Abstract
Objective:
To quantify polyneuropathy impairments and comorbidities utilizing the Rochester Epidemiology Project (2010 census = 148,201).
Methods:
ICD-9-CM coding identified polyneuropathy cases (2006–2010) and their 5:1 age- and sex-matched controls. Mortality and impairments were evaluated while identifying and adjusting for Charlson Index comorbidities.
Results:
Overall prevalence of polyneuropathy was 1.66%, and markedly rose to 6.6% in persons older than 60 years. Cases (n = 2,892) had more comorbidities than controls (n = 14,435) with higher median Charlson Index (6 vs 3, p < 0.001). Diabetes with end-organ disease represented the largest increased comorbidity in cases compared with controls (46.8% vs 6.5%). Diabetic polyneuropathy was the most common specific subtype (38.2%). Miscoded idiopathic cases and false-negative controls also commonly had diabetic polyneuropathy. Median modified Rankin Scale score was considerably higher for cases than controls (4 vs 1, p < 0.001). Multiple comorbidities were found associated with polyneuropathy after adjusting for diabetes co-occurrence, including pulmonary disease, dementia, and others. Polyneuropathy was an independent contributor to multiple functional impairments including difficulty walking (odds ratio [OR] = 1.9), climbing stairs (OR = 2.0), using an assistive device (OR = 2.0), fall tendency (OR = 2.4), work disability (OR = 4.2), lower limb amputations (OR = 3.9), and opioid use (OR = 2.7). Prevalent cases had a younger median age at death than controls (80 vs 86 years, p < 0.001), and incident cases had a 6-month shorter survival.
Conclusions:
Polyneuropathies have notable neurologic impairments beyond their identified multiple comorbidities. Life expectancy is shortened. Diabetic polyneuropathy is underidentified. The quantified extent of the disease burden and refined comorbidity associations emphasize that greater research efforts and health care initiatives are needed.
Underrecognition of common contributors to poor health and physical impairments poses an obstacle to establishing best health care policies.1 Previous studies assessing polyneuropathy prevalence2–10 or disease burden11–15 have focused on specific polyneuropathy subtypes or used small cohorts with limited demographics. From those studies, certain polyneuropathies have been suggested to have substantial disease burden from physical impairments16,17 or even mortality.18,19 However, mortality and disease burden quantification of polyneuropathies irrespective of etiology through a large population-based study has not been performed.
Because diabetes9,10 and other vascular risk factors are common in patients with polyneuropathy, associated impairments could be related to nonneuromuscular sources (i.e., peripheral vascular, cardiovascular, and cerebrovascular disease). It is therefore important to assess whether polyneuropathy is independently a risk factor for impairment and mortality beyond its comorbidities. Such quantification is warranted as world populations are shifting into older and heavier populations with greater diabetes prevalence.20,21
The Rochester Epidemiology Project (REP) is an NIH-funded resource that comprehensively links the medical records of 95% of Olmsted County, MN, residents, irrespective of their health care provider.22,23 It has been used extensively in many important population-based epidemiologic disease studies including several specific peripheral nerve diseases10,24–28 and has been shown to be representative of US populations.22 In the present study, we estimated the prevalence of disease burden and mortality for all polyneuropathy cases in Olmsted County, MN. Disease burden was measured using death and multiple markers of impairment while adjusting for Charlson Index comorbidities29 compared with age- and sex-matched controls.
METHODS
Health care visit dates are linked to addresses within the REP database, and this information has been used to define who resided in Olmsted County (REP Census) at any given point in time since 1966.22,23,30 The population counts obtained by the REP Census exceed those obtained by the US Census, indicating that virtually the entire population of the county is captured by the system.23,30 We used the REP Census to identify all individuals who resided in Olmsted County from January 1, 2006, through December 31, 2010 (the “study period”).
ICD-9-CM administrative codes31 that most clearly represented polyneuropathy (table e-1 on the Neurology® Web site at Neurology.org) were used to screen the study population for prevalent cases. These codes were assigned by providers or professional coders. Others have used a similar method with slightly different lists of codes on 2 other populations.8,9 Up to 5 age- and sex-matched controls per case were identified from the REP population within the study period. Population point prevalence was calculated for December 31, 2010; however, all prevalent cases (even those that died before study's end) were included for disease burden analysis. To assess the effect of comorbid medical conditions on impairment markers, Charlson Comorbidity Index29 was calculated for each patient. The effect of diabetes contribution to peripheral neuropathy comorbidities was also assessed.
