscopy
Capsule endoscopy is an alternative method for evaluation of celiac disease and identification of complications. Markers of celiac disease seems to be more accurately identified by capsule compared than conventional endoscopy.44 Capsule endoscopy is also able to recognize the patchy distribution of damage and the longitudinal extension of the mucosal compromise. The main limitation of the test is the lack of ability to perform a biopsy. Currently, use of capsule endoscopy for diagnosis of celiac disease is limited to patients who refuse upper endoscopy, to equivocal cases, and to evaluate patients with non-responsive disease (to investigate complications such as ulcerative jejunitis or neoplasia).
Genetic testing
The class II HLA types DQ2 and/or DQ8 are found in almost all patients with celiac disease, but also in 30%-40% of the western Caucasian population; only 3% of individuals with these haplotypes develop celiac disease.45 HLA type analysis has a high negative predictive value (>99%). This is valuable for analysis of subjects with an equivocal diagnosis (eg, seronegative for anti-tTG with enteropathy) or those already on GFDs. Genetic analysis can also be used to rule out celiac disease, and the need for further testing, in individuals at high-risk because of family history. Although only one third of family members will be spared repeated testing, particular combinations (eg, homozygocity for DQ2) increase risk for celiac disease (by up to 40%).46
Patients already on a GFD without testing
Frequently in clinical practice, patients present for evaluation of possible celiac disease after a variable time on a GFD. The most practical approach to diagnosis begins with serologic tests (for anti-tTG and/or DGP) and HLA typing. Positive results from serologic tests support a diagnosis of celiac disease and indicate the need for duodenal biopsy analysis. However, negative results from serologic tests are of limited value. Conversely, celiac disease is excluded for patients who are negative for HLA DQ2 or DQ8. If serologic (and biopsy) analyses produce are negative results but individuals are carriers of HLA DQ2 or DQ8, gluten challenge is appropriate. Recent studies have helped to re-define the timing, doses, and duration of the gluten challenge.47, 48 Interestingly, these studies demonstrate that mucosal damage can be detected in most patients after as few as 2 weeks of challenge and before seroconversion. Furthermore, a lower level of gliadin (2-3 g/day, equivalent to 1-2 slices of bread) is better tolerated yet still effective for diagnosis.
Is diagnosis possible without intestinal biopsy?
Small intestinal biopsy histology has long been considered an essential step for diagnosis of celiac disease. New assays for anti-tTG and DGP have substantially improved the diagnostic accuracy of serologic analysis. Researchers have therefore explored the possibility of celiac disease diagnosis without endoscopy or biopsy analyses. Most of these studies, based on retrospective analysis of case-control cohorts, found this strategy to be acceptable for symptomatic patients. More recently, at least 2 prospective studies explored the value of specific serologic analyses of populations with high and low pre-test probability, where diagnosis of the disorder was based on histological findings. The studies concluded that the concomitant positive results of 2 or 3 specific immunoassay tests are highly predictive of celiac atrophy (Marsh III damage).49, 50
A consensus guideline produced by the European Society of Pediatric Gastroenterology, Hepatology and Nutrition recently proposed a triple test strategy to avoid intestinal biopsies for children.51 Candidates for diagnosis without biopsy should be symptomatic, have level of IgA tTG >10-fold above the upper limit of normal, test positive for anti-EmA in a separate blood sample, and have the HLA DQ2 haplotype.
Since 2012, 4 guidelines for celiac disease diagnosis have been proposed and published under the sponsorship of relevant institutions.45, 51-53 Table 2 summarizes some of the relevant comparative characteristics of these guidelines. All guidelines emphasize the combined use of biopsy and serologic analyses for diagnosis. However, there are some discrepancies in the serologic tests recommended and the use of HLA type analyses. Furthermore, a recent World Gastroenterological Association guideline recommended a no-biopsy algorithm for countries with limited healthcare resources.52
Table 2. Guidelines for Diagnosis of Celiac Disease.
| Guideline | Guideline design [Population] | Intestinal biopsy | Recommended blood tests | Comments and remarks | Ref. |
|---|---|---|---|---|---|
| ESPGHAN | Expert consensus (Children) | Not mandatory | anti-tTG, anti-EmA, total IgA, and tests for HLA DQ2 and DQ8 | Allows for diagnosis without biopsy under certain conditions (see text). | 39 |
| WGO | Expert consensus (Adults) | Not mandatory | anti-tTG, anti-EmA, and anti-DGP | Uses a diagnostic cascade based on available local resources. Allows for diagnosis without biopsy under certain conditions | 40 |
| ACG | Expert consensus Evidence-based (Children and adults) | Mandatory | anti-tTG and anti-DGP | Recommends IgG DGP antibodies in children under 2 years | 22 |
| BSG | Expert consensus Evidence-based (Adults) | Mandatory | anti-tTG, anti-EmA, and anti-DGP | Holds to the position that serology cannot replace biopsy. | 41 |
Treatment
The only available therapy for celiac disease is the GFD which usually reduces clinical symptoms and morbidity and increases nutritional parameters including body weight and bone density.54-57 However, studies have reported low patient satisfaction, high costs, and continued symptoms and histologic signs of intestinal damage, indicating that the GFD is not always optimal.58-63 Nonetheless, the concept that the GFD is an ideal therapy has contributed to the lack of effective alternative and adjunct treatments.
