Abstract
Sodium retention is a major clinical feature of nephrotic syndrome. The mechanisms responsible for sodium retention in this setting have been a subject of debate for years. Excessive sodium retention occurs in some individuals with nephrotic syndrome in the absence of activation of the renin-angiotensin-aldosterone system, suggesting an intrinsic defect in sodium excretion by the kidney. Recent studies have provided new insights regarding mechanisms by which sodium transporters are activated by factors present in nephrotic urine. These mechanisms likely have a role in the development of hypertension in nephrotic syndrome, where hypertension may be difficult to control, and provide new therapeutic options for the management of blood pressure and edema in the setting of nephrotic syndrome.
Keywords: nephrotic syndrome, proteinuria, epithelial sodium channel, serine proteases, potassium sparing diuretics
Introduction
Two main hypotheses have been posited to explain the development of sodium retention in nephrotic syndrome: the underfill hypothesis and the overfill hypothesis. The premise of the underfill hypothesis is that sodium retention in nephrotic syndrome is primarily due to decreased effective circulating volume caused by fluid shifts from the intravascular to the interstitial compartment as a direct consequence of a decrease in plasma oncotic pressure by hypoalbuminemia. These changes activate sodium and water retention in the kidney. The overfill hypothesis postulates that sodium retention reflects an intrinsic defect in kidney sodium handling, which in turn causes volume expansion. After a brief review of evidence in support of these general hypotheses, we address basic mechanisms that may contribute to sodium retention and hypertension in nephrotic syndrome.
Evidence in support of the underfill and overfill hypotheses
It is clear that some individuals with nephrotic syndrome exhibit renal sodium retention in the setting of an activated renin-angiotensin-aldosterone system, consistent with the central tenet of the underfill hypothesis 1–3. Capillary oncotic pressure is reduced in patients with nephrotic syndrome. According to Starling’s law, a decrease in capillary oncotic pressure favors net movement of fluid across the capillary wall. This is particularly true in patients with severe hypoalbuminemia 3. However, a parallel decrease in interstitial oncotic pressure may blunt this effect. There is evidence that the latter phenomenon occurs in some patients with nephrotic syndrome, potentially secondary to increased lymphatic drainage 4,5. Regardless, there is sufficient underfilling of regions of the vascular bed in some nephrotic individuals to activate pathways that stimulate renal sodium retention 1–3. However, there is heterogeneity in intravascular volume status in patients with nephrotic syndrome. Meltzer et al. studied a group of 16 consecutive adults with untreated idiopathic nephrotic syndrome and observed that, at presentation, some individuals had a renin and aldosterone profile suggestive of an underfilled intravascular space 6. However, other individuals exhibited suppressed plasma renin activity and urinary aldosterone levels, suggesting intravascular volume expansion. In these individuals with nephrotic syndrome, intravascular volume depletion was not a prerequisite for sodium retention.
Likewise, low serum albumin does not appear to be sufficient to induce renal sodium retention in some individuals with nephrotic syndrome. It is well known that individuals lacking plasma albumin do not usually develop significant sodium retention. A large pediatric European case series of congenital analbuminemia showed that edema occurred only in a minority of these individuals. When it occurred, it was not a prominent feature of the syndrome 7. Furthermore, Oliver et al. observed that natriuresis occurs in the recovery phase of nephrotic syndrome, even in the presence of hypoalbuminemia 8. They studied 14 children with nephrotic syndrome on a low sodium diet, and after a variable period of observation, initiated corticosteroid therapy. The onset of natriuresis occurred before serum albumin normalized. Moreover, volume expansion with intravenous albumin does not enhance natriuresis in selected individuals with nephrotic syndrome. Koomans and colleagues studied the effect of albumin infusion in 10 individuals with nephrotic syndrome 9. Although blood volume increased up to 120% following a single infusion of 75 g of albumin, effectively suppressing renin and aldosterone, there was no change in urine sodium excretion. Finally, measured plasma and blood volumes are mostly normal or increased in nephrotic syndrome. Geers et al. measured plasma volume by administration of radioactive albumin in 88 individuals with nephrotic syndrome and 51 controls 10. Plasma and blood volumes were normal or increased in the majority of individuals and low in only 2% of cases. These findings suggest that low serum albumin and intravascular volume are not the primary mediators of sodium retention in some individuals with nephrotic syndrome.