To estimate the false-positive and -negative rates of ICD-9-CM ascertainment for polyneuropathy, electronic health records of 289 (10%) randomly selected polyneuropathy cases and 289 of our nonneuropathy controls were reviewed by the investigators for documentation supporting a diagnosis of distal symmetric polyneuropathy, as defined by the American Academy of Neurology.32 Clinical documentation of symptoms, examination findings, and neurophysiologic test results were reviewed (table e-2). When coexisting polyneuropathy subset codes existed with idiopathic diagnosis, the more specific code was selected away from idiopathic. In the instance of 2 or more specific codes, chart review was utilized to place most accurate single coding. All cases were included in subsequent analyses of electronically extracted data.
Current procedural terminology (CPT) codes were used to identify which patients had the following neurophysiologic testing from 2000 to 2011: nerve conduction studies (CPT codes 95860–95864, 95870), EMG (CPT codes 95900, 95903, 95904, 95934, 95936), autonomic testing (CPT codes 95921–95923), and quantitative sensory testing (0106 T-0110T). Whether results of such testing supported a diagnosis of polyneuropathy was ascertained for the 10% of randomly selected prevalent cases whose charts were reviewed.
The REP was electronically queried for prescriptions for neuropathic pain medications prescribed to polyneuropathy cases and controls. Prescription of any dose of one of the queried medications for any length of time within the study period was considered positive use. The REP was also electronically queried for ICD-9-CM procedural or diagnostic codes for lower limb ulcers (707.1x), lower limb amputation (84.1x), excision and repair of bunion and other toe deformities (77.5x), and arthrodesis and arthroereisis of foot and ankle (81.1x) for all cases and controls. A single occurrence of one of these procedures or diagnoses within the study period was considered a presence of that complication.
Mayo Clinic patients are administered standardized questionnaires periodically at clinical encounters, which, among other things, ask about ability to perform activities of daily living (ADL), limb weakness, numbness, fall tendency, pain, stair-climbing difficulty, reliance on assistance from gait aid or others, employment status, and living environment. Answers from the most recent questionnaire within the study period were obtained via automated electronic retrieval for cases and controls. Each self-reported marker of impairment was assigned an equivalent modified Rankin Scale (mRS)33 score; the highest equivalent mRS score for each patient was set as that patient's mRS score (table e-3).
All analyses were performed with JMP Pro 9.0.3 software (SAS Institute, Cary, NC). Prevalence estimates were calculated using the REP population census rather than the US Census estimates. The Wilcoxon rank sum test was used to compare Charlson Comorbidity Indices, mRS scores, and age at death between cases and controls with interquartile ranges (IQRs) calculated. Logistic regression models were used to estimate associations between polyneuropathy and surrogate markers of impairment with ORs and 95% confidence intervals (CIs). Multivariate models were used to adjust for potentially confounding effects of Charlson comorbidities. Effect of diabetes on each Charlson comorbidity was assessed for all polyneuropathy subtypes in consideration of its contribution to the comorbidity (table e-4). Accuracy of the type of polyneuropathy documentation and ICD-9 coding was reviewed among the 289 randomly selected cases (table e-5). Specific question to surrogate markers of impairment for idiopathic polyneuropathy and diabetic neuropathy were reviewed compared with matched controls (tables e-6 and e-7).
Death data were obtained beyond the case ascertainment period (through September 30, 2013) for all cases and controls. Survival analyses were performed to determine whether patients with newly diagnosed (incident) polyneuropathy had shorter survival times compared with patients without polyneuropathy. Incident polyneuropathy was defined as a polyneuropathy diagnosis between January 1, 2006, through December 31, 2010, without a previous polyneuropathy ICD-9-CM code between January 1, 1995, and December 31, 2005. Patients were right-censored on the date of their last documented day of Olmsted County residence or September 30, 2013, whichever was later. Kaplan–Meier survival curves were plotted for those with and without polyneuropathy. Cox proportional hazards regression was used to estimate hazards ratios for survival. Multivariate models were adjusted for potentially confounding effects of Charlson comorbidities.
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of both Mayo Clinic and Olmsted Medical Center approved the study.
RESULTS
There were 2,892 prevalent polyneuropathy cases in Olmsted County from 2006 to 2010, and on December 31, 2010, there were 2,455 patients alive with polyneuropathy, making the point prevalence 1.66% (table 1). Polyneuropathy prevalence consistently increased with age and was more common in male patients. In patients 60 years and older, the point prevalence was markedly increased to 6.6%. To assess the accuracy of polyneuropathy diagnosis through administrative coding, we randomly selected 289 cases (10%) and carefully reviewed their clinical documentation. Among them, 270 (93.4%) had sufficient documentation to meet the diagnosis criteria (table e-2), whereas 19 cases (6.6%) either did not have sufficient documentation or had another diagnosis after testing (table e-2). The false-negative rate of case ascertainment by ICD-9 coding among the controls was 5.1%, but only 38% of the reviewed controls had a comprehensive neurologic examination in the study period (table e-2). Subtypes were assigned as per table 2. Approximately half (50.1%) of all polyneuropathy cases were coded as idiopathic, and diabetic polyneuropathy was the second most common subtype, accounting for 38.2% of all cases. Idiopathic polyneuropathy coding was the most frequently identified inaccurate classification based on subset chart review (19% inaccurate) with diabetic polyneuropathy identified to be most frequently missed (52%) among those patients (table e-5).