For the GFD to be effective, all wheat (gluten), rye (secalin), and barley (hoerdin) products must be strictly avoided. As little as 50 mg of gluten, an amount present in a few crumbs of bread or a small piece of pasta, can increase enteropathy.64 In addition to obvious sources of gluten such as bread and pasta, many products are contaminated with gluten during harvesting, processing, and packaging.65, 66 A good example is oats, which do not contain gluten, but are often heavily contaminated with wheat or barley.67 Certified gluten-free oats are well tolerated by most people with celiac disease and are now an accepted part of the GFD.68 Due to the combination of contamination of gluten-free foods and accidental and intentional gluten exposures, it is not possible for most people to remain totally gluten free.69, 70 The best that can be accomplished is a diet that is highly gluten restricted. Although mucosal healing occurs routinely in pediatric patients, it is much less common in adults with celiac disease, for unknown reasons.71 Most individuals still have intermittent symptoms related to intermittent or ongoing gluten exposure.60, 72 The negative social effects of a highly restricted diet, the constant vigilance required to avoid gluten, and the high frequency of inadvertent exposure are major determinants of the low patient satisfaction and large burden of the GFD.
In addition to the extra costs and difficulties of the GFD, it can also lead to nutritional deficiencies that cause new or continued symptoms.73 The most common of these is constipation, due to the lack of fiber in many gluten-free foods. Other common nutritional consequences include a lack of fortification with B vitamins and a high content of fat and simple carbohydrates, compared with non-gluten free foods, often leading to unwanted weight gain.74 At the time of diagnosis, patients should receive dietary counselling, ideally by a dietician with expertise in celiac disease; they can significantly improve the nutritional quality of a patient's GFD, and guide their use of gluten-free whole grains.
Non-dietary therapy
Non-dietary therapy, with local or systemic corticosteroids or immune modulators, is largely confined to the treatment of refractory celiac disease. However the limitations of the GFD and the realization that celiac disease is common in many parts of the world have prompted a search for therapies to augment the GFD.58-63, 75 Our understanding of the pathogenesis of celiac disease is far more detailed than for most other autoimmune disorders, so a multitude of therapeutic targets are available (refer to Celiac disease Pathogenesis article also in Gastro 13th Special Supplement).
Strategies include developing reagents to degrade or alter dietary gluten, prevent gluten peptides from crossing the epithelial barrier, inhibit tTG-induced potentiation of gliadin peptides, or block gliadin binding to HLA DQ2. Immune-based strategies involve those to prevent T-cell activation or innate and adaptive immune responses (such as by inducing tolerance to gluten).75 However, only 2 agents are in late Phase 2 clinical trials. ALV003 (2 recombinant, orally administered, gluten-specific proteases) reduced the small intestinal mucosal injury caused by 6 weeks of gluten challenge.76 Larazotide acetate, an oral peptide that modulates intestinal tight junctions, reduced symptoms in patients in response to gluten challenge.77 Additional studies are underway to determine if these agents are safe and effective for patients with persistent symptoms and mucosal injury despite a continued GFD. These, or other effective therapies could reduce the burden of GFD by decreasing the effects of inadvertent gluten exposure.
Monitoring
Celiac disease is a lifelong inflammatory condition that affects multiple organ systems, so patients should be followed routinely. There are no differences in recommendations for monitoring symptomatic vs asymptomatic patients. Based on expert consensus, at the time of diagnosis, patients should be evaluated for common coexisting autoimmune conditions, such as thyroid and liver diseases, as well as deficiencies in iron, vitamin D, and vitamin B12. It is also important to consider zinc, folate, and other deficiencies, based on regional trends and patient symptoms. There should be a low threshold to test patients for other autoimmune disorders based on their symptoms or signs.
There is general agreement among guidelines (Tables 2 & 3) that patients should be examined at least twice in their first year after diagnosis, to monitor symptoms, dietary adherence, nutrition, body mass index, and serologic features.52, 53, 78. Although it takes varying amounts of time for serologic features of celiac disease to normalize, a significant decrease over the first year is a sign of GFD adherence; patients whose serologic features do not improve should be re-evaluated for continued gluten exposure. Low bone mineral density is one of the more common extra-intestinal manifestations of celiac disease, so DXA evaluation is generally recommended in the first year after diagnosis.53
Table 3. Monitoring in Celiac Disease.
