Abstract
A light-regulated adenylyl cyclase, mPAC, was previously identified from the cyanobacterium Microcoleus chthonoplastes PCC7420. MPAC consists of a flavin-based blue light-sensing LOV domain and a catalytic domain. In this work, we expressed mPAC in an adenylate cyclase A null mutant (aca-) of the eukaryote Dictyostelium discoideum and tested to what extent light activation of mPAC could restore the cAMP-dependent developmental programme of this organism. Amoebas of Dictyostelium, a well-established model organism, generate and respond to cAMP pulses, which cause them to aggregate and construct fruiting bodies. mPAC was expressed under control of a constitutive actin-15 promoter in D. discoideum and displayed low basal adenylyl cyclase activity in darkness that was about five-fold stimulated by blue light. mPAC expression in aca- cells marginally restored aggregation and fruiting body formation in darkness. However, more and larger fruiting bodies were formed when mPAC expressing cells were incubated in light. Extending former applications of light-regulated AC, these results demonstrate that mPAC can be used to manipulate multicellular development in eukaryotes in a light dependent manner.
Keywords: Cyclic AMP, flavin chromophore, light-regulated enzyme activity, LOV domain, optogenetics, photoreceptors
INTRODUCTION
The second messenger molecule adenosine 3′,5′-cyclic monophosphate (cAMP) mediates a broad range of cellular responses to environmental stimuli and secreted signals in all domains of life (Beavo and Brunton, 2002). cAMP plays a particularly large role in the life cycle of the social amoeba Dictyostelium discoideum. Here cAMP not only acts as an intracellular second messenger, but is also secreted as a chemoattractant by the cells to coordinate cell movement and to induce prespore cell differentiation. Social amoebas live in forest soil feeding on bacteria and move together in aggregates when their food source is depleted. The aggregate goes through a number of shape changes forming at first a migrating “slug” and next an erect fruiting body. During this process, the amoebas differentiate into spores and stalk cells. All Dictyostelids use secreted cAMP pulses to coordinate cell movement during fruiting body morphogenesis (Alvarez-Curto et al., 2005), but some species, such as D. discoideum also use cAMP pulses to coordinate aggregation (Tomchik and Devreotes, 1981). The cAMP pulses are generated by adenylyl cyclase A, which is controlled by both positive and negative feedback of cAMP binding to cell surface cAMP receptors (Saran et al., 2002). The prominent role of cAMP has been demonstrated using D.discoideum mutants that lack ACA (aca-) and consequently are completely defective in aggregation and fruiting body formation (Pitt et al., 1992). Adenylyl cyclases have also been identified in recent years as signaling domains in biological photoreceptors. Light – dark transitions regulate physiology and/or behaviour of most organisms, but are particularly important for photosynthetic organisms, such as photosynthetic bacteria, algae and plants. Ambient light conditions therefore have to be detected and processed by the organism in order to induce the appropiate adjustments of its lifestyle. In some photoreceptors, enzyme activities, fused to the light-sensing domain, are employed to translate the photophysical/photochemical event into a biological signal. Whereas the majority of enzyme functions identified so far in photoreceptors are histidine kinases acting together with response regulators in the well-characterized two component signalling system (Wolanin et al., 2002), nucleotide cyclases constitute still rare cases. MPAC (Microcoleus Photoactivated Adenylate Cyclase) is a blue light-photoreceptor composed of a light sensing LOV domain (LOV, light, oxygen, voltage) and a photoactivated adenylyl cyclase that was isolated from the cyanobacterium Microcoleus chthonoplastes. The adenylate cyclase domain displays low basal activity in darkness that was amplified 30-fold after blue light irradiation and returned to basal levels after about 14 s in darkness (Raffelberg et al., 2013). These properties make mPAC a potentially useful tool for studying cellular responses to acute inducible increases of cAMP. The non-invasive regulation of enzyme activities by light makes biological photoreceptors interesting candidates for optogenetics. The functionality of PAC was already demonstrated in-vitro for heterologously expressed proteins in Xenopus laevis oocytes, and also in various in-vivo applications. Despite the fact that they were only recently discovered, PAC proteins have already been widely employed to probe regulation of cAMP-gated channels in neuronal cells (Stierl et al., 2011), investigate differentiation in zebrafish (de Marco et al., 2013), and study host-cell invasion by pathogens (Hartmann et al., 2013). Thus far, however, applications of PAC were only performed with proteins carrying a BLUF domain (BLUF, blue light-sensing using FAD). Similar to LOV domains, BLUF are also flavin-based photoreceptors, but they undergo a significantly different photochemical activity than LOV domains. The signaling state of these protein domains is generated simply by a rearrangement of the hydrogen-bonding network around the flavin chromophore, manifesting itself as a small bathochromic shift of the absorption maximum.