Table 1.
Demographics of all polyneuropathy cases and matched controls from 2006 to 2010
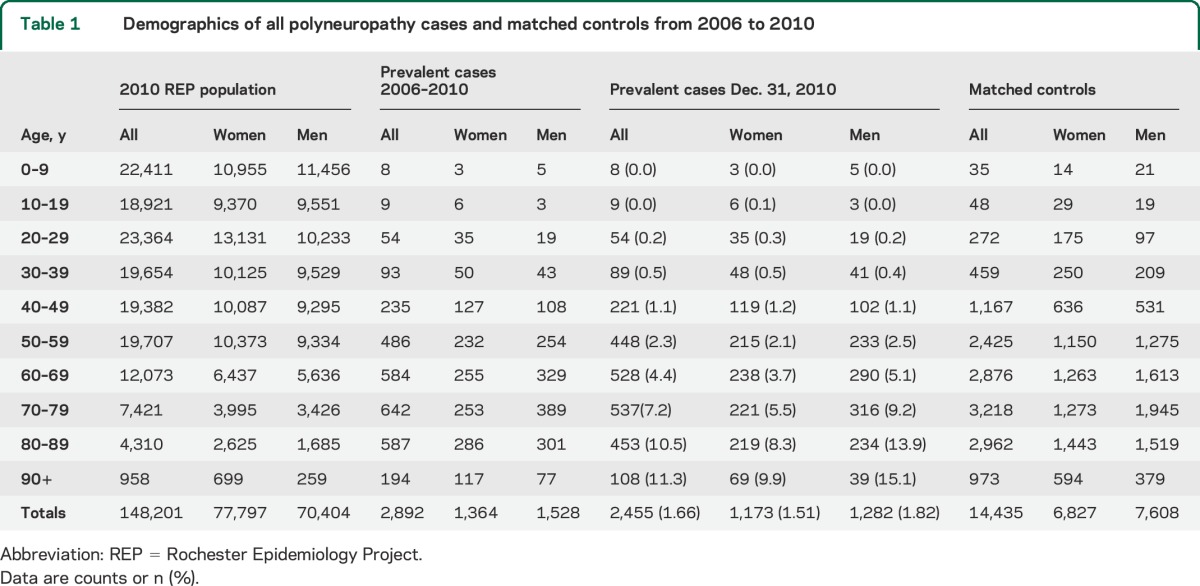
Table 2.
Distribution of polyneuropathy cases by subtype
From 2000 through 2011, 1,421 polyneuropathy cases (49.1%) had nerve conduction and/or EMG studies, 166 (5.7%) had autonomic testing, and 75 (2.6%) had quantitative sensory testing. The review of 289 randomly selected polyneuropathy cases showed that 115 (39.8%) had nerve conduction studies, and in 94 (81.7%), polyneuropathy was confirmed.
