Abstract
Objective:
To provide evidence-based recommendations for treatment of adults with an unprovoked first seizure.
Methods:
We defined relevant questions and systematically reviewed published studies according to the American Academy of Neurology's classification of evidence criteria; we based recommendations on evidence level.
Results and recommendations:
Adults with an unprovoked first seizure should be informed that their seizure recurrence risk is greatest early within the first 2 years (21%–45%) (Level A), and clinical variables associated with increased risk may include a prior brain insult (Level A), an EEG with epileptiform abnormalities (Level A), a significant brain-imaging abnormality (Level B), and a nocturnal seizure (Level B). Immediate antiepileptic drug (AED) therapy, as compared with delay of treatment pending a second seizure, is likely to reduce recurrence risk within the first 2 years (Level B) but may not improve quality of life (Level C). Over a longer term (>3 years), immediate AED treatment is unlikely to improve prognosis as measured by sustained seizure remission (Level B). Patients should be advised that risk of AED adverse events (AEs) may range from 7% to 31% (Level B) and that these AEs are likely predominantly mild and reversible. Clinicians' recommendations whether to initiate immediate AED treatment after a first seizure should be based on individualized assessments that weigh the risk of recurrence against the AEs of AED therapy, consider educated patient preferences, and advise that immediate treatment will not improve the long-term prognosis for seizure remission but will reduce seizure risk over the subsequent 2 years.
An estimated 150,000 adults present annually with an unprovoked first seizure in the United States.1 Even one seizure is a traumatic physical and psychological event that poses difficult diagnostic and treatment questions, and has major social consequences (e.g., loss of driving privileges, limitations for employment).2,3 Recurrent seizures pose even more serious and costly problems.2–4 Therefore, optimal evidence-based approaches for evaluating and managing adults after a first seizure and preventing recurrences with antiepileptic drug (AED) therapy are important. A 2007 practice guideline addresses the evaluation of an unprovoked first seizure in adults3; the present practice guideline analyzes evidence regarding prognosis and therapy.
We included studies of adults with an unprovoked first seizure and excluded those of patients with more than one seizure at the time of presentation.3,5,6 Unprovoked seizures are classified in 1 of 2 broad categories: (1) a seizure of unknown etiology, or (2) a seizure in relation to a demonstrated preexisting brain lesion or progressive CNS disorder (so-called “remote symptomatic” seizure).5 We excluded studies of provoked seizures, which are defined as seizures due to an acute symptomatic condition (e.g., a metabolic or toxic disturbance, cerebral trauma, stroke) and differ in prognosis from unprovoked seizures.3,5–7
This practice guideline considers the evidence for prognosis and treatment of adults with an unprovoked first seizure; a 2003 guideline addresses this for children.8 We posed 3 questions: (1) What are the risks for seizure recurrence after a first seizure? (2) Does immediate treatment with an AED reduce or change (a) short-term risks for a seizure recurrence or (b) long-term prognosis for seizure freedom or remission? (3) For those patients prescribed AEDs immediately, what are the risks for adverse events (AEs)?
DESCRIPTION OF THE ANALYTIC PROCESS
This is an evidence-based appraisal from a systematic review of the literature published in English and based on established 2004 process standards from the American Academy of Neurology (AAN) Guideline Development Subcommittee9 (see appendices e-1 and e-2 on the Neurology® Web site at Neurology.org). We searched MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases (1966 to March 2013), and reviewed the literature for relevant publications using established criteria. See appendix e-3 for complete search strategy and appendix e-4 for inclusion and exclusion criteria.9
We identified 2,613 articles, obtained all in abstract form, and selected 281 for full-text review. Of the selected articles, 47 were judged relevant and acceptable. We systematically reviewed and rated the 47 articles according to the AAN classification of evidence scheme for prognostic or therapeutic articles (appendix e-5). We linked recommendations to evidence strength based primarily on studies rated Class I or II (appendix e-6). Appendix e-7 presents all rated articles. Tables e-1 through e-4 show the data.
ANALYSIS OF EVIDENCE
Risk of seizure recurrence.
Question.
For the adult who presents with an unprovoked first seizure, what are the risks for seizure recurrence?
Evidence.
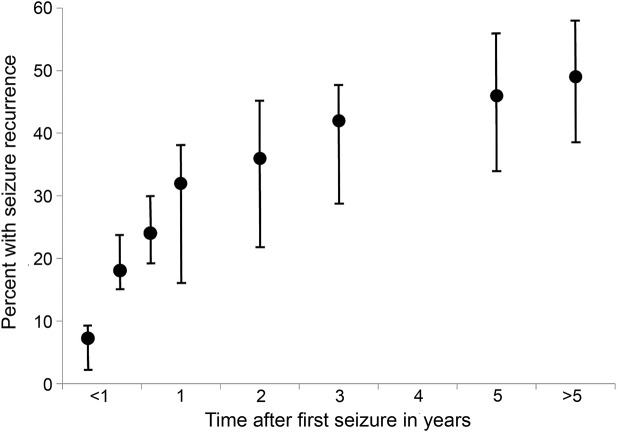
We identified 2 prognostic Class I10–14 and 8 prognostic Class II studies15–22 addressing the probability that an adult with an unprovoked first seizure would have recurrent seizures, and estimated the recurrence risk from these pooled data, which included studies wherein AED treatment was not randomized or controlled (table 1, figure 1). Generalized tonic–clonic convulsive seizures comprise the major seizure type, with some studies including only patients with such seizures.12,13,17,22 These studies, including both AED-treated and untreated subjects, demonstrate that the cumulative incidence of seizure recurrence increases over time, with the great majority of recurrences occurring within the first 1 to 2 years after the initial seizure and the greatest risk in the first year (i.e., 32% at 1 year, compared with just 46% by 5 years) (table 1, figures 1 and 2). Patient ascertainment and treatment differences among studies may account for some of the wide variation in recurrence rates observed. If recruitment into trials is delayed by weeks or months, patients may experience a recurrence and become ineligible.11,12,15,16 Also, seizure recurrence was lower for patients treated with AEDs in most of these studies, but treatment often was not randomized.10,11,16,17,19,20 These 2 factors would lead to variability and underestimation of recurrence risk.