| Test | Interval | Comments |
|---|---|---|
| Clinical evaluation | Annually or if recurrent symptoms | |
| Serology | Every 3 to 6 months until normal Then every 1 to 2 years |
Normal titers are insensitive for ongoing gluten exposure or enteropathy. Persistently increased or increasing titers indicate significant gluten exposure |
| Nutritional evaluation | Every 3 to 6 months until normal Then every 1 to 2 years |
Common deficiencies in iron, 25-OH vitamin D, vitamin B12, folate, and zinc. Monitor for weight gain, low fiber intake, and constipation. |
| Bone Density | Once within first two years | Significant increases in bone density are often observed in the first year after diagnosis. Many experts therefore advocate testing for the first time after 1 year on the GFD. |
| Liver transaminases | At diagnosis Then every 1 to 2 years |
Increased levels of AST and ALT are a common manifestation of celiac disease. Persistent increases or increasing levels indicate a comorbid liver disorder |
| Thyroid function tests | At diagnosis Then every 1 to 2 years |
Autoimmune thyroid disease is the most common co-morbid autoimmune disorder found in approximately 15%–20% of adults with celiac disease. |
| Duodenal biopsy | Consider 1 to 2 years after diagnosis | Repeated biopsies, to evaluate healing, are frequently performed. However it is not clear if this is necessary for patients with well-treated, clinically responsive celiac disease. |
| Cancer screening | As for general population | Although rates of certain cancers are increased, these are not sufficiently common to warrant disease-specific screening. |
One of the more controversial aspects of celiac disease monitoring is the role and timing of repeated endoscopic biopsy analyses. Repeating biopsy analysis 6 months to 2 years after diagnosis allows physicians to assess a patient's response to therapy, and GFD adherence for patients with little mucosal healing. However, intestinal healing is often slow, incomplete, and age dependent.71 Furthermore, persistent enteropathy does not always predict long-term outcome, and histologic abnormalities can have other causes.59, 79 However, there is no doubt that intestinal biopsy is an important component in the evaluation of patients with persistent symptoms (see Figure 2).
Figure 2. Evaluation of NRCD.
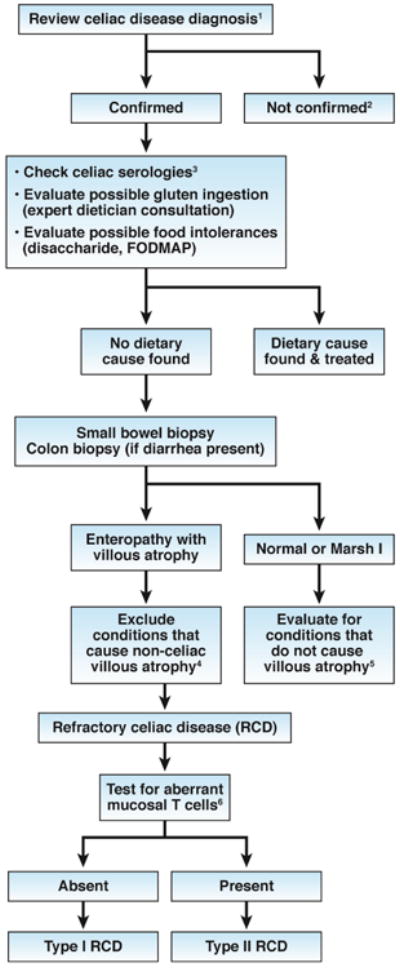
- Confirm the diagnosis of celiac disease by reviewing findings from serologic tests (not anti-gliadin antibody tests) and small bowel histology findings. If patients tested negative for tTG and EMA antibodies, perform HLA DQ2 DQ8 typing.
- Investigate other possible etiologies for clinical presentation and/or abnormal histology findings.
- Increased serum levels of IgA against tTG indciate continued gluten ingestion as a cause
- Non-celiac villous atrophy can be caused by intestinal infections (eg, giardiasis, small intestinal bacterial overgrowth, and viral enteritis, including HIV enteropathy), autoimmune enteropathy, hypogammaglobulinemia, as well as combined variable immunodeficiency, tropical sprue, Crohn' s disease, peptic duodenitis, or collagenous sprue.
- Conditions that present as NRCD without villous atrophy include irritable bowel syndrome, microscopic colitis, food intolerances, small intestinal bacterial overgrowth, Crohn's disease, and microscopic colitis.
- Aberrant small intestinal mucosal and intraepithelial lymphocytes in patients with RCD Type II can be identified by immunohistochemistry or flow cytometry (an excess of CD3+ cells without CD4 or CD8 surface proteins) or by T-cell receptor gene rearrangement analysis showing clonal expansion.
Non-responsive celiac disease (NRCD)
NRCD can be defined as persistent or recurrent symptoms, signs, or laboratory findings consistent with active celiac disease, despite at least 12 months of treatment with the GFD.1, 60, 72, 80 A substantial proportion of patients with celiac disease develop NRCD (7%-30% in different series studies). NRCD has multiple and diverse etiologies; a thorough and systematic evaluation is needed to determine the correct diagnosis and management plan for each patient (see Figure 2). The first and essential step is to carefully review the initial diagnosis of celiac disease, because patients with other disorders will not respond to GFDs. HLA typing may be required, especially for patients with consistently negative serologic tests for celiac disease.
