Abstract
Atherosclerotic renal artery stenosis (ARAS) remains a major cause of secondary hypertension and renal failure. Randomized, prospective trials show that medical treatment should constitute the main therapeutic approach in ARAS. Regardless of intensive treatment and adequate blood pressure control, however, renal and extra-renal complications are not uncommon. Yet, the precise mechanisms, accurate detection, and optimal treatment in ARAS remain elusive. Strategies oriented to early detection and targeting these pathogenic pathways might prevent development of clinical endpoints. Here, we review the results of recent clinical trials, current understanding of the pathogenic mechanisms, novel imaging techniques to assess renal damage in ARAS, and treatment options.
Keywords: Atherosclerosis, Renal artery obstruction, Ischemia, Inflammation, Hypertension
Introduction
Atherosclerotic renal artery stenosis (ARAS) (>60% lumen occlusion) is present in almost 7% of elderly people.1 Attribution of ARAS as an etiology of end-stage renal disease is often difficult, especially in patients with vascular diseases, who often have increased burden of risk factors for parenchymal renal disease.2 Nevertheless, experimental and observational cohort studies confirm that ARAS is an important contributor to renal failure and aggravating hypertension.3–5 In addition, ARAS with chronic kidney disease (CKD) poses a risk for exacerbation of cardiovascular disease and multiple long-term complications.6 Several cohort and clinical trials suggest therapeutic regimens such as angiotensin blockade and statins may slow the rate of loss of renal function over time.7,8 However, sub-groups of patients with ARAS experience rapid renal functional decline,9 although its determinants are difficult to establish.10 Several lines of evidence highlight the pathophysiological complexity contributing to renal and cardiovascular damage in ARAS, which warrant detailed examination and design of effective therapeutic strategies. Recent randomized clinical trials of renal artery revascularization showed no benefit compared to medical treatment.9,11 Among the troublesome results from these studies was an unrelenting high incidence of clinical end-point, implying that more effective strategies of screening, monitoring and treatment are needed in ARAS. While small studies reported that renal revascularization sometimes can reverse accelerated hypertension and restore kidney function, how best to identify these sub-groups and recognize the potentially “viable kidney” remains unknown. To this end, several imaging methods have been developed in an attempt to probe the post-stenotic kidney in ARAS.
This review highlights conclusions gleaned from recent clinical trials and new understanding of ARAS, as well as cutting edge imaging techniques applied for detecting and monitoring ARAS.
Recent clinical trials
Recent randomized clinical trials show that renal artery revascularization does not confer a significant benefit with respect to preservation of kidney function or prevention of adverse renal and cardiovascular events in ARAS patients. Two randomized treatment trials were published in 2009. The Stent Placement in Patients with Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) and Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trials failed to detect any benefit regarding glomerular filtration rate (GFR) decline, blood pressure (BP), renal function, mortality, or cardiovascular events.9,11 The authors concluded that renal revascularization carries substantial procedure-related complications without adding benefit compared to medical treatment. However, these studies have limitations. The ASTRAL study restricted participation to patients in whom the treating physicians was uncertain about the appropriate treatment strategy (patients who would definitely “benefit’ from renal revascularization were excluded). In addition, about 40% had a likely non-hemodynamically significant stenosis under 70%. In the STAR trial, among 64 patients allocated to stent therapy, 30% did not undergo revascularization because of non-significant lesion (under 50%) and follow-up loss. These design flaws might have underpowered the results of these trials.
The more recent Cardiovascular Outcomes in Renal Atherosclerotic Lesion (CORAL) study published in 2014 was a large, multicenter, open-label, randomized, controlled trial comparing optimal medical therapy alone to medical therapy plus stenting.12 CORAL enrolled and followed 947 patients for a median of 43 months. Optimal medical therapy included an angiotensin-receptor blocker (ARB), with or without thiazide-type diuretics, and calcium channel blocker for BP control. Participants also took antiplatelet and a lipid-lowering agent, and some were also randomized to renal revascularization. The rate of the primary composite endpoints, including death and major cardiovascular outcomes, did not differ between medical therapy alone and medical and stenting therapy (35.8% and 35.1%, respectively; hazard ratio, 0.94; 95% confidence interval, 0.76 to 1.17; p=0.58). Compared to the medical treatment group, a lower systolic BP was observed in the stented group, but the number of antihypertensive medications did not differ between the two groups. The authors concluded that renal vascularization with stenting does not have a significant benefit for prevention of clinical events in ARAS patients. However, this well conducted study had some limitations. Enrollment did not require true resistant hypertension. Exclusion criteria precluded patients with recent episodes of congestive heart failure, who might have specifically benefitted from stenting. A recent meta-analysis of several ARAS treatment trials including the CORAL study showed that overall patients receiving endovascular treatment required fewer anti-hypertensive medications, but systolic BP, serum creatinine, and incident cardiovascular event rate were unaffected.13 This finding is consistent with the conclusions of previous meta-analyses.14,15
From these studies, it becomes clear that medical treatment should be the preferred management strategy for ARAS patients and that revascularization should be reserved for carefully selected subgroups of patients. However, how to select patients who stand to benefit from stenting is yet a challenge. Clearly, treatment outcomes of medically treated patients remain suboptimal. Furthermore, in recent trials, BP control was aggressive, whereas in real clinical practice, achieving and sustaining optimal BP levels are challenging. Therefore, development of innovative strategies targeting disease mechanisms activated in ARAS is critically needed.