Table 1.
Risk of seizure recurrence after an unprovoked first seizure in adults (Class I and II studies)
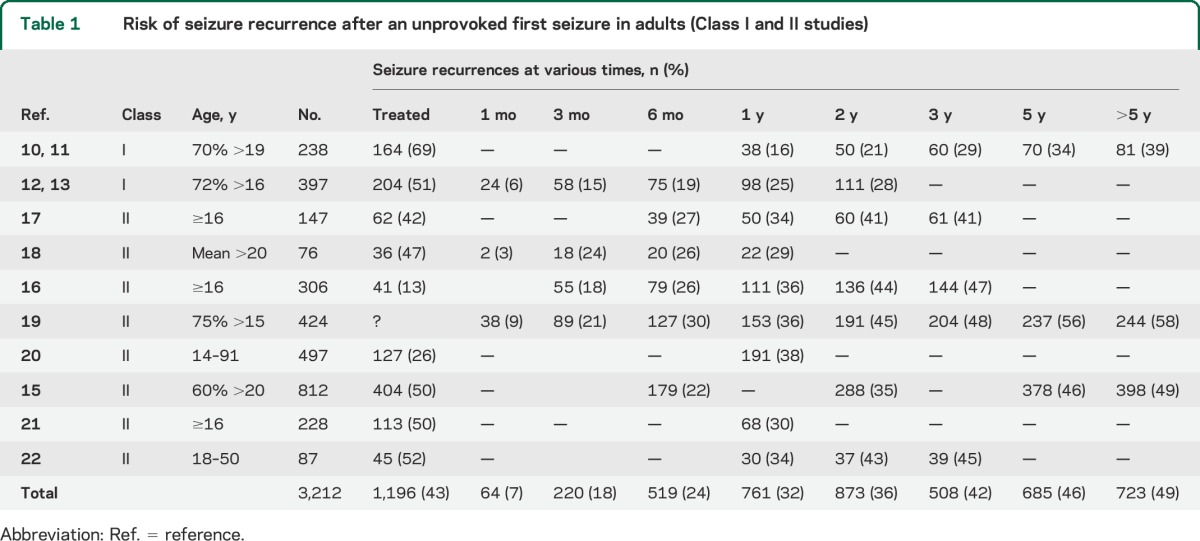
Figure 1. Percentages of patients with first seizure experiencing a recurrent seizure over time.
This graph is based on a fixed-effect pooled percentage model from data in table 1 and shows the cumulative average and the range for each time period from 1 month to more than 5 years.
Figure 2. Cumulative proportion of patients experiencing a seizure recurrence after randomization, comparing immediate vs deferred treatment.
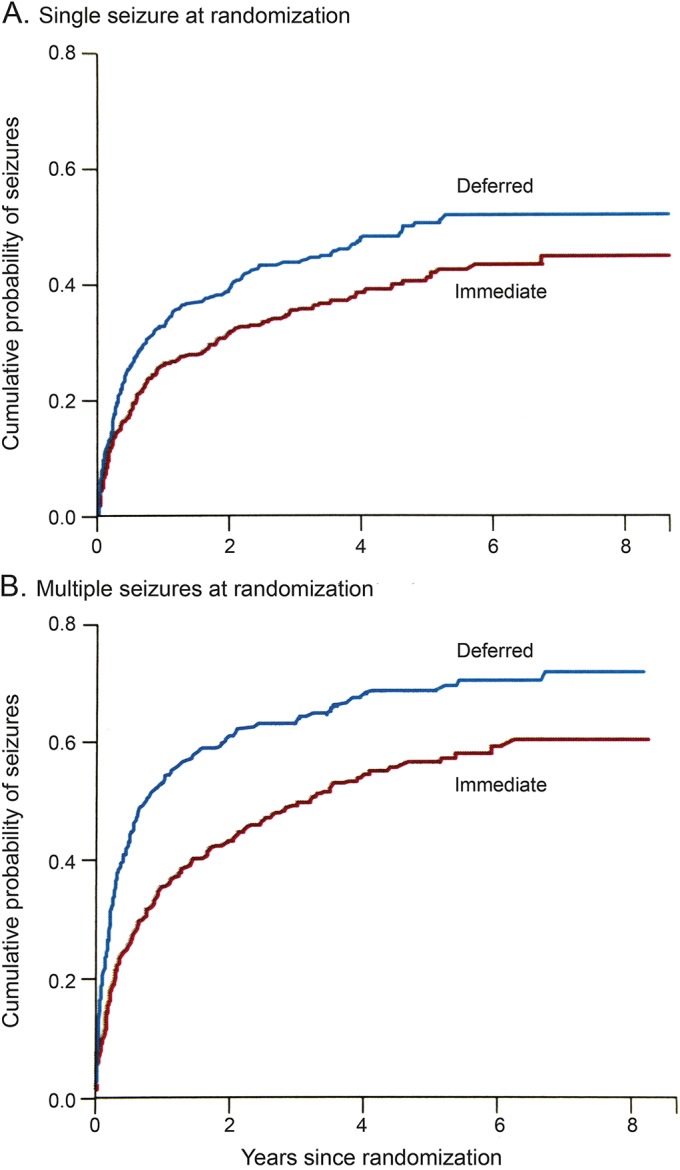
Cumulative proportion of patients with an unprovoked first seizure experiencing a seizure recurrence after randomization and comparing patients with immediate antiepileptic drug treatment vs patients with treatment deferred pending a seizure recurrence (A), and in this specific study, comparing those individuals with patients who had multiple seizures before randomization to treatment (B). Reprinted from The Lancet (Marson et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 2005;365:2007–2013), © 2005, with permission from Elsevier.15
We also identified clinical variables found in studies to be associated with an increased risk of seizure recurrence. The most consistently noted factors associated with an increased risk of seizure recurrence following an unprovoked first seizure include a prior brain lesion or insult causing the seizure,3,11,13,19,23,24 an EEG with epileptiform abnormalities (characterized by spikes or sharp waves),3,11–13,16–19,23,24 a significant brain-imaging abnormality (judged the cause of the seizure),3,6,9,20,24 and a nocturnal seizure.16,17 Of 2 Class I11,13 and 2 Class II studies,19,20 most confirm that individuals with a seizure related to a prior brain lesion11,13,19,20 (including those due to stroke, trauma, CNS infection, cerebral palsy, and cognitive developmental disability), a so-called “remote symptomatic” seizure,5,11,23,24 demonstrate an approximately 2-fold-higher risk of seizure recurrence. That increased risk is illustrated in a representative study with seizure recurrence rates of 26%, 41%, and 48% at 1, 3, and 5 years, respectively, as compared with 10%, 24%, and 29% at these same intervals for patients with a seizure of unknown cause.11 Regarding EEG findings, a majority of 2 Class I11,12 and 4 Class II studies16–19 confirm a similar increased recurrence risk for an epileptiform abnormality. Comparably increased recurrence risk is noted with respect to abnormal brain imaging, such as MRI or CT in 2 Class II studies16,20 and 1 Class III study,25 with approximately 10% of subjects manifesting clinically relevant structural lesions.3,16,20 Likewise, 2 Class II studies16,17 report a similarly increased risk for patients experiencing a nocturnal seizure as compared with those whose first seizure occurred while they were awake. The following are representative examples of increased seizure recurrence risks or hazard ratios from studies of mixed cohorts of AED-treated and untreated subjects:
A prior brain insult as a seizure cause was associated with an increased relative rate for seizure recurrence at 1 to 5 years of 2.55 (95% confidence interval [CI] 1.44–4.51) as compared with that in patients with seizures of unknown cause.11
An EEG with epileptiform abnormalities was associated with a relative rate increase for seizure recurrence at 1 to 5 years of 2.16 (95% CI 1.07–4.38) as compared with that in patients without such EEG abnormalities.11
Abnormal brain imaging was associated with a hazard ratio increase for seizure recurrence at 1 to 4 years of 2.44 (95% CI 1.09–5.44) as compared with that in patients without imaging abnormalities.25
A nocturnal seizure was associated with an increased recurrence risk odds ratio at 1 to 4 years of 2.1 (95% CI 1.0–4.3) as compared with a seizure while the patient was awake.17
In contrast, clinical variables that were not consistently associated with an increased seizure recurrence risk after an unprovoked first seizure in adults include the patient's age, sex, family history of seizures, seizure type, and presentation with status epilepticus or multiple (2 or more) discrete seizures within 24 hours with recovery between them.3,10,11,20,23,24
Conclusion.
Based on data from studies including mixed cohorts of both AED-treated and untreated subjects, an adult with an unprovoked first seizure is at greatest risk of a recurrence relatively early, within the first 2 years (21%–45%), and especially in the first year (2 Class I studies, 8 Class II studies), and this risk appears to be lower for patients treated with AEDs.
The risk of seizure recurrence increases in certain clinical circumstances. These include a prior brain lesion or insult causing the seizure (2 Class I studies, 2 Class II studies), an EEG with epileptiform abnormalities (2 Class I studies, 4 Class II studies), a significant brain-imaging abnormality (2 Class II studies, 1 Class III study), and a nocturnal seizure (2 Class II studies).
Management.
To evaluate evidence as to whether immediate AED treatment of an unprovoked first seizure in adults changes prognosis, we considered (1) the short-term risk for a seizure recurrence, and (2) the longer-term potential for seizure remission.
Question.
For the adult presenting with an unprovoked first seizure, does immediate treatment with an AED change the short-term (2-year) prognosis for seizure recurrence?
Evidence.