The most common causes of NRCD are related to diet—the most common of all is continued or intermittent, purposeful or inadvertent, gluten ingestion.80 Patients' diets should therefore be carefully reviewed, ideally with a specialized celiac dietician. Persistent increases in serum levels of IgA–tTG, or other markers, is often an indicator of gluten exposure. Conversely, normal results from serologic tests do not exclude continued gluten exposure, because the tests are not sensitive enough to detect low levels of gluten ingestion. Other dietary factors can also contribute to NRCD, including fermentable, oligo-, di-, mono-saccharides and plyols (FODMAPs), so avoidance might be tested. Small intestinal bacterial overgrowth can complicate celiac disease and should be considered, especially for patients with bloating, excess gas, or diarrhea.
Once dietary causes of NRCD have been excluded, a small-bowel biopsy should be performed. For patients with diarrhea, colonic biopsies should be evaluated for microscopic colitis, which develops in 4% of patients with celiac disease.81 Improvements in histologic features from baseline indicate that patients' persistent symptoms and signs could have alternative causes. Possibilities in patients with normal (Marsh 0) or near normal (Marsh I) histology findings include irritable bowel syndrome, microscopic colitis, small intestinal bacterial overgrowth, pancreatic exocrine insufficiency, and food allergies or intolerances. 1, 60, 72, 80 Persisting duodenal villous atrophy and other histological features of active celiac disease raise the possibility of refractory celiac disease. However, before this diagnosis is made, it is important to again consider other causes for small-intestinal villous atrophy, especially if patients have had negative results from serologic tests for celiac disease (see Figure 2).36 The possibility of continued gluten ingestion should be re-visited and a period of extremely strict gluten avoidance advocated.82
Refractory celiac disease (RCD)
RCD can be defined as persistent or recurrent small intestinal villous atrophy with symptoms of malabsorption, despite ≥12 months of a strict GFD, in the absence of an overt lymphoma or another condition that causes villous atrophy.1 RCD makes up a small subset (approximately 10%) of NRCDs and occurs in 1% to 2% of patients with celiac disease.60, 72, 83 Severe diarrhea and weight loss in a patients with NRCD increase the risk for RCD.
RCD is characterized by the absence (Type I) or presence (Type II) of an aberrant population of IELs that lack lineage differentiation surface markers (eg, CD4, CD8, or the interleukin-2 receptor) but are positive for cytoplasmic CD3, indicating a T-cell phenotype.84-86 This abnormal population of T cells can be identified by immunohistochemistry, flow cytometry, or T-cell receptor analysis of small-bowel biopsy tissue. RCD I is more commonly diagnosed in the US, whereas RCD II predominates in Europe.
The prognoses for patients with RCD I and for RCD II differ markedly.83-87 RCD I is associated with severe symptoms and malabsorption, but life expectancy is not greatly reduced; the disease often responds to treatment with topical steroids and enteric delivery of budesonide.88 Less commonly, treatment with a systemic steroid, immunosuppressant. or a biologic agent (eg, prednisone, azathioprine, or inflixamab) is required. A strict GFD should be maintained and nutritional supplementation given when needed.82
Five-year mortality for patients with RCD II is ∼50%.83, 85, 86 The disease is often complicated by transition to enteropathy-associated T-cell lymphoma, ulcerative jejuno-ileitis, and severe malabsorption with intestinal failure necessitating total parenteral nutrition.83-87 The initial treatment approaches are the same as for RCD I, but fewer patients with RCD II respond, and responses are often short lived.89 Because of the poor response to therapy and high mortality, it has been advocated that RCD II be treated with a cytotoxic chemotherapeutic agent such as cladribine (2-chlorodeoxyadenosine). However, there have been no controlled trials of treatments for RCD II.89
Malignancy
Mortality risk is increased in adult celiac patients (hazard ratio 1.31; 95% CI 1.13-1.51 in one study) due to increased risk for fatal malignancy.90, 91 Mortality risk was highest shortly after diagnosis and in those with active malabsorption and enteropathy suggesting a beneficial effect of the GFD.79, 90-92 Celiac disease was first associated with small intestinal adenocarcinoma, and then with non-Hodgkins lymphoma and, more specifically, small intestinal T-cell lymphomas, now known as enteropathy-associated T-cell lymphoma (EATL). Risks for other gastrointestinal cancers, including gastric and colon cancer, are not substantially increased, and risk for a few cancers, including breast cancer, may be lower. 93-95
Non-Hodgkins lymphoma is the most common celiac-associated malignancy. Early studies suggesting a high risk of non-Hodgkins lymphoma were based on relatively small case series. Recent larger and well-designed case series have estimated a 2–3-fold increase in risk for non-Hodgkins lymphoma in patients with celiac disease.94, 95 The risk of malignancy is highest in the first years after diagnosis and, similar to overall mortality, decreases to normal or near-normal levels by 5 years after diagnosis. The post-diagnosis decrease in lymphoma risk distinguishes celiac disease from many other autoimmune disorders, including rheumatoid arthritis, Sjögrens syndrome, and Crohn's disease, for which risk of lymphoma remains high or increases with time.96
Small intestinal adenocarcinomas are rare in the general population (estimated incidence of 1/100,000); but the risk is increased more than 10-fold in patients with celiac disease.94, 97, 98 Unlike EATL, small intestinal adenocarcinoma is not associated with refractory celiac disease. Patients with celiac disease and obscure gastrointestinal bleeding, new or persistent anemia, or obstructive symptoms should have a careful small bowel examination, by computed tomorgraphy or magnetic resonance enterography or capsule endoscopy, depending on local resources and expertise.