New understanding on the pathogenesis of ARAS
ARAS involves exposure of the post-stenotic kidney to coexisting hypoperfusion, atherosclerosis, and cardiovascular risk factors, which in concert aggravate renal dysfunction and impair kidney architecture. The decline in renal perfusion and function in patients with ARAS does not correlate with the angiographic degree of stenosis,16,17 and recent data suggest that its manifestations are far more complex than previously thought. Studies with blood oxygen level-dependent (BOLD) magnetic resonance imaging (MRI) indicate the kidneys can adapt to substantial reduction in blood flow without developing tissue hypoxia,18 but extreme vascular compromise overwhelms these adaptations and leads to cortical hypoxia and microvascular injury.19 Furthermore, regional patches of hypoxia likely develop earlier, but are more difficult to detect.20,21 Well established animal models and human studies have clarified the pathogenesis of ARAS distal to the stenosis.1,22–24 ARAS not only induces hypoxia in the kidney, but also activates the renin-angiotensin-aldosterone system (RAAS), oxidative stress, inflammation, and microvascular rarefaction, eventuating in kidney failure. (Figure 1)
Figure 1.
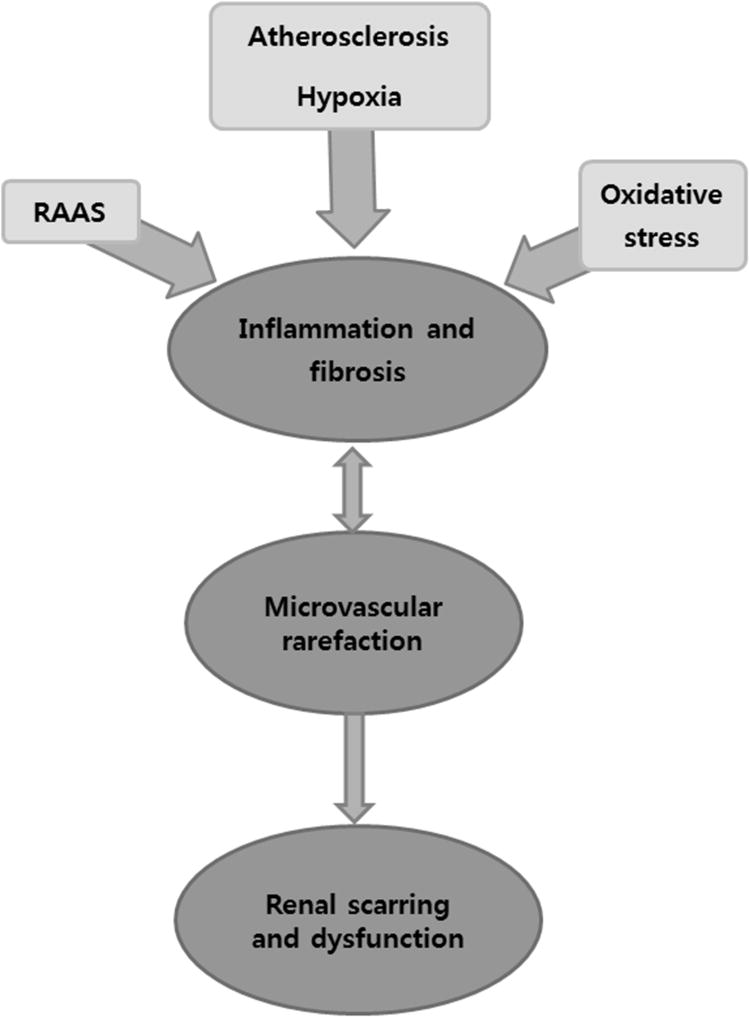
Schematic of interactive mechanisms responsible for kidney injury in atherosclerotic renal artery stenosis (ARAS). A critical renovascular occlusion activates the renin-angiotensin-aldosterone system (RAAS), elicits hypoxia, and increases oxidative stress. Together with atherosclerosis this process induces parenchymal inflammation, fibrosis, microvascular loss, and kidney dysfunction.