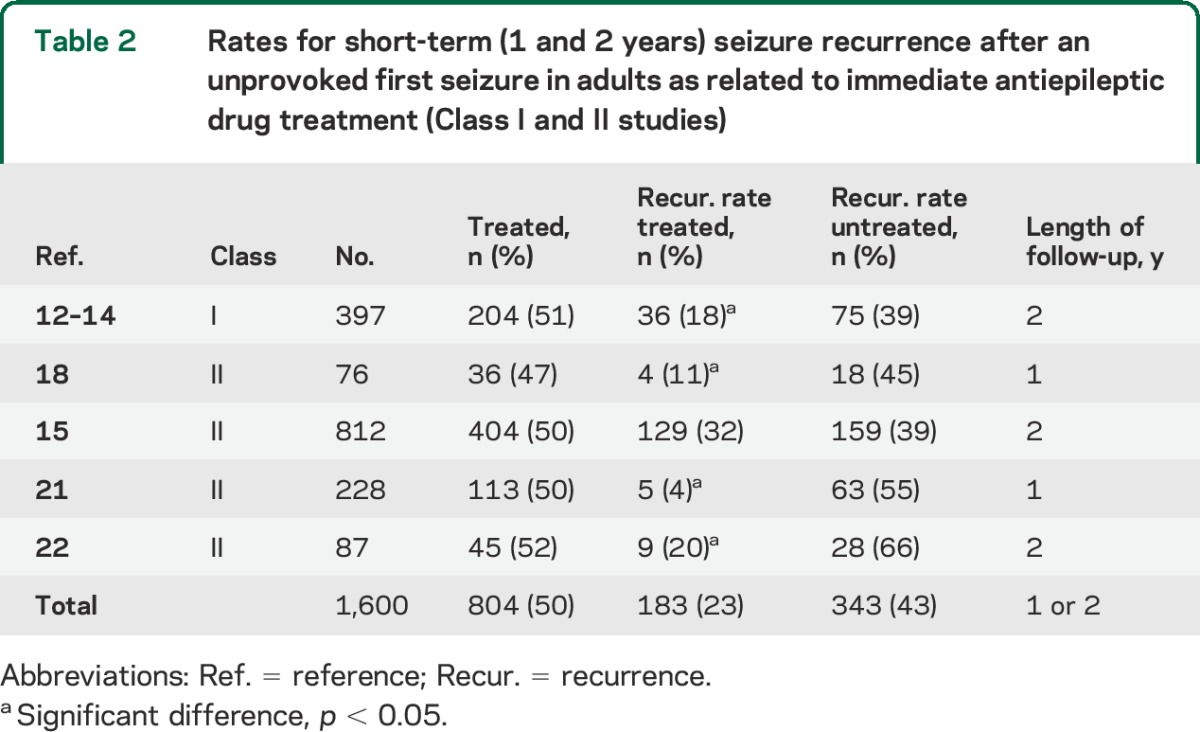
We identified 1 Class I study12–14 and 4 Class II studies15,18,21,22,26 addressing this issue (table 2). Immediate therapy with an AED after an unprovoked first seizure in adults significantly reduces seizure risk in the short term, which we define as within 2 years (figure 1). Although cumulative risk of seizure recurrence increases over time, most recurrences happen within the first year (table 1, figures 1 and 2). Immediate AED treatment convincingly reduces that risk within the first 2 years of the initial seizure, with the only Class I study and 3 of 4 Class II studies demonstrating significantly fewer seizure recurrences (table 2). A random-effects meta-analysis of data from the studies in table 2 indicates an absolute risk reduction in seizure recurrence of 35% (95% CI 23%–46%) in a comparison of immediate and delayed AED treatment for pooled 2-year data. Immediate treatment in the only Class I study meant AED therapy started within 1 week of the index seizure,12,13 whereas in 1 Class II study, it was within 1 week in 30% of subjects, by 1 month in 55%, and by 3 months in 81%.15 Details regarding what constituted immediate AED treatment were not provided in the other Class II studies.18,21,22
Table 2.
Rates for short-term (1 and 2 years) seizure recurrence after an unprovoked first seizure in adults as related to immediate antiepileptic drug treatment (Class I and II studies)
Despite this reduced risk of early seizure recurrence, the only quality of life (QOL) analysis, which is from a Class II study, demonstrates no significant differences in standard, validated 2-year QOL measures.15,26
Conclusion.
For adults presenting with an unprovoked first seizure, immediate AED therapy as compared with no treatment is likely to reduce absolute risk by about 35% for a seizure recurrence within the subsequent 2 years (1 Class I study, 4 Class II studies) but might not affect QOL (1 Class II study).
Question.
For the adult presenting with an unprovoked first seizure, does immediate treatment with an AED as compared with delay pending a seizure recurrence influence prognosis, such as the potential for seizure remission over the longer term (>3 years)?
Evidence.