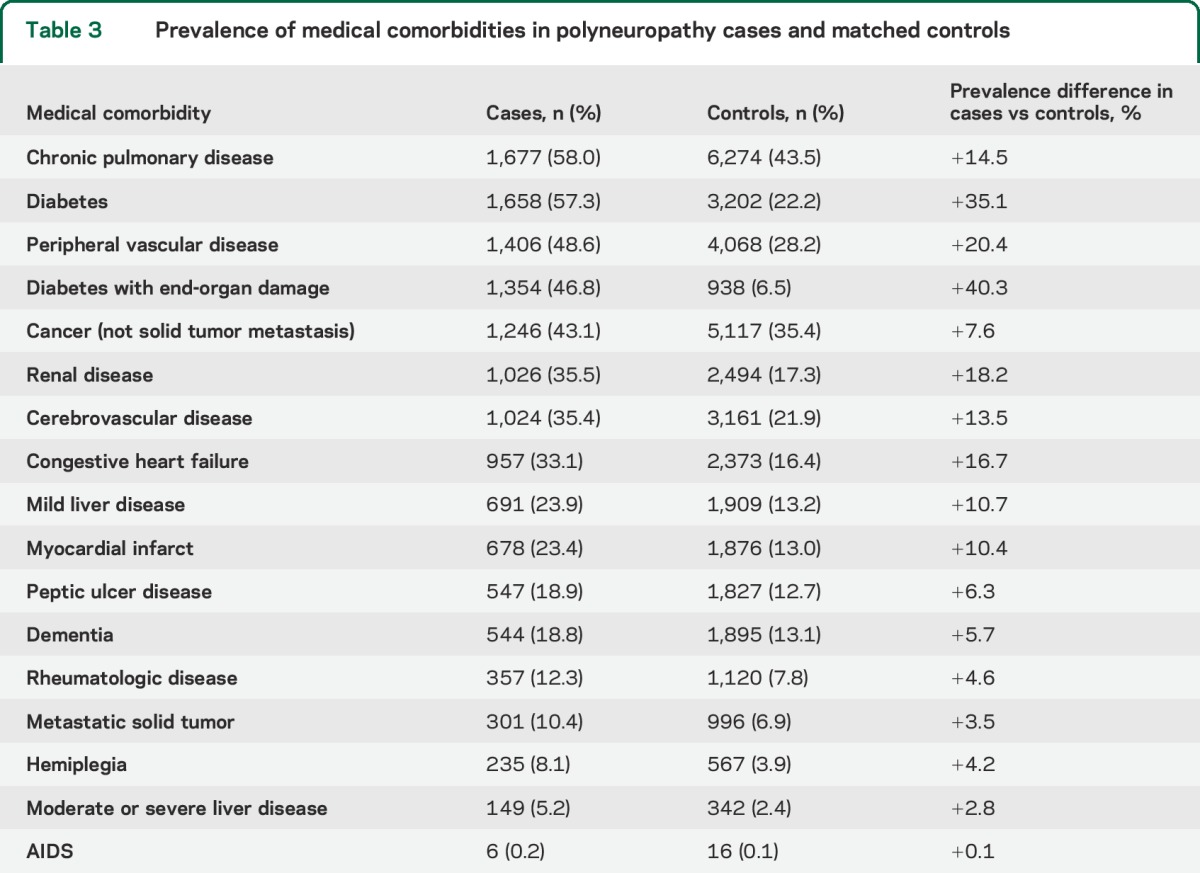
All 17 medical comorbidities were more common in polyneuropathy cases than in controls (table 3). The median Charlson Comorbidity Index was significantly greater for cases (6; IQR 3–10) than controls (3; IQR 1–6; p < 0.001). Chronic pulmonary disease was the most prevalent comorbidity in both cases and controls, but significantly more common in polyneuropathy. Diabetes with and without end-organ damage was the comorbidity with the largest prevalence difference between polyneuropathy cases and controls. Diabetes was present in 57.3% of polyneuropathy cases, but only 38.2% of polyneuropathy cases were coded as diabetic polyneuropathy. Peripheral vascular disease, myocardial infarct, renal disease, and congestive heart failure were notably overrepresented in polyneuropathy cases with diabetes (table e-4). However, not accountable by co-occurrence of diabetes, multiple comorbidities were also overrepresented in polyneuropathy, including pulmonary disease, dementia, metastatic solid tumor cancer, and others.
Table 3.
Prevalence of medical comorbidities in polyneuropathy cases and matched controls
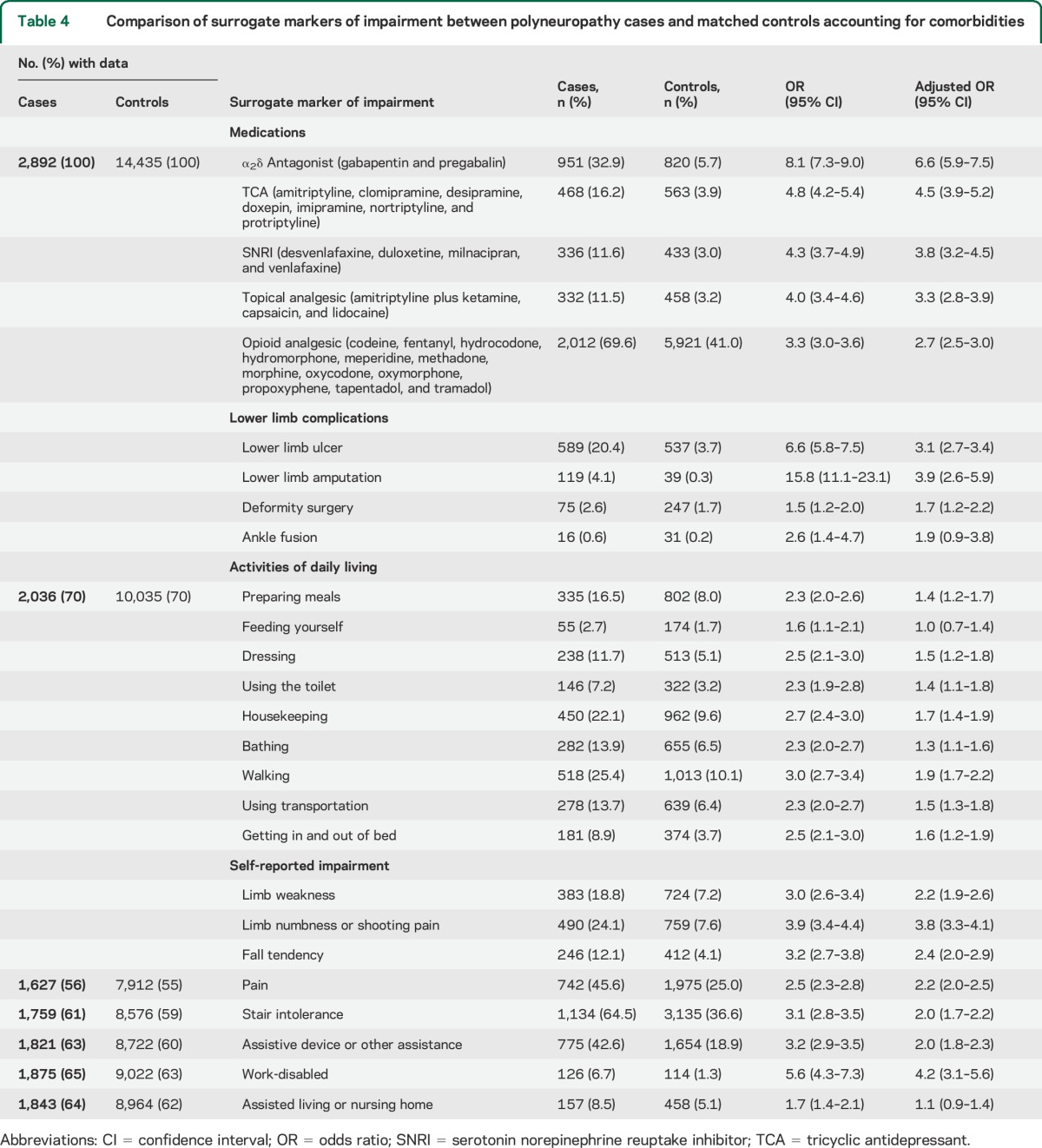
Morbidity data on analgesic usage and lower limb complications were available for 100% of cases and controls. Individuals with polyneuropathy were more likely to be prescribed pain medications even after adjusting for medical comorbidities (table 4). Except for ankle fusions, all other lower limb complications had significant independent associations with polyneuropathy, even after adjustment for comorbidities. Data for ADLs and self-reported disabling symptoms were less available than analgesic usage and lower limb complications, but missing data were similar for each metric between cases and controls. Impairment in performing most ADLs (except for feeding oneself) was more likely in those with polyneuropathy than controls. Disabling symptoms, such as limb weakness, numbness, fall tendency, pain, stair intolerance, reliance on assistive devices or assistance from others, or work-disabled status, were all more likely to be reported by polyneuropathy cases than controls, independent of comorbidities. Those with polyneuropathy were also more likely to report living in a skilled nursing or assisted-living facility, but this effect was attenuated after adjusting for comorbidities. The median mRS score was higher for cases than controls (4 vs 1), which was statistically significant both before (OR per 1-point mRS increase 1.27, 95% CI 1.25–1.30) and after (OR 1.18, 95% CI 1.15–1.21) adjustment for comorbidities. Patients with idiopathic polyneuropathy had comparable impairments to the entire cohort for analgesic use, lower limb complications, and self-reported impairments, but with fewer significant difficulties of ADL (table e-6). Of interest, it is evident that increased risk of “lower limb amputation” and “work-disabled” is largely attributable to diabetic neuropathy (table e-7).
Table 4.