We here present the first application of a LOV-domain PAC in a living organism and demonstrate the capability to induce differentiation of D. discoideum, transformed with mPAC, in a light-regulated fashion. As the inherent adenylyl cyclases of Dictyostelium would interfere with such experiments, an AC-knock out mutant (D.discoideum aca-) has been used.
METHODS
Cell Lines and Culture
D.discoideum cells were cultured in HL5 medium that was supplemented with 5 μg/ml blasticidin for null mutants and with 10 to 100 μg/ml G418 for cells harbouring expression constructs. Cultures are routinely grown under full spectrum fluorescent room lights. For culture in darkness, flasks were wrapped in aluminium foil. Multicellular development was induced by incubating cells, freed from bacteria or growth medium, at 1.5 × 106 cells/cm2 on non-nutrient (NN) agar (1.5% agar in 10 mm Na/K phosphate buffer, pH 6.5) in either darkness or under white fluorescence light at about 10 μmol photons/m2/s).
MPAC Expression Construct
The full-length mPAC cDNA was excised with KpnI from a pET28a plasmid (Novagen/Merck), into which the mPAC-encoding gene had been ligated (NdeI-site at the 5′-end and SacI-site at the 3′ end) (Raffelberg et al., 2013), and inserted into the KpnI digested Dictyostelium expression vector pB17S-EYFP (Meima et al., 2002). A plasmid was selected that harboured mPAC in the proper orientation, i.e. with mPAC fused at its 5′ end to the constitutively active A15 promoter and at its 3′ end to an enhanced yellow fluorescent protein (YFP) tag. This vector, act15::mPAC-YFP, was transformed into both aca- cells and acarg- cells in which all three Dictyostelium adenylyl cyclases ACA, AcrA and ACG had been sequentially knocked out by homologous recombination (Z.-H. Chen, unpublished results).
Cellular cAMP assay
Cells grown in darkness were developed on NN agar for 10 h in dark or light and harvested in PB (10 mm Na/K phosphate buffer, pH 6.5). Pellets of 108 cells were resuspended in 200 μl of PB and lysed with 300 μl 3.5% (v/v) HClO4. The lysates were neutralised by the addition of 150 μl 50% saturated KHCO3 and 200 μl of cAMP-assay buffer (4 mM EDTA in 150 mM Na phosphate pH 7.5). After 5 min of centrifugation at 3000 × g, cAMP was assayed in 40 μl of the supernatant fraction by isotope-dilution assay (Gilman and Murad, 1974), using purified protein kinase A regulatory subunit (PKA-R) from beef muscle as a cAMP-binding protein and [2,8-3H]cAMP as the competitor.