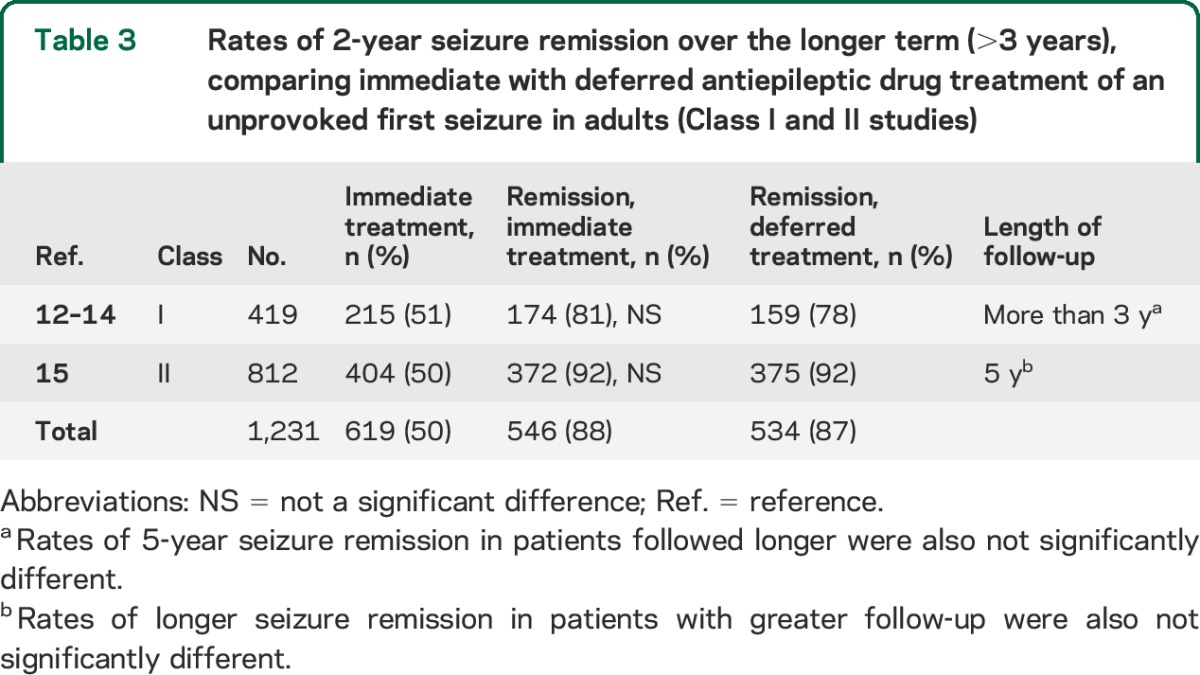
We identified 1 Class I study12–14 and 1 Class II study15 addressing this issue and longer-term prognosis (table 3). The generally accepted metric for assessing long-term outcome and prognosis is the seizure remission rate, which is a measure of maintaining seizure freedom for a specified time duration, typically 2 to 5 years.12–15 Studies demonstrate that immediate AED treatment after an unprovoked first seizure as compared with treatment delayed pending another seizure does not increase the incidence of sustained seizure remission (table 3). Long-term survival is addressed only in 1 Class III study,27 which, although limited by sample size, notes that immediate treatment does not affect mortality over a 20-year period.
Table 3.
Rates of 2-year seizure remission over the longer term (>3 years), comparing immediate with deferred antiepileptic drug treatment of an unprovoked first seizure in adults (Class I and II studies)
Conclusion.
For adults presenting with an unprovoked first seizure, immediate AED treatment as compared with treatment delayed until a second seizure occurs is unlikely to improve the chance of attaining sustained seizure remission over the longer term (>3 years) (1 Class I study, 1 Class II study).
Risks of AED treatment.
Question.
For the adult who presents with an unprovoked first seizure, what are the nature and frequency of AEs with AED treatment?
Evidence.
We identified 4 Class II studies12,15,21,22 and 1 Class III study28 addressing the nature and frequency of AEs (table e-5). These studies focused on the effects of AEDs on seizure recurrence risk and included data on medication AEs. Although those articles specifically addressed populations with unprovoked first seizures, comparable findings are reported by studies in patients with new-onset epilepsy initially treated similarly with AEDs.2,29–32
The incidence of AEs from AEDs in adults initially treated with a single AED for an unprovoked first seizure is reported to range from 7% to 31% for a variety of AEDs (table e-5). No AED-related deaths or life-threatening allergic reactions were described, but population size was limited. Reported AEs in these studies appear to be mild and reversible when an affected patient is switched to another AED.13,15 AEDs included phenytoin, phenobarbital, carbamazepine, valproic acid, and lamotrigine,12,15,21,22,28 some of which may now be considered older AEDs but were standard, commonly used AEDs at the time of the studies.30 Although AEDs may cause different AEs, most events are known to be dose-related and reversible through dose reduction or discontinuation of the responsible drug.30,31
Evidence from reports of AED AEs in comparable patient populations with epilepsy, some treated with newer drugs, supports low AED risks.30 For example, a study of patients with new-onset epilepsy found that initial AED monotherapy with either phenytoin or topiramate was associated with AEs leading to AED discontinuation in only 7% to 13% of patients.32 Another study in a mixed group of patients with epilepsy initially treated with AEDs found that AEs were no more likely to occur than in untreated controls and proposed that this was because the drugs were typically started as monotherapy and at low doses.31
Conclusion.
For adults with an unprovoked first seizure immediately treated with AEDs, studies of the nature and incidence of AEs indicate a wide range of predominantly mild and reversible AEs that occur in approximately 7% to 31% of patients (4 Class II studies, 1 Class III study).
RECOMMENDATIONS
Adults presenting with an unprovoked first seizure should be informed that the chance for a recurrent seizure is greatest within the first 2 years after a first seizure (21%–45%) (Level A).
Clinicians should also advise such patients that clinical factors associated with an increased risk of seizure recurrence include a prior brain insult such as a stroke or trauma (Level A), an EEG with epileptiform abnormalities (Level A), a significant brain-imaging abnormality (Level B), or a nocturnal seizure (Level B).
Clinicians should advise patients that, although immediate AED therapy, as compared with delay of treatment pending a second seizure, is likely to reduce the risk of a seizure recurrence in the 2 years subsequent to a first seizure (Level B), it may not improve QOL (Level C).
Clinicians should advise patients that over the longer term (>3 years), immediate AED treatment is unlikely to improve the prognosis for sustained seizure remission (Level B).
Patients should be advised that their risk for AED AEs ranges from 7% to 31% (Level B) and that these AEs are predominantly mild and reversible.