Comparison of surrogate markers of impairment between polyneuropathy cases and matched controls accounting for comorbidities
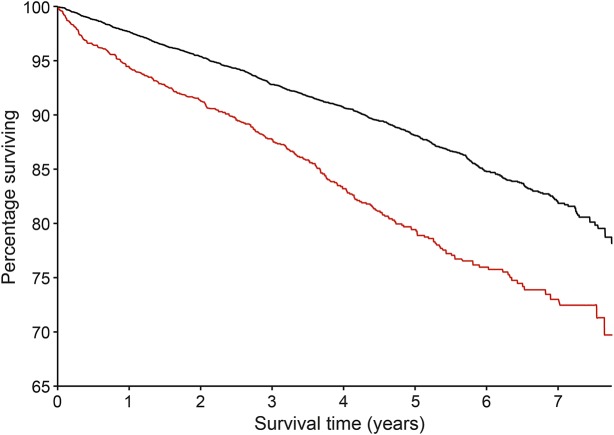
Median age at death was younger for prevalent cases (80 years; IQR 69–88; n = 602) than controls (86 years; IQR 80–91; n = 1,722; p < 0.001). By September 30, 2013, 307 (19.5%) of 1,574 incident polyneuropathy cases and 901 (11.8%) of 7,660 controls matched to incident polyneuropathy cases had died. Mean survival for incident polyneuropathy cases and controls over the follow-up period was 6.5 and 7.0 years, respectively (figure). The unadjusted hazard ratio was 1.74 (CI 1.53–1.98), whereas the hazard ratio adjusted for medical comorbidities was 1.11 (CI 0.97–1.28).
Figure. Survival curves for incident polyneuropathy cases and matched controls.
Incident cases of polyneuropathy (n = 1,574; red line) identified during the study period had significantly shorter survival than matched controls (n = 7,669; black line) from diagnosis and through a 33-month follow-up after the study period.
DISCUSSION
This large geographical population-based, case-control study of polyneuropathy provides important new insights. The present study assessed the most comprehensive range of impairment markers to date irrespective of polyneuropathy subtype, utilized a unique database representative of national demographics, and conducted statistical analysis in 5:1 ratio of age- and sex-matched controls and polyneuropathy cases correcting for comorbidity associations. Prior prevalence estimates (2.3%–12.2%) excluded younger individuals,3,5–8 are sex-specific,11,15 or are from a consanguineous population.2 Our study screened all demographics of a large population with documented generalizability22 uniformly for not only polyneuropathy, but also functional impairments corrected for associated comorbidities. Very few administratively coded polyneuropathy cases did not meet established criteria32 of polyneuropathy, with 49.1% of all polyneuropathy cases in our population having had nerve conduction studies and/or needle EMG during the study period. Our estimate of polyneuropathy point prevalence (1.66%) is less than earlier studies. However, in persons older than 60 years of age, there was a dramatic increase in point prevalence to 6.6%, which is consistent with studies of older populations.3,5–7,9 Some patients (5.1%) with polyneuropathy were missed in our diagnostic coding, most commonly having diabetic polyneuropathy. Therefore, the prevalence of polyneuropathy may be slightly higher, especially for asymptomatic forms. This is offset by 6.6% of patients coded as polyneuropathy who were underdocumented or believed to be inaccurately coded. Accuracy to the type of polyneuropathy was fairly high, i.e., 90% or higher for most forms apart from idiopathic at 81%. Most important, however, multiple quantified functional impairments are significantly more common in polyneuropathy cases than their age- and sex-matched controls even after adjusting for comorbidities, and these impairments translate to higher mortalities. These results clearly indicate that polyneuropathy disease burden will worsen as world populations are evolving to older demographics,20 and greater attention should be directed to the care of polyneuropathy.
Diabetic polyneuropathy was the most frequently coded specific diagnosis (38.2%). Review of the other polyneuropathy subtypes by administrative coding is informative. A majority of polyneuropathy cases (50.1%) carried idiopathic diagnoses and this was despite many having concomitant diabetes (57.3% of all polyneuropathy cases). This observation is emphasized by the relatively low occurrence of diabetes in our nonpolyneuropathy controls (22.3%). In fact, we realized many persons coded as idiopathic polyneuropathy had diabetic polyneuropathy, i.e., 52% of those miscoded (table e-5). Indeed, 485 of the 1,449 patients coded as idiopathic polyneuropathy also had diabetes, and 226 (46.8%) of those had end-organ damage. This finding supports the earlier contention that diabetes is the most commonly identified cause of polyneuropathy.34 In addition, a previous study that used administrative coding for case ascertainment estimated that 66% of polyneuropathy cases in an elderly population are diagnosed as idiopathic and 47.8% of patients with polyneuropathy and concomitant diabetes are coded as idiopathic, supporting our finding that administrative coding artificially increases the proportion of idiopathic cases.9
Predictively, among patients with diabetes and polyneuropathy, known comorbidities or end-organ disease of diabetes were more common (peripheral vascular disease, myocardial infarction, renal disease, and congestive heart failure). Diabetes, however, among patients with polyneuropathy did not account for the increased comorbidities in patients with polyneuropathy for pulmonary disease, dementia, and many other Charlson comorbidity indices. The average number of Charlson comorbidities in patients with polyneuropathy was 6 vs 3 in controls. These findings warrant a new area of investigation to study disease associations beyond diabetes. Investigation of the temporal onset development of comorbidities among this cohort's nondiabetic idiopathic polyneuropathy cases in relation to polyneuropathy symptoms could considerably improve the understanding of polyneuropathy pathophysiology and diagnosis. For example, chronic pulmonary disease was found to be 14.5% more common in cases compared with controls. Evaluating the association with smoking and other risks would be helpful in discovery of a shared pathogenesis. In addition, the association among obesity, sleep apnea, and chronic pulmonary disease in patients with polyneuropathy is also an interesting subject for future investigation. Nevertheless, the clear importance of identifying and treating diabetes in polyneuropathy is further emphasized by this study, especially as world populations are shifting to older and heavier weight demographics with increased prevalence of diabetes.27