Adenylyl cyclase assay
Cells grown in darkness were harvested during exponential growth, washed once with PB and resuspended in lysis buffer (2 mm MgCl2 and 250 mm sucrose in 10 mm Tris, pH 8.0) to 108 cells/ml. Cells were lysed by passage through 3-μm pore size Whatman® nuclepore filters. Aliquots of 10 μl cell lysate were added to 10 μl of 2× assay mix (1 mm ATP, 16 mm MgCl2, 0.4 mm 3-isobutyl-1-methylxanthine (IBMX), and 20 mm dithiothreitol (DTT) in lysis buffer) (Chen et al., 2010). IBMX and DTT are inhibitors for the Dicytostelium cAMP phosphodiesterases RegA and PdsA, respectively. After 5 min of incubation on ice in darkness, reactions were started by transferring samples into a 22 °C water bath and incubating them in darkness or under blue light irradiation, provided by a Decospot® LED GU10. Reactions were terminated after 0, 5, 10 and 20 min by addition of 10 μl of 0.4 M EDTA (pH 8.0), followed by boiling for 1 min. cAMP was assayed directly in the boiled lysate by isotope dilution assay (Gilman and Murad, 1974).
RESULTS AND DISCUSSION
Light activated mPAC activity in Dictyostelium
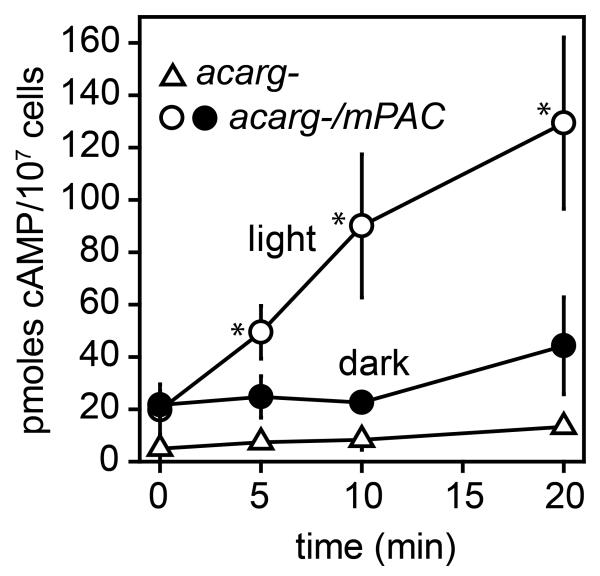
We first investigated whether mPAC showed basal and light-activated adenylyl cyclase activity when expressed in D. discoideum cells. A construct in which mPAC was fused at the N-terminus to the constitutive actin 15 promoter and at the C-terminus to YFP (act15::mPAC-YFP) was transformed into the D. discoideum acarg- mutant from which the three endogenous adenylyl cyclase genes AcaA, AcrA and AcgA had been deleted (Z.-H. Chen and P. Schaap, manuscript in preparation). Lysates of acarg-/act15::mPAC-YFP cells were incubated in darkness or under blue light irradiation with ATP/Mg2+ and PDE inhibitors for 20 min, and accumulated cAMP levels were measured after 5, 10 and 20 minutes. In darkness cAMP levels increased slowly from 20 to 40 pmoles cAMP/107 cells during 20 min of incubation (Figure 1). Under blue light irradiation, cAMP accumulation was much more rapid and reached 130 pmol/107 cells after 20 min. These data confirm the formerly reported constitutive activity of mPAC in the dark (Raffelberg et al., 2013) and its blue light mediated activity increase. However, the observed 5-fold stimulation of mPAC by blue light in Dictyostelium is rather low compared to the 30-fold stimulation observed in M. chthonoplastes. This is probably due to the activity of the cAMP-phosphodiesterase PdeE, which is directly activated by cAMP (Meima et al., 2003). RegA, another cAMP phosphodiesterase that is indirectly activated by cAMP (Maeda et al., 2004) should be inhibited in the in vitro assay by IBMX, but may reduce light-induced cAMP levels in intact cells.
Figure 1. mPAC activity in D.discoideum acarg- cell lysates.