CLINICAL CONTEXT
For an adult with a first seizure, the risk of a recurrence poses major concerns and raises the question of whether immediate AED treatment is advisable.33,34 It is a proposed and now generally accepted principle that when a patient with a first seizure has one or more ensuing seizures, an AED should be initiated because the risk of yet additional seizures is very high (57% by 1 year and 73% by 4 years), with risk increasing proportionally after each subsequent recurrence as the time interval between seizures decreases.33 In contrast, immediate AED treatment at the time of the first unprovoked seizure is not well accepted and is debated.34,35
For a patient with a first unprovoked seizure, the chance for a seizure recurrence can be estimated and stratified on the basis of clinical factors, with greater risk associated with a prior brain insult or lesion as the cause of the seizure, an EEG with epileptiform abnormalities, a significant brain-imaging abnormality, or a nocturnal seizure.3,16,17,23,24 Such risk stratification may help guide physicians counseling patients about their risks for seizure recurrence and options for management. In some instances, a patient's statistical risk for a seizure recurrence may approach that of patients for whom immediate AED treatment is generally accepted, such as those who have already experienced multiple seizures.12,13,15 A recent report from the International League Against Epilepsy (ILAE) promotes a new practical clinical definition of epilepsy that emphasizes the importance of estimating recurrence risk for individuals with a first unprovoked seizure.34 The ILAE expanded the diagnosis of epilepsy beyond the prior standard requiring at least 2 unprovoked seizures to encompass people with an unprovoked seizure and a high (at least 60%) risk of seizure recurrence over the subsequent 10 years. However, as our analysis indicates and the ILAE cautions, the lack of evidence regarding specific risk factors and their interactions poses limitations.
Some of these risk factors may be independent predictors for risk of recurrence, whereas others (e.g., a prior brain lesion as a seizure cause, or a brain-imaging abnormality) likely are related.3,8,12,23,24 The relatively small numbers of subjects in studies addressing this issue limit the strength of evidence.2,3,34 Only 2 studies analyzed evidence specifically regarding additive effects or covariance of the risk factors for seizure recurrence after a first seizure, and reached somewhat different conclusions. One study noted that the only independent risk factor for seizure recurrence was an EEG with epileptiform abnormalities,12 and the other reported a remote symptomatic seizure etiology as the only independent risk factor.20 Because of this lack of evidence, caution is urged regarding the calculation of additive risk of seizure recurrence after a first unprovoked seizure. The ILAE report states as much: “No formula can be applied for additive risks since data are lacking on how such risks combine; such risks will have to be decided by individualized considerations.”34 Such caution also applies to decisions in AED treatment.34
Indications for immediate AED treatment are based largely but not only on estimations of an individual's risk of a seizure recurrence.33,34 Physicians planning to prescribe an AED for treatment should also carefully consider the drug's specific therapeutic and AE profiles on an individualized basis.30 Evidence indicates that immediate AED therapy is likely to reduce seizure recurrence risk for individuals with an unprovoked first seizure, particularly within the first 2 years. Such seizure recurrence prevention, even in the short term, may be important, with potentially greater implications for adults than for children. For adults, seizure recurrences may cause such serious psychological and social consequences as loss of driving privileges and limitations on employment.2 Still, one controlled Class II study comparing immediate AED treatment with treatment deferred until after a seizure recurrence found no significant difference in standard 2-year QOL measures. However, that study also noted that patients who were not immediately treated with AEDs were more likely to be restricted from driving.26
The longer-term prognosis for patients with a first seizure as measured by whether patients maintain seizure freedom demonstrates no benefit for immediate AED treatment.13,15 Moreover, although individual seizure recurrences pose some risk for physical harm and even death,2,15 there is no evidence that immediate AED treatment reduces that risk or improves QOL.12,14,15,26 Also, the only study appraising the incidence of sudden unexplained death after an unprovoked first seizure demonstrates no advantage with immediate AED therapy.15
FUTURE RESEARCH RECOMMENDATIONS
For patients with a first seizure, rigorous, well-designed studies analyzing patient management techniques, interventions, and counseling that focus on objective outcomes, such as QOL, are limited and needed.2 For example, it would be helpful to know when, how, and by whom a patient would be best advised regarding driving laws and other social issues such as employment. Such matters also may guide a patient's personal preference for AEDs. One study noted that only 21% of all patients with first seizures received correct advice about driving limitations.35 Also, further studies of patient preferences, psychosocial factors, and QOL measures are encouraged and should be incorporated into decision-making and guideline development.2,34,36
The issue of exactly how to use complicated risk data of recurrences and seizure remission to guide management is a question that warrants further research and clarification.34 Predictive statistical models to analyze such risks, although complex and difficult, have been demonstrated to be feasible and potentially useful.25,37 These types of predictive models and analyses would benefit from additional data on the extent, potential additive effects, and timing of seizure recurrence risks associated with specific clinical variables, such as seizure etiology, EEG findings, and brain imaging. It would also be important to determine the degree to which AED treatment may influence the risk of seizure recurrence for each of those clinical factors taken individually or together.
There also is a need for better and more focused research on the nature and risks of AEs with AED treatment for patients with an initial seizure. The existing studies of AED AEs for such patients report results using mostly older AEDs (table e-5), but newer AEDs may have fewer and different AEs.2,30 Therefore, updated studies utilizing newer AEDs for initial therapy are warranted and encouraged for this and comparable patient populations.30
Research on AED discontinuation in patients with a first unprovoked seizure or recurrence who receive AEDs is also lacking. It is important for patients to appreciate how long they may need to be on an AED once it has been started and the risks of AED discontinuation, as this type of information may help guide a patient's decision-making about AED initiation. There are some data on such matters in mixed groups of patients with various epilepsy or seizure types who have become seizure-free and who have discontinued AEDs; however, further studies are warranted because these data may not apply to individuals who experience only an initial seizure.38
Supplementary Material
GLOSSARY
- AAN
American Academy of Neurology
- AE
adverse event
- AED
antiepileptic drug
- CI
confidence interval
- ILAE
International League Against Epilepsy
- QOL
quality of life
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Allan Krumholz: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision. Sam Wiebe: acquisition of data, analysis or interpretation of data, critical revision of the manuscript for important intellectual content. Gary Gronseth: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. David Gloss: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Ana Sanchez: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Arif Kabir: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Aisha Liferidge: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Justin Martello: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Andres Kanner: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Shlomo Shinnar: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Jennifer Hopp: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Jacqueline French: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This guideline was developed with financial support from the American Academy of Neurology.