As suggested by its name, EATL was initially described based on its strong association with celiac disease. More than half of cases of EATL are diagnosed simultaneously with celiac disease. Type I EATL is associated with celiac disease and accounts for 80% of cases in western countries. Its major risk factor is Type 2 refractory celiac disease.99 Type II EATL is less well characterized and not associated with celiac disease or the associated HLA haplotypes DQ2 or DQ8. Fewer than 25% of patients with EATL survive for 5 years, although surgical debulking, combined with chemotherapy or bone marrow transplant, can occasionally produce prolonged remission.100, 101
Although the risk of certain malignancies is increased in patients with celiac disease, their most frequent causes of morbidity and mortality are the same as those of the general population: cardiovascular disease, breast cancer (in women), prostate cancer (in men), and colon cancers in both sexes. So, there are no celiac-specific cancer screening recommendations.
Future Directions
The last decade has deepened our appreciation of the protean manifestations of celiac disease, which presents at all stages of life, has a diverse geographical distribution, and is a common autoimmune disease. Advances in our understanding of pathogenesis and genetic factors that affect risk have led to development and refinements to diagnostic tools. Challenges for the next decade include reducing the burden of treatment by providing easier access to inexpensive gluten-free foods and developing non-dietary approaches to increase the efficacy of treatment. Disease prevention, through modification of childhood risk factors, and cure, through induction of immune tolerance to gluten, are important long-term goals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ciarán P. Kelly, Email: ckelly2@bidmc.harvard.edu, Celiac Program, Harvard Medical School & Beth Israel Deaconess Medical Center, 330 Brookline Ave, Gastroenterology, Dana 601E, Boston, MA 02215-5400, United States, Phone: (617) 667-1272.
Julio C. Bai, Email: jbai@intramed.net, Hospital Gastroenterologia, Dr. Bonorino Udaondo, Av. Casero 2061, Buenos Aires, 1264, Argentina, Phone: +549114404-8022.
Edwin Liu, Email: edwin.liu@childrenscolorado.org, Colorado Center for Celiac Disease, Digestive Health Institute, Children's Hospital Colorado and the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045. United States, Phone: (720) 777-6669.
Daniel A. Leffler, Email: dleffler@caregroup.harvard.edu, Celiac Program, Harvard Medical School & Beth Israel Deaconess Medical Center, 330 Brookline Ave, Gastroenterology, Dana 501, Boston, MA 02215-5400, United States, Phone: (617) 667-1272.
References
- 1.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 4.Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295–303. doi: 10.1056/NEJMoa1400697. [DOI] [PubMed] [Google Scholar]
- 5.Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304–15. doi: 10.1056/NEJMoa1404172. [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol. 2013;108:818–24. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 8.Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530–8. doi: 10.3109/07853890.2010.514285. [DOI] [PubMed] [Google Scholar]
- 9.Zipser RD, Patel S, Yahya KZ, et al. Presentations of adult celiac disease in a nationwide patient support group. Dig Dis Sci. 2003;48:761–4. doi: 10.1023/a:1022897028030. [DOI] [PubMed] [Google Scholar]
- 10.Norsa L, Shamir R, Zevit N, et al. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J Gastroenterol. 2013;19:5658–64. doi: 10.3748/wjg.v19.i34.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeds JS, Hopper AD, Hadjivassiliou M, et al. High prevalence of microvascular complications in adults with type 1 diabetes and newly diagnosed celiac disease. Diabetes Care. 2011;34:2158–63. doi: 10.2337/dc11-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasa JS, Zubiaurre I, Soifer LO. Risk of infertility in patients with celiac disease: a meta-analysis of observational studies. Arq Gastroenterol. 2014;51:144–50. doi: 10.1590/s0004-28032014000200014. [DOI] [PubMed] [Google Scholar]
- 13.Tersigni C, Castellani R, de Waure C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update. 2014;20:582–93. doi: 10.1093/humupd/dmu007. [DOI] [PubMed] [Google Scholar]
- 14.Dhalwani NN, West J, Sultan AA, et al. Women with Celiac Disease Present with Fertility Problems No More often than Women in the General Population. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Freeman HJ. Neurological disorders in adult celiac disease. Can J Gastroenterol. 2008;22:909–11. doi: 10.1155/2008/824631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lionetti E, Francavilla R, Pavone P, et al. The neurology of coeliac disease in childhood: what is the evidence? A systematic review and meta-analysis. Dev Med Child Neurol. 2010;52:700–7. doi: 10.1111/j.1469-8749.2010.03647.x. [DOI] [PubMed] [Google Scholar]