RAAS activation
The RAAS is one of the major control systems for BP and fluid balance. In a hemodynamically significant stenosis, reduced renal perfusion stimulates release of angiotensin II, which exerts deleterious effects such as oxidative stress, apoptosis, and inflammation.25 In turn, it activates several mechanisms that promote vascular and tissue remodeling. Angiotensin II exerts a strong stimulatory effect on transcription of profibrotic factors like transforming growth factor-ß (TGF-ß) and plasminogen activator inhibitor-1, leading to accumulation of extracellular matrix. Hence, prolonged activation of angiotensin II can directly lead to renal fibrosis. Angiotensin II also stimulates nuclear factor-kB (NF-kB), leading to activation of inflammatory gene transcription.26 Clinically, RAAS inhibition with either ARB or angiotensin converting enzyme inhibitors (ACEi) has a favorable impact on the kidney, which is thought to be additive to or independent of lowering BP.27 Pharmacological and genetic studies have confirmed that deleterious effects of the RAAS effects are mediated by angiotensin II type-1 receptor (AT1R).28–30 ACEi decrease production of angiotensin II, while ARB block AT1R and thereby not only blunt the deleterious effects of angiotensin II, but also allow it to bind to its type-2 receptor (AT2R), which counters the effects of AT1R by inducing vasodilation, anti-proliferation, and anti-apoptosis.25 Moreover, AT2R stimulation in this setting reduces organ damage by both inhibiting arachidonic acid synthesis and limiting activation of NF-kB.31 Recently, we demonstrated that ARBs in a swine ARAS model also preserve the microcirculation of both the kidney32 and heart.33 The role of angiotensin II in activating the immune system is also under investigation.34,35
Oxidative stress
Much evidence implicates reactive oxygen species (ROS) in the pathophysiology of ARAS.24,32,36,37 Oxidative stress, defined as an excessive production of ROS surpassing existing anti-oxidative defense mechanisms, plays a critical role in the development and progression of diabetic vascular complications, including nephropathy.38 Oxidative stress in experimental ARAS has been evidenced by a progressive increase in systemic levels of the oxidative stress markers prostaglandin F2α (PGF2α)-isoprostanes, associated with decreased endogenous radical-scavenger levels in both the stenotic and contralateral kidneys.24 Activation of the RAAS is also a major determinant of ROS production and may interfere with endogenous adaptive mechanisms of oxidative stress in ARAS.24 Physiologically, a fall in oxygen supply upregulates hypoxia-inducible-factor-1α and vascular endothelial growth-factor (VEGF) to induce new vessel formation. However, this compensatory capability can be impaired in chronic ischemia by amplified oxidative stress and inflammation.39 ROS also directly increase vascular tone, producing structural and functional impairment in the stenotic kidney, evoking monocyte and lymphocyte infiltration40, and hampering the response to revascularization.23,41 Indeed, animal experiments showed an ability to reverse these effects using anti-oxidants.23,42,43 Notably, several factors account for the consistent failure of human trials to identify a clinical benefit of antioxidants in blunting cardiovascular disease.44 First, compared to human cardiovascular disease, animal models are of relatively shorter disease duration and have minimal co-morbidities. Secondly, the benefits of antioxidants might necessitate early administration for successful prevention. Lastly, in many clinical trials, the basal redox status has not been ascertained, and supplementation of subjects without oxidative stress with high-dose antioxidants might paradoxically be harmful.
Inflammation
Mounting data highlight inflammatory cells as important mediators of irreversible kidney damage.45,46 Experimental and human studies demonstrate increased release of pro-inflammatory cytokines and decreased anti-inflammatory cytokines in post stenotic kidneys.47,48 Biopsies of human ARAS kidneys identifies altered inflammatory cell populations,49,50 and renal vein samples reveal multiple markers such as tumor necrosis factor (TNF)-α, interferon-γ, interleukin (IL)-6 and neutrophil gelatinase-associated lipocalin (NGAL), reflecting active inflammation.47,48 These observations are supported by previous studies indicating elevated systemic levels of TNF-α and IL-6 in patients22 and pigs51 with renovascular hypertension. TNF-α has been implicated in renal inflammation by inducing expression of cell adhesion molecules, monocyte chemoattractant protein (MCP-1), IL-1, IL-6, and IL-8.52 In experimental renovascular disease, upregulated expression of MCP-1 in the stenotic kidney was accompanied by inflammatory cell infiltration,40 and MCP-1 blockade decreased inflammation and fibrosis and improved renal function and microvascular density.
In contrast to activation of pro-inflammatory pathways, anti-inflammatory mechanisms are downregulated in ARAS. Renal vein levels of the anti-inflammatory cytokine IL-10 are decreased in ARAS patients compared with essential hypertension and healthy volunteers.47 Furthermore, a recent study demonstrated increased number of M1 (pro inflammatory) and decreased number of M2 (reparative) macrophages in the post stenotic swine kidney.37
Interestingly, renal injury and inflammation within the ARAS kidney persist after revascularization despite reversal of hypoxia. In patients with ARAS, stent revascularization failed to decrease inflammatory markers such as NGAL, MCP-1 and TNF-α,53 which might be linked to the failure to improve renal function compared to medical treatment.
Taken together, these findings underscore the important role of inflammation and the innate immune system in the pathogenesis of ARAS.
Fibrosis
Ongoing inflammation and hypoxia stimulate production and activity of growth factors that modulate renal tissue response to ischemia, like collagen deposition, extracellular matrix turnover, fibrosis, and defective angiogenesis.54,55 Excessive matrix accumulation finally leads to renal fibrosis, which in turn promotes irreversible damage and loss of functioning nephrons, a major pathological feature of progressive kidney disease.56 Renal fibrosis is observed in experimental and clinical ARAS, associated with vascular remodeling and microvascular rarefaction.36,50,57 TGF-ß has long been considered a key mediator in renal fibrosis and induces renal scarring. A major rise in TGF-ß was observed in the post-stenotic kidney of mice,58 and in human kidney biopsies, increased tissue TGF-ß was accompanied by CD68+ macrophage infiltration.50 TGF-ß mediates progressive renal fibrosis by stimulating extracellular matrix accumulation and inhibiting matrix metalloproteinases (MMP),59,60 likely mediated through its small-mother-against-decapentaplegic (Smad)-3 effector.61 In addition, recent studies have implicated the p38-MAPK pathway in development of renal inflammation and fibrosis.62,63 Several stimuli, including angiotensin II, ROS, TNF-α, IL-1 and TGF-ß, activate p38 to induce renal damage, and its blockade attenuates renal atrophy and fibrosis in stenotic murine kidneys.63 Therefore, treatments targeting these pathways might prevent irreversible kidney damage.