DISCLOSURE
A. Krumholz serves on the editorial board for Clinical EEG and Neuroscience, and has received royalties from UpToDate. S. Wiebe has received research funding from the Alberta Heritage Medical Research Foundation, the Canadian Institutes for Health Research, the M.S.I. Foundation of Alberta, and the Hotchkiss Brain Institute of the University of Calgary. G. Gronseth reports no disclosures relevant to the manuscript. D. Gloss is a paid evidence-based medicine consultant for the American Academy of Neurology. A. Sanchez, A. Kabir, A. Liferidge, and J. Martello report no disclosures relevant to the manuscript. A. Kanner serves as a journal editor for Epilepsy Currents and as a regional editor for Epileptology; serves on the editorial boards of Epilepsy & Behavior and CNS Spectrums; and has received royalties for Psychiatric Issues in Epilepsy, Second Edition: A Practical Guide to Diagnosis and Treatment; Psychiatric Controversies in Epilepsy; and Depression in Neurologic Disorders. S. Shinnar has served on scientific advisory boards for Acorda, Questcor, and Upsher-Smith; has received royalties for Febrile Seizures and honoraria from Questcor, UCB, and Upsher-Smith; has received research funding from the National Institute of Neurological Disorders and Stroke and the Citizens United for Research in Epilepsy Foundation; and has given expert testimony. J. Hopp has received royalties from publishing from UpToDate and honoraria from lectures for UCB Pharma, has served on speakers bureaus for UCB Pharma and GlaxoSmithKline, and has given expert testimony. J. French has served as a consultant for Acorda, Biotie, Eisai Medical Research, GlaxoSmithKline, Impax, Johnson & Johnson, LCGH, Marinus, Novartis, Pfizer, Sunovion, SK Life Science, Supernus Pharmaceuticals, UCB, Upsher-Smith, and Vertex; has received grants from Eisai Medical Research, Epilepsy Research Foundation, Epilepsy Study Consortium, Epilepsy Therapy Project, Lundbeck, Pfizer, and UCB; and is president of the Epilepsy Study Consortium. All consulting is done on behalf of the Consortium, and fees are paid to the Consortium. New York University receives salary support from the Consortium. Go to Neurology.org for full disclosures.
DISCLAIMER
Clinical practice guidelines, practice advisories, systematic reviews, and other guidance published by the American Academy of Neurology and its affiliates are assessments of current scientific and clinical information provided as an educational service. The information: (1) should not be considered inclusive of all proper treatments, methods of care, or as a statement of the standard of care; (2) is not continually updated and may not reflect the most recent evidence (new evidence may emerge between the time information is developed and when it is published or read); (3) addresses only the question(s) specifically identified; (4) does not mandate any particular course of medical care; and (5) is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. AAN provides this information on an “as is” basis, and makes no warranty, expressed or implied, regarding the information. AAN specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. AAN assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information or for any errors or omissions.
CONFLICT OF INTEREST
The American Academy of Neurology (AAN) and the American Epilepsy Society (AES) are committed to producing independent, critical, and truthful clinical practice guidelines (CPGs). Significant efforts are made to minimize the potential for conflicts of interest to influence the recommendations of this CPG. To the extent possible, the AAN and AES keep separate those who have a financial stake in the success or failure of the products appraised in the CPGs and the developers of the guidelines. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee before project initiation. AAN and AES limit the participation of authors with substantial conflicts of interest. The AAN and AES forbid commercial participation in, or funding of, guideline projects. Drafts of the guideline have been reviewed by at least 3 AAN committees, at least one AES committee, a network of neurologists, Neurology peer reviewers, and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at www.aan.com. For complete information on this process, access the 2004 AAN process manual.9
REFERENCES
- 1.Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia 2008;49(suppl 1):8–12. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) Committee on the Public Health Dimensions of the Epilepsies; England MJ, Livermari CT, Schultz AM, Strawbridge LM, editors. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 3.Krumholz A, Wiebe S, Gronseth G, et al. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007;69:1996–2007. [DOI] [PubMed] [Google Scholar]
- 4.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 2000;41:342–351. [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 1993;34:592–596. [DOI] [PubMed] [Google Scholar]
- 6.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CORALE study. Coordination Active du Réseau Observatoire Longitudinal de l'Epilepsie. Epilepsia 2001;42:464–475. [DOI] [PubMed] [Google Scholar]
- 7.Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009;50:1102–1108. [DOI] [PubMed] [Google Scholar]
- 8.Hirtz D, Berg A, Bettis D, et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2003;60:166–175. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Neurology. Clinical Practice Guideline Process Manual, 2004 ed St. Paul, MN: American Academy of Neurology; 2004. [Google Scholar]