Our results not only further emphasized that polyneuropathy is disabling,11–15,35 but also provided new insights that polyneuropathy was independently associated with greater pain and prescription of analgesics, increased lower limb complication rates (including ulcers, 20.4%, and amputations, 4.1%), greater impairment of most ADLs, higher fall tendency, stair climbing difficulty, and reliance on assistive devices or assistance from others. The degrees of impairment associations were often striking with ORs frequently greater than 3.0 even after correction for the associated multiple Charlson comorbidities. This translated into major work impairment with a comorbidity-corrected OR of 4.2 and mRS score of 4 for cases (moderately disabled) vs 1 for controls (some impairment symptoms). There were only a few impairment markers not independently associated with polyneuropathy after adjusting for the 17 Charlson comorbidities. While the effect size of most other surrogate markers of impairment was reduced after adjustment for comorbidities, it is still statistically significant (table 4). In addition, this study showed that individuals with polyneuropathy had significantly shorter lives than controls by 6 years and the rate of death during the relatively short duration of follow-up was nearly doubled in cases compared with controls (figure). Although this effect on mortality was attenuated after adjusting for comorbidities, the results suggest that polyneuropathy may independently contribute to increased mortality, but longer prospective study is needed to define this better.
As an inherent limitation to all retrospective studies, asymptomatic cases not detected by the health care provider would not have been reported, but our approach would identify the majority of clinically relevant polyneuropathy cases. Accurate case ascertainment requires that providers or medical coders assign appropriate administrative codes. Through a random review of 10% of the entire polyneuropathy cases and an equal number of controls, we found a very good agreement with administratively coded polyneuropathy diagnosis. In addition, we recognize that comorbidities not included in the Charlson index could have confounded the analysis. However, given the comprehensive list of comorbidities and impairments associated with morbidity and mortality we included in this study, that likelihood is very low. Further validation of the extent of morbidity and mortality will continue to be of major interest, and will need confirmation in additional US and world populations.
This population-based study shows that polyneuropathies are associated with multiple impairments beyond their associated comorbidities, and it is likely overlooked as a major disabling medical condition. Our results demonstrate that patients with polyneuropathy are often work-disabled, lose independence, have pain requiring multiple medications, and die younger, emphasizing the significance of this problem. The likely underrecognition of diabetic cause provides a specific focus for additional research, as do the newly identified comorbidity associations. Greater public attention and resources are needed to address polyneuropathy as a major health issue.
Supplementary Material
GLOSSARY
- ADL
activities of daily living
- CI
confidence interval
- CPT
current procedural terminology
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- mRS
modified Rankin Scale
- OR
odds ratio
- REP
Rochester Epidemiology Project
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
E.M.H. and N.P.S.: data collection, analysis and interpretation of data, drafting and revising the manuscript. J.M.R.: data collection and analysis. J.L.S.: analysis and interpretation of data. P.J.D.: drafting and revising the manuscript. C.J.K.: concept, supervision, data collection, analysis and interpretation of data, drafting and revising the manuscript.
STUDY FUNDING
The Mayo Foundation for Medical Education and Research, the Mayo Clinic Center for Individualized Medicine, Rochester Epidemiology Project, and NIH: RO1 AG034676 (Rochester Epidemiology Project), K08 NS065007 (C.J.K.), K08 CA169443-02 (N.P.S.), UL1 RR000135 (Clinical and Translational Sciences Award).
DISCLOSURE
E. Hoffman, N. Staff, J. Robb, and J. St. Sauver report no disclosures relevant to the manuscript. P. Dyck is an associate editor of Diabetes and receives an honorarium for this work. He receives personal and laboratory support from ISIS Pharmaceuticals Inc. and Alnylam Pharmaceuticals Inc. C. Klein is a coeditor of Journal of the Peripheral Nervous System. Go to Neurology.org for full disclosures.
REFERENCES
- 1.U.S. Department of Health and Human Services. HHS announces the nation's new health promotion and disease prevention agenda [online]. Available at: http://www.healthypeople.gov/sites/default/files/DefaultPressRelease_1.pdf. Accessed October 11, 2014.