Lysates of vegetative acarg- and acarg-/A15::mPAC-YFP cells were incubated with ATP/Mg2+ and cAMP phosphodiesterase inhibitors for 20 minutes at 22°C in either darkness or under blue light irradiation for acarg-/A15::mPAC_YFP and only blue light for acarg-. Accumulated cAMP levels were measured at the indicated time points by isotope dilution assay. Means and SD of two experiments performed in triplicate are presented. An asterix denotes a significant difference between cAMP levels in light and dark samples at P<0.002, as determined by a rank sum test (Mann and Whitney, 1947).
Developmental rescue of aca- by mPAC
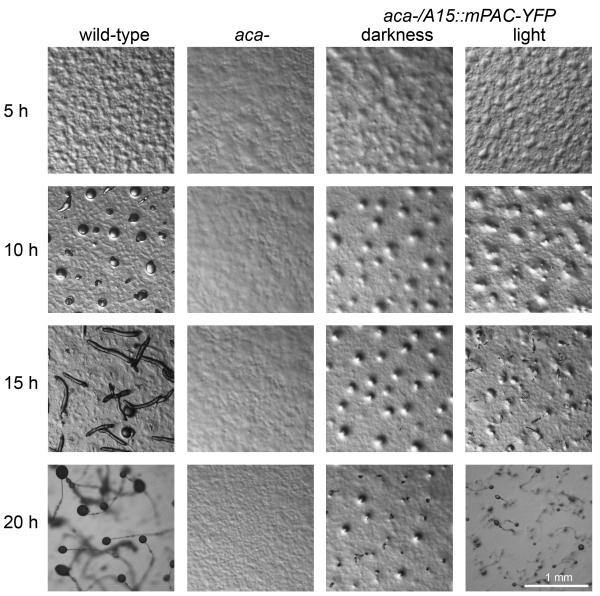
Expression of act15::mPAC-YFP did not fully restore development of the acarg- mutant. Small aggregates with projecting finger-like structures were formed, but these structures never progressed to form fruiting bodies. We therefore transformed the construct in a mutant that only lacked the aggregative adenylyl cyclase ACA (Pitt et al., 1992). Wild-type, aca- and aca- /act15::mPAC-YFP were plated on non-nutrient agar and incubated either in darkness or under full spectrum fluorescent room lights at 22 °C. Under these conditions wild-type cells have aggregated after 10 hours and formed fruiting bodies after 20 hours, while the aca- cells did not aggregate at all (Fig. 2). In darkness, the aca-/act15::mPAC-YFP had formed small aggregates after 15 h, from which some tiny fruiting bodies started to emerge after 20 hours. Light did not markedly improve aggregation of the aca-/act15::mPAC-YFP cells, but fruiting bodies emerged about 5 hours earlier and were considerably larger than when cells were kept in darkness. This experiment shows that the constitutive activity of mPAC can restore aggregation and further development to some extent, but that the light-activated activity particularly improves fruiting body formation. During wild-type development ACA synthesizes cAMP in pulses with 6 min intervals, and we therefore also attempted to restore development of the aca-/act15::mPAC-YFP with 6 s pulses of blue light at 6 min intervals. However, light pulses did not cause aggregation (data not shown). This could be due to a light pulse causing all cells to synthesize a cAMP pulse at once, depriving the cAMP signal of directionality. ACA mediated oscillations normally first emerge in a few cells in the starving population and are propagated through the population by cAMP-induced ACA activation, known as cAMP relay (Tomchik and Devreotes, 1981). Mixing in 0.1 % aca-/act15::mPAC-YFP with aca- cells also did not cause aggregation (data not shown), most likely because there is no cAMP relay.
Figure 2. Complementation of a D.discoideum aca- mutant by mPAC.
Wild-type D.discoideum, aca- and aca-/A15::mPAC-YFP cells were plated on non-nutrient agar at a density of 1.5×106 cells/cm2 and incubated under ambient room lighting or in darkness as indicated. Developmental progression was recorded at 5 h intervals. For wild-type and aca- cells, development or lack thereof was the same in darkness or in light.