- 10.Hauser WA, Anderson VE, Loewenson RB, McRoberts SM. Seizure recurrence after a first unprovoked seizure. N Engl J Med 1982;307:522–528. [DOI] [PubMed] [Google Scholar]
- 11.Hauser WA, Rich SS, Annegers JF, Anderson VE. Seizure recurrence after a 1st unprovoked seizure: an extended follow-up. Neurology 1990;40:1163–1170. [DOI] [PubMed] [Google Scholar]
- 12.First Seizure Trial Group (FIR.S.T. Group). Randomized clinical trial on the efficacy of antiepileptic drugs in reducing the risk of relapse after a first unprovoked tonic-clonic seizure. Neurology 1993;43:478–483. [DOI] [PubMed] [Google Scholar]
- 13.Musicco M, Behgi E, Solari A, Viani F; First Seizure Trial Group (FIRST Group). Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. Neurology 1997;49:991–998. [DOI] [PubMed] [Google Scholar]
- 14.Leone MA, Solari A, Beghi E; FIRST Group. Treatment of the first tonic-clonic seizure does not affect the long-term remission of epilepsy. Neurology 2006;67:2227–2229. [DOI] [PubMed] [Google Scholar]
- 15.Marson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D; Medical Research Council MESS Study Group. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 2005;365:2007–2013. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins A, Garman A, Clarke C. The first seizure in adult life: value of clinical features, electroencephalography, and computerised tomographic scanning in prediction of seizure recurrence. Lancet 1988;1:721–726. [DOI] [PubMed] [Google Scholar]
- 17.Bora I, Seckin B, Zarifoglu M, Turan F, Sadikoglu S, Ogul E. Risk of recurrence after first unprovoked tonic-clonic seizure in adults. J Neurol 1995;242:157–163. [DOI] [PubMed] [Google Scholar]
- 18.Das CP, Sawhney IM, Lal V, Prabhakar S. Risk of recurrence of seizures following a single unprovoked idiopathic seizure. Neurol India 2000;48:357–360. [PubMed] [Google Scholar]
- 19.Annegers JF, Shirts SB, Hauser WA, Kurland LT. Risk of recurrence after an initial unprovoked seizure. Epilepsia 1986;27:43–50. [DOI] [PubMed] [Google Scholar]
- 20.Kho LK, Lawn ND, Dunne JW, Linto J. First seizure presentation: do multiple seizures within 24 hours predict recurrence? Neurology 2006;67:1047–1049. [DOI] [PubMed] [Google Scholar]
- 21.Chandra B. First seizure in adults: to treat or not to treat. Clin Neurol Neurosurg 1992;94(suppl):S61–S63. [DOI] [PubMed] [Google Scholar]
- 22.Gilad R, Lampl Y, Gabbay U, Eshel Y, Sarova-Pinchas I. Early treatment of a single generalized tonic-clonic seizure to prevent recurrence. Arch Neurol 1996;53:1149–1152. [DOI] [PubMed] [Google Scholar]
- 23.Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 1991;41:965–972. [DOI] [PubMed] [Google Scholar]
- 24.Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia 2008;49(suppl 1):13–18. [DOI] [PubMed] [Google Scholar]
- 25.Hui AC, Tang A, Wong KS, Mok V, Kay R. Recurrence after a first untreated seizure in the Hong Kong Chinese population. Epilepsia 2001;42:94–97. [DOI] [PubMed] [Google Scholar]
- 26.Jacoby A, Gamble C, Doughty J, Marson A, Chadwick D; Medical Research Council MESS Study Group. Quality of life outcomes of immediate or delayed treatment of early epilepsy and single seizures. Neurology 2007;68:1188–1196. [DOI] [PubMed] [Google Scholar]
- 27.Leone MA, Vallalta R, Solari A, Beghi E; FIRST Group. Treatment of a first tonic-clonic seizure does not affect mortality: long-term follow-up of a randomised clinical trial. J Neurol Neurosurg Psychiatry 2011;82:924–927. [DOI] [PubMed] [Google Scholar]
- 28.Ruggles KH, Haessly SM, Berg RL. Prospective study of seizures in the elderly in the Marshfield Epidemiologic Study Area (MESA). Epilepsia 2001;42:1594–1599. [DOI] [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality (AHRQ). Management of Newly Diagnosed Patients with Epilepsy: A Systematic Review of the Literature (AHRQ Publication No. 01-E038). Rockville, MD: Agency for Health Research Quality, U.S. Department of Health and Human Services; 2001. [Google Scholar]
- 30.French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2004;62:1252–1260. [DOI] [PubMed] [Google Scholar]
- 31.Perruca P, Jacoby A, Marson AG, et al. Adverse antiepileptic drug effects in new-onset seizures: a case control study. Neurology 2011;76:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay E, Faught E, Krumholz A, et al. ; CAPSS-272 Study Group. Efficacy, tolerability, and safety of rapid initiation of topiramate versus phenytoin in patients with new-onset epilepsy: a randomized double-blind clinical trial. Epilepsia 2010;51:1970–1977. [DOI] [PubMed] [Google Scholar]
- 33.Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med 1998;338:429–434. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RS, Acevedo C, Arzimanoglou A, et al. A practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- 35.Edmondstone WM. How do we manage the first seizure in adults? J R Coll Physicians Lond 1995;29:289–294. [PMC free article] [PubMed] [Google Scholar]
- 36.Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA 2008;300:436–438. [DOI] [PubMed] [Google Scholar]
- 37.Kim LG, Johnson TL, Marson AG, Chadwick DW; MRC MESS Study Group. Prediction of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurol 2006;5:317–322. [DOI] [PubMed] [Google Scholar]
- 38.Bonnett LJ, Shukralla A, Tudur-Smith C, Williamson PR, Marson AG. Seizure recurrence after antiepileptic drug withdrawal and the implications for driving: further results from the MRD Antiepileptic Drug Withdrawal Study and a systematic review. J Neurol Neurosurg Psychiatry 2011;82:1328–1333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.