Microvascular rarefaction
Patients with ARAS exhibit intra-renal microvascular disease leading to vascular rarefaction.50,57 Prolonged periods of reduction of renal blood flow, and increased inflammation, endothelial dysfunction, oxidative stress, and fibrosis can alter the microcirculation, most prominently inducing microvascular remodeling (e.g., thickening) and rarefaction (reduced number or combined length).36,64 In an experimental swine model, 3D micro-computed tomography images obtained from the stenotic kidney of ARAS confirm significant impairment of microvascular architecture and spatial density (Figure 2). Although inflammatory mediators stimulate angiogenesis in early disease,54 new vessels may be highly permeable, allowing inflammatory cells to migrate to the interstitium, thereby aggravating renal parenchymal injury. In addition, microvascular remodeling correlates with renal fibrosis,65 which constrains vascular growth and increases tortuosity.36 Consequent microvascular rarefaction aggravates renal damage and compromises renal functional outcomes after revascularization.3
Figure 2.
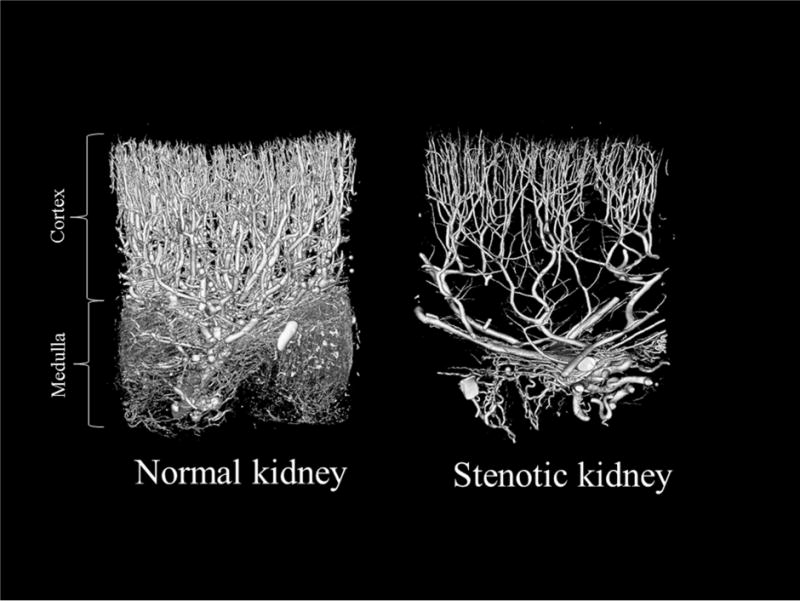
Representative 3D micro-computed tomography images from a normal (left) and a stenotic swine kidney, showing a decreased number of cortical and medullary microvessels in the hypo-perfused kidney.
New imaging techniques of ARAS
The diagnosis of ARAS often relies on imaging studies. The CORAL study enrollment criteria included Duplex Doppler Ultrasonography velocity above 300 cm/sec, with computed tomography (CT) or magnetic resonance (MR) angiography stenosis greater than 60% in one or both renal arteries.12 Because these criteria do not necessarily reflect the degree of injury in the renal parenchyma, these techniques merely evaluate the stenosis and macro-anatomy of the kidney. However, the severity of parenchymal injury is an important determinant of renal outcomes in ARAS.17 Recently emerging novel imaging tools show promise for evaluation of post-stenotic circulation and function. These might also provide individualized and unique information to stratify patients who might benefit from revascularization, medical treatment only, or new developing treatment strategies such as regenerative therapy.
Multi Detector Computed Tomography (MDCT)
During the past decade, CT angiography has become a standard noninvasive imaging modality for exploration of renal vascular anatomy and pathology. Moreover, newer applications of CT also provide reliable assessment of functional parameters of kidney function such as renal blood flow (RBF), GFR, and tubular dynamics of each kidney4,66 by recording changes in renal tissue image density, which is consequent to transit of a bolus of contrast media in the kidneys.4,67 CT derived GFR and RBF correlated with measurements obtained by intravascular Doppler and inulin clearance.4 In patients undergoing stenting, renal revascularization improved CT-measured cortical and medullary RBF and perfusion, whereas GFR remained unchanged.53 Because quantitative functional measurements can be obtained during routine clinical examination, MDCT could provide valuable functional information. However, CT is limited by the need for contrast media and by the radiation burden.