- 17.Shen TC, Lebwohl B, Verma H, et al. Peripheral neuropathic symptoms in celiac disease and inflammatory bowel disease. J Clin Neuromuscul Dis. 2012;13:137–45. doi: 10.1097/CND.0b013e31821c55a1. [DOI] [PubMed] [Google Scholar]
- 18.Zelnik N, Pacht A, Obeid R, et al. Range of neurologic disorders in patients with celiac disease. Pediatrics. 2004;113:1672–6. doi: 10.1542/peds.113.6.1672. [DOI] [PubMed] [Google Scholar]
- 19.Burk K, Farecki ML, Lamprecht G, et al. Neurological symptoms in patients with biopsy proven celiac disease. Mov Disord. 2009;24:2358–62. doi: 10.1002/mds.22821. [DOI] [PubMed] [Google Scholar]
- 20.Hadjivassiliou M, Sanders DS, Grunewald RA, et al. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010;9:318–30. doi: 10.1016/S1474-4422(09)70290-X. [DOI] [PubMed] [Google Scholar]
- 21.Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, et al. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975–92. doi: 10.3390/nu5103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper JW, Holleran SF, Ramakrishnan R, et al. Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82:996–1000. doi: 10.1002/ajh.20996. [DOI] [PubMed] [Google Scholar]
- 23.Annibale B, Severi C, Chistolini A, et al. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am J Gastroenterol. 2001;96:132–7. doi: 10.1111/j.1572-0241.2001.03463.x. [DOI] [PubMed] [Google Scholar]
- 24.Rawal P, Thapa BR, Prasad R, et al. Zinc supplementation to patients with celiac disease--is it required? J Trop Pediatr. 2010;56:391–7. doi: 10.1093/tropej/fmq011. [DOI] [PubMed] [Google Scholar]
- 25.McKeon A, Lennon VA, Pittock SJ, et al. The neurologic significance of celiac disease biomarkers. Neurology. 2014 doi: 10.1212/WNL.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Ludvigsson J, Ekbom A, et al. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care. 2006;29:2483–8. doi: 10.2337/dc06-0794. [DOI] [PubMed] [Google Scholar]
- 27.Elfstrom P, Montgomery SM, Kampe O, et al. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab. 2008;93:3915–21. doi: 10.1210/jc.2008-0798. [DOI] [PubMed] [Google Scholar]
- 28.Casella G, Antonelli E, Di Bella C, et al. Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int. 2013;33:1128–31. doi: 10.1111/liv.12178. [DOI] [PubMed] [Google Scholar]
- 29.Leffler DA, Kelly CP. The cost of a loaf of bread in symptomless celiac disease. Gastroenterology. 2014;147:557–9. doi: 10.1053/j.gastro.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Marsh MN. Gluten, major histocompatibility complex, and the small intestine A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 31.Oxentenko AS, Grisolano SW, Murray JA, et al. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol. 2002;97:933–8. doi: 10.1111/j.1572-0241.2002.05612.x. [DOI] [PubMed] [Google Scholar]
- 32.Niveloni S, Fiorini A, Dezi R, et al. Usefulness of videoduodenoscopy and vital dye staining as indicators of mucosal atrophy of celiac disease: assessment of interobserver agreement. Gastrointest Endosc. 1998;47:223–9. doi: 10.1016/s0016-5107(98)70317-7. [DOI] [PubMed] [Google Scholar]
- 33.Prasad KK, Thapa BR, Nain CK, et al. The frequency of histologic lesion variability of the duodenal mucosa in children with celiac disease. World J Pediatr. 2010;6:60–4. doi: 10.1007/s12519-010-0008-3. [DOI] [PubMed] [Google Scholar]
- 34.Nenna R, Pontone S, Mennini M, et al. Duodenal bulb for diagnosing adult celiac disease: much more than an optimal biopsy site. Gastrointest Endosc. 2012;76:1081–2. doi: 10.1016/j.gie.2012.06.019. author reply 1082. [DOI] [PubMed] [Google Scholar]
- 35.Lebwohl B, Kapel RC, Neugut AI, et al. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–9. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallav K, Leffler DA, Tariq S, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2012;35:380–90. doi: 10.1111/j.1365-2036.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- 37.Aziz I, Key T, Goodwin JG, et al. Predictors for Celiac Disease in Adult Cases of Duodenal Intraepithelial Lymphocytosis. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 38.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520–4. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 39.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 40.Sugai E, Vazquez H, Nachman F, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4:1112–7. doi: 10.1016/j.cgh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Amarri S, Alvisi P, De Giorgio R, et al. Antibodies to deamidated gliadin peptides: an accurate predictor of coeliac disease in infancy. J Clin Immunol. 2013;33:1027–30. doi: 10.1007/s10875-013-9888-z. [DOI] [PubMed] [Google Scholar]
- 42.Korponay-Szabo IR, Szabados K, Pusztai J, et al. Population screening for coeliac disease in primary care by district nurses using a rapid antibody test: diagnostic accuracy and feasibility study. BMJ. 2007;335:1244–7. doi: 10.1136/bmj.39405.472975.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li M, Yu L, Tiberti C, et al. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. Am J Gastroenterol. 2009;104:154–63. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:303–8. doi: 10.1097/MEG.0b013e32834fa914. [DOI] [PubMed] [Google Scholar]