- 2.Bharucha NE, Bharucha AE, Bharucha EP. Prevalence of peripheral neuropathy in the Parsi community of Bombay. Neurology 1991;41:1315–1317. [DOI] [PubMed] [Google Scholar]
- 3.Monticelli ML, Beghi E. Chronic symmetric polyneuropathy in the elderly: a field screening investigation in two regions of Italy: background and methods of assessment. The Italian General Practitioner Study Group (IGPSG). Neuroepidemiology 1993;12:96–105. [DOI] [PubMed] [Google Scholar]
- 4.Savettieri G, Rocca WA, Salemi G, et al. Prevalence of diabetic neuropathy with somatic symptoms: a door-to-door survey in two Sicilian municipalities. Sicilian Neuro-Epidemiologic Study (SNES) Group. Neurology 1993;43:1115–1120. [DOI] [PubMed] [Google Scholar]
- 5.Italian General Practitioner Study Group. Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation in two Italian regions: I: prevalence and general characteristics of the sample. Neurology 1995;45:1832–1836. [DOI] [PubMed] [Google Scholar]
- 6.Beghi E, Monticelli ML. Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation of risk factors for polyneuropathy in two Italian communities. Italian General Practitioner Study Group (IGPST). J Clin Epidemiol 1998;51:697–702. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract 2007;77:485–488. [DOI] [PubMed] [Google Scholar]
- 8.Callaghan B, Kerber K, Longoria R, Feldman E, Lisabeth L. Capturing cases of distal symmetric polyneuropathy in a community. Muscle Nerve 2012;46:943–947. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan B, McCammon R, Kerber K, Xu X, Langa KM, Feldman E. Tests and expenditures in the initial evaluation of peripheral neuropathy. Arch Intern Med 2012;172:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993;43:817–824. [DOI] [PubMed] [Google Scholar]
- 11.Karvonen-Gutierrez CA, Ylitalo KR. Prevalence and correlates of disability in a late middle-aged population of women. J Aging Health 2013;25:701–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mold JW, Lawler F, Roberts M; Oklahoma Physicians Resource/Research Network Study. The health consequences of peripheral neurological deficits in an elderly cohort: an Oklahoma Physicians Resource/Research Network Study. J Am Geriatr Soc 2008;56:1259–1264. [DOI] [PubMed] [Google Scholar]
- 13.Resnick HE, Vinik AI, Schwartz AV, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women's Health and Aging Study. Diabetes Care 2000;23:1642–1647. [DOI] [PubMed] [Google Scholar]
- 14.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) Study. Diabetes Care 2008;31:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ylitalo KR, Herman WH, Harlow SD. Performance-based physical functioning and peripheral neuropathy in a population-based cohort of women at midlife. Am J Epidemiol 2013;177:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes RA. Epidemiology of peripheral neuropathy. Curr Opin Neurol 1995;8:335–338. [DOI] [PubMed] [Google Scholar]
- 17.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry 1997;62:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stosovic M, Nikolic A, Stanojevic M, et al. Nerve conduction studies and prediction of mortality in hemodialysis patients. Ren Fail 2008;30:695–699. [DOI] [PubMed] [Google Scholar]
- 19.Gertz MA, Kyle RA, Greipp PR. Response rates and survival in primary systemic amyloidosis. Blood 1991;77:257–262. [PubMed] [Google Scholar]
- 20.United Nations. World population ageing: 1950–2050. Department of Economics and Social Affairs, Population Division. New York: United Nations Publications; 2002:11–13. [Google Scholar]
- 21.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic Proc 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beghi E, Kurland LT, Mulder DW, Nicolosi A. Brachial plexus neuropathy in the population of Rochester, Minnesota, 1970–1981. Ann Neurol 1985;18:320–323. [DOI] [PubMed] [Google Scholar]
- 25.Gelfman R, Melton LJ, III, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology 2009;72:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughlin RS, Dyck PJ, Melton LJ, III, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology 2009;73:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parisi TJ, Mandrekar J, Dyck PJ, Klein CJ. Meralgia paresthetica: relation to obesity, advanced age, and diabetes mellitus. Neurology 2011;77:1538–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radhakrishnan K, Litchy WJ, O'Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976 through 1990. Brain 1994;117:325–335. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 30.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clinic Proc 1996;71:266–274. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [online]. Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed January 5, 2014. [Google Scholar]
- 32.England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207. [DOI] [PubMed] [Google Scholar]
- 33.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 34.England JD, Asbury AK. Peripheral neuropathy. Lancet 2004;363:2151–2161. [DOI] [PubMed] [Google Scholar]
- 35.Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract 2004;17:309–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.