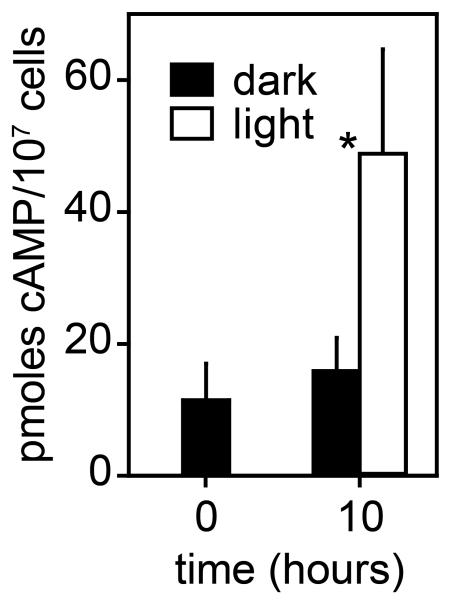
In addition to extracellular cAMP acting on cAMP receptors, Dictyostelium development also strongly depends on PKA activation by intracellular cAMP (Saran et al., 2002), and development of the aca- mutant was previously shown to be partially rescued by PKA overexpression (Wang and Kuspa, 1997). The partial recovery of development in the aca-/act15::mPAC-YFP cells may therefore be due to increased intracellular cAMP levels and PKA activation rather than restoration of chemotactic signalling. To assess whether blue light increased cellular cAMP levels after prolonged stimulation, we measured cell-associated cAMP levels in the acarg-/act15::mPAC-YFP cells after 10 h of incubation in darkness and light. When acarg-/act15::mPAC-YFP cells develop under dark conditions for 10 h, cell associated cAMP levels increased from 11 to 16 pmoles/107 cells, while under continuous blue light irradiation cAMP increases from 11 to 49 pmols/107 cells (Figure 3). These data demonstrate that mPAC causes both a constitutive and light-induced increase in cellular cAMP levels, which is probably the cause of improved developmental rescue of the aca- mutant in the light.
Figure 3. Cell-associated cAMP levels in mPAC transformed cells.
acarg-/A15::mPAC-YFP cells were cultured in darkness, then harvested and incubated for 10 h on non-nutrient agar in darkness or under blue light irradiation. At 0 and 10 h, cells were lysed in perchloric acid and cAMP was measured in the neutralized cell lysates. Means and SD of a three experiments performed in triplicate are shown. The asterix denotes a significant difference between cAMP levels in light and dark samples at P=0.00054, as determined by a t-test.
CONCLUSION
The presented results demonstrate the functional expression of a blue light-regulated adenylyl cyclase from Microcoleus chthonoplastes mPAC in Dictyostelium discoideum cells. The demonstration of both constitutive mPAC activity in the dark and increased activity under blue light irradiation in this organism goes beyond former reports where mPAC activity was studied in frog oocytes (Raffelberg et al., 2013). In addition, the use of an organism that depends on cAMP for its developmental programme extends the application range of the light-regulated enzyme approach from not only oocytes or homogeneous cell cultures, e.g., nerve cell cultures (Stierl et al., 2011), but also to the regulation of complex morphogenetic processes, as has recently been demonstrated for bPAC in zebrafish (de Marco et al., 2013).
ACKNOWLEDGEMENT
This work was supported by grants WT090276 and WT100293 from the Wellcome Trust (Z.-h. C., P.S.). S.R. is grateful for a Ph.D. grant from the ‘Biostruct’ Initiative of the University of Düsseldorf. W.G. thanks the Max-Planck-Society for continuous financial support.