- 45.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–28. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu E, Lee HS, Agardh D. Risk of celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:1074. doi: 10.1056/NEJMc1409252. [DOI] [PubMed] [Google Scholar]
- 47.Leffler D, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62:996–1004. doi: 10.1136/gutjnl-2012-302196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahdeaho ML, Maki M, Laurila K, et al. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011;11:129. doi: 10.1186/1471-230X-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hopper AD, Cross SS, Hurlstone DP, et al. Pre-endoscopy serological testing for coeliac disease: evaluation of a clinical decision tool. BMJ. 2007;334:729. doi: 10.1136/bmj.39133.668681.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugai E, Moreno ML, Hwang HJ, et al. Celiac disease serology in patients with different pretest probabilities: is biopsy avoidable? World J Gastroenterol. 2010;16:3144–52. doi: 10.3748/wjg.v16.i25.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 52.Bai JC, Fried M, Corazza GR, et al. World Gastroenterology Organisation global guidelines on celiac disease. J Clin Gastroenterol. 2013;47:121–6. doi: 10.1097/MCG.0b013e31827a6f83. [DOI] [PubMed] [Google Scholar]
- 53.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–76. doi: 10.1038/ajg.2013.79. quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bardella MT, Fredella C, Prampolini L, et al. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am J Clin Nutr. 2000;72:937–9. doi: 10.1093/ajcn/72.4.937. [DOI] [PubMed] [Google Scholar]
- 55.Kabbani TA, Goldberg A, Kelly CP, et al. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther. 2012;35:723–9. doi: 10.1111/j.1365-2036.2012.05001.x. [DOI] [PubMed] [Google Scholar]
- 56.Kemppainen T, Kroger H, Janatuinen E, et al. Osteoporosis in adult patients with celiac disease. Bone. 1999;24:249–55. doi: 10.1016/s8756-3282(98)00178-1. [DOI] [PubMed] [Google Scholar]
- 57.Kalayci AG, Kansu A, Girgin N, et al. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics. 2001;108:E89. doi: 10.1542/peds.108.5.e89. [DOI] [PubMed] [Google Scholar]
- 58.Aziz I, Evans KE, Papageorgiou V, et al. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis. 2011;20:27–31. [PubMed] [Google Scholar]
- 59.Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37:332–9. doi: 10.1111/apt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leffler DA, Dennis M, Hyett B, et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Shah S, Akbari M, Vanga R, et al. Patient Perception of Treatment Burden Is High in Celiac Disease Compared With Other Common Conditions. Am J Gastroenterol. 2014 doi: 10.1038/ajg.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh J, Whelan K. Limited availability and higher cost of gluten-free foods. J Hum Nutr Diet. 2011;24:479–86. doi: 10.1111/j.1365-277X.2011.01160.x. [DOI] [PubMed] [Google Scholar]
- 63.MacCulloch K, Rashid M. Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr Child Health. 2014;19:305–9. doi: 10.1093/pch/19.6.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–6. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 65.Kupper C. Dietary guidelines and implementation for celiac disease. Gastroenterology. 2005;128:S121–7. doi: 10.1053/j.gastro.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 66.Thompson T. Contaminated oats and other gluten-free foods in the United States. J Am Diet Assoc. 2005;105:348. doi: 10.1016/j.jada.2005.01.024. author reply 348-9. [DOI] [PubMed] [Google Scholar]
- 67.Koerner TB, Cleroux C, Poirier C, et al. Gluten contamination in the Canadian commercial oat supply. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:705–10. doi: 10.1080/19440049.2011.579626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koskinen O, Villanen M, Korponay-Szabo I, et al. Oats do not induce systemic or mucosal autoantibody response in children with coeliac disease. J Pediatr Gastroenterol Nutr. 2009;48:559–65. doi: 10.1097/MPG.0b013e3181668635. [DOI] [PubMed] [Google Scholar]
- 69.Thompson T, Simpson S. A comparison of gluten levels in labeled gluten-free and certified gluten-free foods sold in the United States. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.211. [DOI] [PubMed] [Google Scholar]