BOLD-MRI
BOLD-MR imaging is a unique noninvasive tool capable of monitoring changes in intra-renal oxygenation. The endogenous contrast agent in BOLD-MRI is paramagnetic deoxyhemoglobin, which accelerates signal decay (shortens T2*) as its concentration increases, and reciprocally increases the BOLD index, R2* (1/T2*), which correlates with tissue hypoxia (Figure 3).68 Studies in a swine model confirmed a linear relationship between measurement of renal R2* by BOLD-MRI and direct measurements of pO2 levels in the renal tissue using oxygen sensitive microelectrodes.69,70 Furthermore, BOLD-MRI can estimate the renal oxygen-dependent functional reserve using diuretics that inhibit oxygen-dependent transport and increase oxygenation. The post-stenotic pig kidney shows increased hypoxia and reduced furosemide-induced suppression of oxygen-consumption.71
Figure 3.
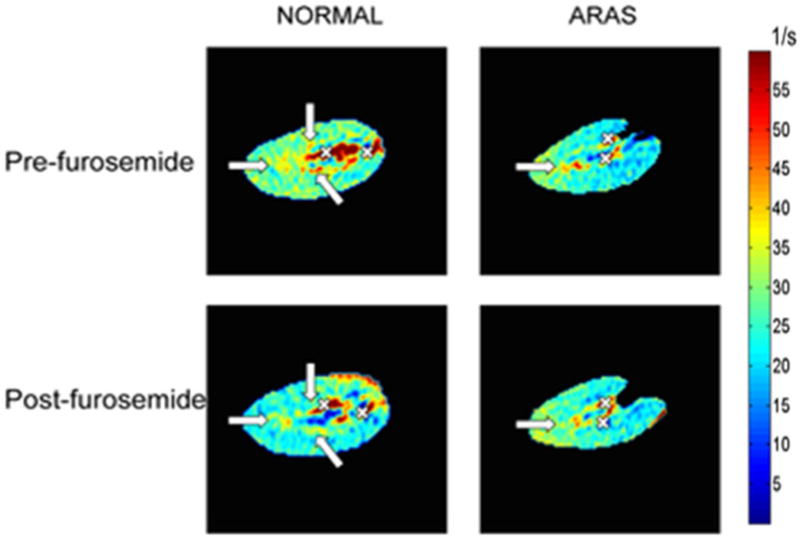
Blood oxygen-level-dependent (BOLD) parametric map in normal pigs (left) and in a swine model of atherosclerotic renal artery stenosis (ARAS) (right) obtained before and after injection of furosemide, which inhibits oxygen-dependent tubular transport and thereby increases kidney oxygenation. Arrows indicate the medullary regions, and crosses blood vessels. Medullary R2* was higher (more red) both at baseline and after furosemide in ARAS, suggesting greater hypoxia and blunted response to furosemide compared to normal pig kidneys.
Can BOLD-MRI be useful in ARAS patients? In patients with a moderate unilateral ARAS, interestingly, cortical and medullary R2* did not differ from those in patients with essential hypertension,72 but the fall in R2* after furosemide was blunted in stenotic compared to contralateral kidneys. Furthermore, kidneys with severe stenosis had elevated R2*,18 and calculation of “fractional kidney hypoxia” with BOLD-MRI demonstrated direct relationship to RBF and GFR.73 Therefore, kidneys can adapt to moderate reduction in blood flow without tissue hypoxia, but have functional impairment, whereas a more severe stenosis produces cortical hypoxia associated with vascular rarefaction.
While unquestionably powerful, application of BOLD-MRI is not routine in ARAS and the complex processes that define R2* require careful interpretation. For example, a recent study showed that BOLD-MRI failed to discriminate patients with various stages of chronic kidney disease.74 Factors such as differences in hematocrit, fibrosis, and oxygen-dependent transport activity may all affect the BOLD signal. Clearly, this technique warrants further investigation, as it may allow the clinician to quantify the severity of renal dysfunction in ARAS and predict its recovery.75
Magnetic Resonance Elastography (MRE)
MRE is an emerging MRI modality that can noninvasively quantify and visualize tissue elasticity76 using an imaging-based counterpart to palpation, commonly used in physical examination. ARAS induces CKD characterized by under-perfusion of the kidney and tissue scarring due to tubulointerstitial fibrosis.45 Renal biopsy is too invasive to employ routinely, and is not representative of the entire kidney due to sample size limitation. The main advantage of MRE is its noninvasiveness and sampling the entire kidney. Our group demonstrated that acute decreases in swine RBF without development of fibrosis led to a fall in renal cortical stiffness in a swine model.77 Consequently, in chronic renal artery stenosis, the stiffness of the stenotic cortex was unchanged despite the presence of fibrosis confirmed by histology. Presumably, a decrease in RBF offsets an increase in stiffness secondary to development of renal fibrosis. MRE of kidney allografts supported this finding.78 Contrarily, 3-dimensional MRE detected increased medullary stiffness in ARAS kidneys,76 which correlated with the histological degree of fibrosis and could potentially be used to track longitudinal ARAS progression. However, further studies are needed to examine the utility of this promising approach in humans with ARAS.
Treatment
All patients with ARAS should implement life style modification (e.g., smoking cessation, exercise, etc.) and medical treatment to prevent disease progression and complications.