ABBREVIATIONS
- AC
adenylyl cyclase
- BLUF
blue light-sensing using FAD
- FAD
flavin-adenine-dinucleotide
- FMN
flavin-mono-nucleotide
- LOV
light-oxygen-voltage (protein domain)
- mPAC
photo-activated AC from Microcoleus chthonoplastes
- YFP
yellow fluorescent protein
Reference List
- Alvarez-Curto E, Rozen DE, Ritchie AV, Fouquet C, Baldauf SL, Schaap P. Evolutionary origin of cAMP-based chemoattraction in the social amoebae. Proc.Natl.Acad.Sci.USA. 2005;102:6385–6390. doi: 10.1073/pnas.0502238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research - still expanding after half a century. Nature Rev.Mol.Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Schilde C, Schaap P. Functional dissection of adenylate cyclase R, an inducer of spore encapsulation. J.Biol.Chem. 2010;285:41724–41731. doi: 10.1074/jbc.M110.156380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco RJ, Groneberg AH, Yeh C-M, Castillo Ramirez LA, Ryu S. Optogenetic elevation of endogenous glucocorticoid level in larval zebrafish. Front.Neural Circuits. 2013 doi: 10.3389/fncir.2013.00082. doi: 10.3389/fncir.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG, Murad F. Assay of cyclic nucleotides by receptor protein binding displacement. Methods Enzymol. 1974;38:49–61. doi: 10.1016/0076-6879(74)38010-x. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Arroyo-Olarte RD, Imkeller K, Hegemann P, Lucius R, Gupta N. Optogenetic modulationof an adenylate cyclase in Toxoplasma gondii demonstrates a requirement of the parasite cAMP for host cell invasion and stage differentiation. J.Biol.Chem. 2013;288:13705–13717. doi: 10.1074/jbc.M113.465583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Lu S, Shaulsky G, Miyazaki Y, Kuwayama H, Tanaka Y, Kuspa A, Loomis WF. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science. 2004;304:875–878. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- Mann HB, Whitney DR. On a test of whether one of 2 random variables is stochastically larger than the other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- Meima ME, Biondi RM, Schaap P. Identification of a novel type of cGMP phosphodiesterase that is defective in the chemotactic stmF mutants. Mol.Biol.Cell. 2002;13:3870–3877. doi: 10.1091/mbc.E02-05-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meima ME, Weening KE, Schaap P. Characterization of a cAMP-stimulated cAMP phosphodiesterase in Dictyostelium discoideum. J Biol Chem. 2003;278:14356–14362. doi: 10.1074/jbc.M209648200. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Milona N, Borleis J, Lin KC, Reed RR, Devreotes PN. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell. 1992;69:305–315. doi: 10.1016/0092-8674(92)90411-5. [DOI] [PubMed] [Google Scholar]
- Raffelberg S, Wang L, Gao S, Losi A, Gärtner W, Nagel G. A LOV-domain-mediated blue-light-activated adenylate (adenylyl) cyclase from the cyanobacterium Microcoleus chthonoplastes PCC 7420. Biochem.J. 2013;455:359–365. doi: 10.1042/BJ20130637. [DOI] [PubMed] [Google Scholar]
- Saran S, Meima ME, Alvarez-Curto E, Weening KE, Rozen DE, Schaap P. cAMP signaling in Dictyostelium - Complexity of cAMP synthesis, degradation and detection. J.Muscle Res.Cell Motility. 2002;23:793–802. doi: 10.1023/a:1024483829878. [DOI] [PubMed] [Google Scholar]
- Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gärtner W, Petereit L, Efetova M, Schwarzel M, Oertner TG, Nagel G, Hegemann P. Light-modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium beggiatoa. J.Biol.Chem. 2011;286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik KJ, Devreotes PN. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: A demonstration by isotope dilution-fluorography. Science. 1981;212:443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- Wang B, Kuspa A. Dictyostelium development in the absence of cAMP. Science. 1997;277:251–254. doi: 10.1126/science.277.5323.251. [DOI] [PubMed] [Google Scholar]
- Wolanin P, Thomason P, Stock J. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-10-reviews3013. reviews3013.3011 - reviews3013.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]