- 70.Hall NJ, Rubin GP, Charnock A. Intentional and inadvertent non-adherence in adult coeliac disease. A cross-sectional survey. Appetite. 2013;68:56–62. doi: 10.1016/j.appet.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Lebwohl B, Murray JA, Rubio-Tapia A, et al. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment Pharmacol Ther. 2014;39:488–95. doi: 10.1111/apt.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdulkarim AS, Burgart LJ, See J, et al. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016–21. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 73.Theethira TG, Dennis M, Leffler DA. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev Gastroenterol Hepatol. 2014;8:123–9. doi: 10.1586/17474124.2014.876360. [DOI] [PubMed] [Google Scholar]
- 74.Thompson T, Dennis M, Higgins LA, et al. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J Hum Nutr Diet. 2005;18:163–9. doi: 10.1111/j.1365-277X.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 75.Mukherjee R, Kelly CP, Schuppan D. Nondietary therapies for celiac disease. Gastrointest Endosc Clin N Am. 2012;22:811–31. doi: 10.1016/j.giec.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Lahdeaho ML, Kaukinen K, Laurila K, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology. 2014;146:1649–58. doi: 10.1053/j.gastro.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 77.Kelly CP, Green PH, Murray JA, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37:252–62. doi: 10.1111/apt.12147. [DOI] [PubMed] [Google Scholar]
- 78.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 79.Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547–57. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155–65. doi: 10.1053/j.gastro.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Hollon JR, Cureton PA, Martin ML, et al. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013;13:40. doi: 10.1186/1471-230X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roshan B, Leffler DA, Jamma S, et al. The incidence and clinical spectrum of refractory celiac disease in a north american referral center. Am J Gastroenterol. 2011;106:923–8. doi: 10.1038/ajg.2011.104. [DOI] [PubMed] [Google Scholar]
- 84.Arguelles-Grande C, Brar P, Green PH, et al. Immunohistochemical and T-cell receptor gene rearrangement analyses as predictors of morbidity and mortality in refractory celiac disease. J Clin Gastroenterol. 2013;47:593–601. doi: 10.1097/MCG.0b013e31828a3c44. [DOI] [PubMed] [Google Scholar]
- 85.Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 86.Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 87.Rubio-Tapia A, Kelly DG, Lahr BD, et al. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brar P, Lee S, Lewis S, et al. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007;102:2265–9. doi: 10.1111/j.1572-0241.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 89.Malamut G, Cellier C. Refractory celiac disease. Expert Rev Gastroenterol Hepatol. 2014;8:323–8. doi: 10.1586/17474124.2014.887438. [DOI] [PubMed] [Google Scholar]
- 90.West J, Logan RF, Smith CJ, et al. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716–9. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corrao G, Corazza GR, Bagnardi V, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–61. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 92.Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013;159:169–75. doi: 10.7326/0003-4819-159-3-201308060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moertel CG, Hargraves MM. Coexistence of adenocarcinoma of the jejunum and nontropical sprue. Jama. 1961;176:612–4. doi: 10.1001/jama.1961.63040200015016a. [DOI] [PubMed] [Google Scholar]
- 94.Grainge MJ, West J, Solaymani-Dodaran M, et al. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2012;35:730–9. doi: 10.1111/j.1365-2036.2012.04998.x. [DOI] [PubMed] [Google Scholar]
- 95.Elfstrom P, Granath F, Ekstrom Smedby K, et al. Risk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac disease. J Natl Cancer Inst. 2011;103:436–44. doi: 10.1093/jnci/djq564. [DOI] [PubMed] [Google Scholar]
- 96.Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125:398–405. doi: 10.1002/ijc.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan SY, Morrison H. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol. 2011;3:33–42. doi: 10.4251/wjgo.v3.i3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peters U, Askling J, Gridley G, et al. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163:1566–72. doi: 10.1001/archinte.163.13.1566. [DOI] [PubMed] [Google Scholar]
- 99.van de Water JM, Cillessen SA, Visser OJ, et al. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24:43–56. doi: 10.1016/j.bpg.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 100.Malamut G, Chandesris O, Verkarre V, et al. Enteropathy associated T cell lymphoma in celiac disease: a large retrospective study. Dig Liver Dis. 2013;45:377–84. doi: 10.1016/j.dld.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Sabatino A, Biagi F, Gobbi PG, et al. How I treat enteropathy-associated T-cell lymphoma. Blood. 2012;119:2458–68. doi: 10.1182/blood-2011-10-385559. [DOI] [PubMed] [Google Scholar]
- 102.Abdallah H, Leffler D, Dennis M, et al. Refractory celiac disease. Curr Gastroenterol Rep. 2007;9:401–5. doi: 10.1007/s11894-007-0049-5. [DOI] [PubMed] [Google Scholar]