Medical treatment
Hypertensive patients with ARAS should be treated with antihypertensive medications. Despite past controversy, ACEi and ARB seem to be well tolerated and effective, unless patients have contraindication to their use. Appropriate laboratory monitoring of kidney function and electrolytes while on therapy is prudent. Moreover, addition of thiazide diuretics, calcium channel blockers, and beta-blockers may be necessary to reach the target BP. The target levels of BP in the CORAL study were under 140/90 mmHg for patients without coexisting conditions and under 130/80 mmHg for patients with diabetes or chronic kidney disease.12 Current guidelines recommend BP goals of <150/90 mmHg for the general population 60 years or older, and under 140/90 mmHg for younger individuals.79 The optimal BP target for patients with ARAS has not been established. The use of statins, antiplatelet drugs, and diabetic medications is pivotal for preventing complications.
Revascularization
Although revascularization is not superior to medical treatment for BP control and prevention of cardiovascular complications, medical regimens may fail in some patients. In high-risk patients with ARAS, who were likely excluded in previous trials, revascularization should be considered. High-risk clinical presentations include recurrent episodes of heart failure or sudden unexplained pulmonary edema, acute or sub-acute kidney injury due to ACEi or ARB therapy, resistant renovascular hypertension, and rapid kidney function decline.80
Novel Treatment Strategies
Recent studies have applied cell-based therapies and novel drugs to the treatment of ARAS. Intra-renal administration of endothelial progenitor cells stimulates proliferation and maturation of new vessels, and attenuates renal microvascular remodeling and fibrosis in an experimental model.5,81 Furthermore, delivery of endothelial progenitor cells at the time of revascularization blunts inflammation and improves renal function recovery.37 Similarly, administration of adipose tissue-derived mesenchymal stem cells into the stenotic kidney restores the renal microcirculation by suppressing inflammatory cytokines and apoptosis, normalizes renal function, and decreases renal fibrosis.82
Endothelin-1 receptor blockers83, and angiogenic factors like VEGF84 or hepatocyte growth factor85, also confer renoprotective effects in the stenotic kidney. Finally, protection of mitochondria in ARAS with mitochondrial-targeted peptides preserves tissue integrity and improves hemodynamics and function in the post-stenotic kidney.43,86
Conclusion
ARAS remains an important cause of CKD, but the mechanisms underlying kidney injury in this disease have not been fully elucidated. Several pathophysiologic mechanisms have been implicated and targeted for treatment in ARAS, but clinical outcomes remain suboptimal. Current management in patients with ARAS includes antihypertensive drugs, risk factor modification, and revascularization. However, given the potential renal and cardiovascular complications, newer therapeutic interventions are needed, such as new drugs, gene, and cell-based therapies. Importantly, novel imaging tools may assist in early detection and monitoring of renal parenchymal injury and viability distal to ARAS.
Clinical summary.
Despite intensive treatment, a substantial number of patients with atherosclerotic renal artery stenosis progress to have renal and cardiovascular events.
The complex pathogenesis of atherosclerotic renal artery stenosis warrants therapeutic interventions targeting atherosclerosis, hypoxia, and inflammation.
Acknowledgments
Funding source: This article was partly supported by NIH grants DK73608, DK104273, HL121561, and DK100081.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36(3):443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 2.Guo H, Kalra PA, Gilbertson DT, et al. Atherosclerotic renovascular disease in older US patients starting dialysis, 1996 to 2001. Circulation. 2007;115(1):50–58. doi: 10.1161/CIRCULATIONAHA.106.637751. [DOI] [PubMed] [Google Scholar]
- 3.Ebrahimi B, Li Z, Eirin A, Zhu XY, Textor SC, Lerman LO. Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am J Physiol Renal Physiol. 2012;302(11):F1478–1485. doi: 10.1152/ajprenal.00563.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281(4):F630–638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 5.Chade AR, Zhu XY, Krier JD, et al. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28(6):1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vashist A, Heller EN, Brown EJ, Jr, Alhaddad IA. Renal artery stenosis: a cardiovascular perspective. Am Heart J. 2002;143(4):559–564. doi: 10.1067/mhj.2002.120769. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CM, Patel A, Shaheen N, et al. The effects of statins on the progression of atherosclerotic renovascular disease. Nephron Clin Pract. 2007;107(2):c35–42. doi: 10.1159/000107552. [DOI] [PubMed] [Google Scholar]
- 8.Chrysochou C, Foley RN, Young JF, Khavandi K, Cheung CM, Kalra PA. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27(4):1403–1409. doi: 10.1093/ndt/gfr496. [DOI] [PubMed] [Google Scholar]
- 9.Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361(20):1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 10.Valluri A, Severn A, Chakraverty S. Do patients undergoing renal revascularization outside of the ASTRAL trial show any benefit? Results of a single-centre observational study. Nephrol Dial Transplant. 2012;27(2):734–738. doi: 10.1093/ndt/gfr356. [DOI] [PubMed] [Google Scholar]
- 11.Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150(12):840–848. W150–841. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370(1):13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caielli P, Frigo AC, Pengo MF, et al. Treatment of atherosclerotic renovascular hypertension: review of observational studies and a meta-analysis of randomized clinical trials. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu072. [DOI] [PubMed] [Google Scholar]
- 14.Shetty R, Biondi-Zoccai GG, Abbate A, Amin MS, Jovin IS. Percutaneous renal artery intervention versus medical therapy in patients with renal artery stenosis: a meta-analysis. EuroIntervention. 2011;7(7):844–851. doi: 10.4244/EIJV7I7A132. [DOI] [PubMed] [Google Scholar]
- 15.Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J. 2011;161(3):622–630. e621. doi: 10.1016/j.ahj.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49(3):846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 17.Suresh M, Laboi P, Mamtora H, Kalra PA. Relationship of renal dysfunction to proximal arterial disease severity in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2000;15(5):631–636. doi: 10.1093/ndt/15.5.631. [DOI] [PubMed] [Google Scholar]
- 18.Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (BOLD) MRI in renovascular hypertension. Curr Hypertens Rep. 2011;13(5):370–377. doi: 10.1007/s11906-011-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung CM, Chrysochou C, Shurrab AE, Buckley DL, Cowie A, Kalra PA. Effects of renal volume and single-kidney glomerular filtration rate on renal functional outcome in atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25(4):1133–1140. doi: 10.1093/ndt/gfp623. [DOI] [PubMed] [Google Scholar]
- 20.Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochim Biophys Acta. 2013;1832(7):931–939. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6(11):667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 22.Alhadad A, Guron G, Fortuna-Nowakowska E, et al. Renal angioplasty causes a rapid transient increase in inflammatory biomarkers, but reduced levels of interleukin-6 and endothelin-1 1 month after intervention. J Hypertens. 2007;25(9):1907–1914. doi: 10.1097/HJH.0b013e328244e2ca. [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Rodriguez-Porcel M, Herrmann J, et al. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15(4):958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 24.Lerman LO, Nath KA, Rodriguez-Porcel M, et al. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37(2 Pt 2):541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 25.Calo LA, Schiavo S, Davis PA, et al. Angiotensin II signaling via type 2 receptors in a human model of vascular hyporeactivity: implications for hypertension. J Hypertens. 2010;28(1):111–118. doi: 10.1097/HJH.0b013e328332b738. [DOI] [PubMed] [Google Scholar]
- 26.Muller DN, Dechend R, Mervaala EM, et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(1 Pt 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull F, Neal B, Pfeffer M, et al. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25(5):951–958. doi: 10.1097/HJH.0b013e3280bad9b4. [DOI] [PubMed] [Google Scholar]
- 28.Crowley SD, Vasievich MP, Ruiz P, et al. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest. 2009;119(4):943–953. doi: 10.1172/JCI34862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowley SD, Tharaux PL, Audoly LP, Coffman TM. Exploring type I angiotensin (AT1) receptor functions through gene targeting. Acta Physiol Scand. 2004;181(4):561–570. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 30.Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119(3):524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rompe F, Artuc M, Hallberg A, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010;55(4):924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Eirin A, Li ZL, et al. Angiotensin receptor blockade has protective effects on the poststenotic porcine kidney. Kidney Int. 2013;84(4):767–775. doi: 10.1038/ki.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Li ZL, Crane JA, et al. Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension. 2014;64(1):87–93. doi: 10.1161/HYPERTENSIONAHA.113.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marvar PJ, Thabet SR, Guzik TJ, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107(2):263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang JD, Patel MB, Song YS, et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110(12):1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu XY, Chade AR, Rodriguez-Porcel M, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24(10):1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 37.Eirin A, Zhu XY, Li Z, et al. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol. 2013;33(5):1006–1013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noh H, Ha H. Reactive oxygen species and oxidative stress. Contrib Nephrol. 2011;170:102–112. doi: 10.1159/000324955. [DOI] [PubMed] [Google Scholar]
- 39.Pialoux V, Mounier R, Brown AD, Steinback CD, Rawling JM, Poulin MJ. Relationship between oxidative stress and HIF-1 alpha mRNA during sustained hypoxia in humans. Free Radic Biol Med. 2009;46(2):321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 40.Zhu XY, Chade AR, Krier JD, et al. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. J Hypertens. 2009;27(10):2063–2073. doi: 10.1097/HJH.0b013e3283300192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30(5):1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palm F, Onozato M, Welch WJ, Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: effects of tempol and Angiotensin blockade. Hypertension. 2010;55(2):298–304. doi: 10.1161/HYPERTENSIONAHA.109.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eirin A, Li Z, Zhang X, et al. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension. 2012;60(5):1242–1249. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 44.Myung SK, Ju W, Cho B, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. Bmj. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009;52(3):196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eirin A, Lerman LO. Darkness at the end of the tunnel: poststenotic kidney injury. Physiology (Bethesda) 2013;28(4):245–253. doi: 10.1152/physiol.00010.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eirin A, Gloviczki ML, Tang H, et al. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34(7):540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eirin A, Gloviczki ML, Tang H, et al. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant. 2012;27(11):4153–4161. doi: 10.1093/ndt/gfs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotliar C, Juncos L, Inserra F, et al. Local and systemic cellular immunity in early renal artery atherosclerosis. Clin J Am Soc Nephrol. 2012;7(2):224–230. doi: 10.2215/CJN.06270611. [DOI] [PubMed] [Google Scholar]
- 50.Gloviczki ML, Keddis MT, Garovic VD, et al. TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol. 2013;8(4):546–553. doi: 10.2215/CJN.06460612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu XY, Daghini E, Chade AR, et al. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol. 2007;18(4):1209–1217. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]
- 52.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76(3):262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 53.Saad A, Herrmann SM, Crane J, et al. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv. 2013;6(4):428–435. doi: 10.1161/CIRCINTERVENTIONS.113.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chade AR, Krier JD, Galili O, Lerman A, Lerman LO. Role of renal cortical neovascularization in experimental hypercholesterolemia. Hypertension. 2007;50(4):729–736. doi: 10.1161/HYPERTENSIONAHA.107.093989. [DOI] [PubMed] [Google Scholar]
- 55.Chade AR, Mushin OP, Zhu X, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46(4):772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 56.Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17(11):2964–2966. doi: 10.1681/ASN.2006070704. [DOI] [PubMed] [Google Scholar]
- 57.Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrol Dial Transplant. 2010;25(11):3615–3622. doi: 10.1093/ndt/gfq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng J, Zhou W, Warner GM, et al. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol. 2009;297(4):F1055–1068. doi: 10.1152/ajprenal.90439.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kopp JB, Factor VM, Mozes M, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest. 1996;74(6):991–1003. [PubMed] [Google Scholar]
- 60.Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J Am Soc Nephrol. 1999;10(4):790–795. doi: 10.1681/ASN.V104790. [DOI] [PubMed] [Google Scholar]
- 61.Warner GM, Cheng J, Knudsen BE, et al. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol. 2012;302(11):F1455–1464. doi: 10.1152/ajprenal.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin N, Wang Q, Zhang X, Jiang D, Cheng H, Zhu K. The selective p38 mitogen-activated protein kinase inhibitor, SB203580, improves renal disease in MRL/lpr mouse model of systemic lupus. Int Immunopharmacol. 2011;11(9):1319–1326. doi: 10.1016/j.intimp.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Warner GM, Yin P, et al. Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in a murine renal artery stenosis model. Am J Physiol Renal Physiol. 2013;304(7):F938–947. doi: 10.1152/ajprenal.00706.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy BI, Schiffrin EL, Mourad JJ, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118(9):968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 65.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13(3):806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 66.Lemoine S, Rognant N, Collet-Benzaquen D, Juillard L. Contribution of X-ray computed tomography in the evaluation of kidney performance. Nephrol Ther. 2012;8(4):206–211. doi: 10.1016/j.nephro.2011.07.413. [DOI] [PubMed] [Google Scholar]
- 67.Hackstein N, Bauer J, Hauck EW, Ludwig M, Kramer HJ, Rau WS. Measuring single-kidney glomerular filtration rate on single-detector helical CT using a two-point Patlak plot technique in patients with increased interstitial space. Am J Roentgenol. 2003;181(1):147–156. doi: 10.2214/ajr.181.1.1810147. [DOI] [PubMed] [Google Scholar]
- 68.Lerman LO. Imaging: BOLD assessment–effects of RAAS inhibition in CKD. Nat Rev Nephrol. 2014;10(5):247–248. doi: 10.1038/nrneph.2014.58. [DOI] [PubMed] [Google Scholar]
- 69.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46(1):41–47. doi: 10.1097/RLI.0b013e3181f0213f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pedersen M, Dissing TH, Morkenborg J, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67(6):2305–2312. doi: 10.1111/j.1523-1755.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 71.Gomez SI, Warner L, Haas JA, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297(4):F981–986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gloviczki ML, Glockner JF, Lerman LO, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55(4):961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saad A, Crane J, Glockner JF, et al. Human renovascular disease: estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology. 2013;268(3):770–778. doi: 10.1148/radiol.13122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81(7):684–689. doi: 10.1038/ki.2011.455. [DOI] [PubMed] [Google Scholar]
- 75.Chrysochou C, Mendichovszky IA, Buckley DL, Cheung CM, Jackson A, Kalra PA. BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27(3):1013–1019. doi: 10.1093/ndt/gfr392. [DOI] [PubMed] [Google Scholar]
- 76.Korsmo MJ, Ebrahimi B, Eirin A, et al. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol. 2013;48(2):61–68. doi: 10.1097/RLI.0b013e31827a4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warner L, Yin M, Glaser KJ, et al. Noninvasive In vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol. 2011;46(8):509–514. doi: 10.1097/RLI.0b013e3182183a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee CU, Glockner JF, Glaser KJ, et al. MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study. Acad Radiol. 2012;19(7):834–841. doi: 10.1016/j.acra.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 80.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Chade AR, Zhu X, Lavi R, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119(4):547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ebrahimi B, Eirin A, Li Z, et al. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8(7):e67474. doi: 10.1371/journal.pone.0067474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelsen S, Hall JE, Chade AR. Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol. 2011;301(1):F218–225. doi: 10.1152/ajprenal.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv. 2010;3(4):376–383. doi: 10.1161/CIRCINTERVENTIONS.110.951277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stewart N, Chade AR. Renoprotective effects of hepatocyte growth factor in the stenotic kidney. Am J Physiol Renal Physiol. 2013;304(6):F625–633. doi: 10.1152/ajprenal.00504.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eirin A, Ebrahimi B, Zhang X, et al. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103(4):461–472. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]