While aldosterone plays a role in sodium retention in some individuals with nephrotic syndrome 11, in other individuals, the role of aldosterone appears minimal. For example, in a study examining individuals with nephrotic syndrome, blockade of the renin-angiotensin-aldosterone axis with captopril failed to change urine sodium excretion, despite suppression of plasma aldosterone 12. Usberti et al. reported similar findings with spironolactone 13. Studies in animals support these findings. de Seigneux et al. studied adrenalectomized rats with experimentally-induced nephrotic syndrome 14. To prevent adrenal insufficiency, they administered dexamethasone, which has potent glucocorticoid activity but does not stimulate the mineralocorticoid receptor. The animals developed sodium retention despite lacking aldosterone, suggesting that mineralocorticoid receptor activation has a minimal role in sodium retention in this rat model of nephrotic syndrome.
Several experimental observations provide compelling evidence for an intrinsic defect in sodium excretion in some individuals with nephrotic syndrome. Ichikawa and et al. studied rats subjected to unilateral renal artery infusion of puromycin aminonucleoside (PAN), where proteinuria developed exclusively in the PAN-treated kidney 15. Only the proteinuric kidney exhibited enhanced sodium retention, which was due to enhanced distal tubular sodium absorption. This observation confirmed that systemic factors, such as hypoalbuminemia or activation of neurohormonal systems are not required for enhanced renal sodium retention in the setting of nephrotic syndrome. Other work showed that PAN-induced volume expansion is responsive to amiloride, suggesting that epithelial sodium channel (ENaC) activation has a role in sodium retention in this setting 16,17. Subsequent studies, discussed below, have provided new insights regarding factors in the tubular fluid in nephrotic kidneys that can activate ENaC.
ENaC activation in nephrotic syndrome
ENaC mediates the absorption of sodium from the ultrafiltrate in the late distal convoluted tubule, connecting tubule, and collecting duct. These channels have a key role in the regulation of extracellular fluid volume and blood pressure, and are activated by volume regulatory hormones such as aldosterone and vasopressin 18. Other factors, including specific proteases, regulate ENaC 19. The first hint that proteases could regulate ENaC was from Orce et al., who showed that protease inhibitors decreased short-circuit current, a measure of ENaC activity, in toad urinary bladders 20. Subsequent work has shown that many proteases are capable of activating ENaC 21–30. In nephrotic syndrome, proteases in the tubular ultrafiltrate appear to have an important role in activating ENaC 31,32.
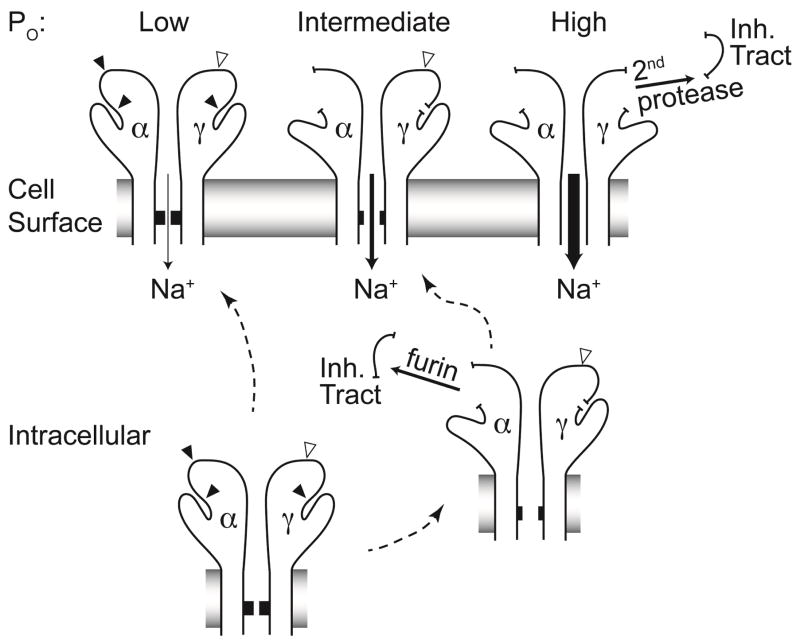
The mechanism by which proteases activate ENaC has been elucidated over the past decade. Individual ENaC channels are composed of three structurally related subunits, termed α, β, and γ 33. Each subunit has two transmembrane segments that contribute to the channel pore and are connected by a large extracellular region. Proteases activate ENaC by cleaving the α and γ subunits at multiple specific sites within their extracellular regions (Figure 1). As nascent channels pass through the cell’s biosynthetic pathway, furin, a serine protease residing in the trans-Golgi network, cleaves the α subunit twice 23. This dual cleavage event releases a small intrinsic inhibitory tract of 26 amino acids, allowing the channel to transition from a low activity state to a moderately active state 23,34,35. Furin cleaves the γ subunit only once 23. Subsequent cleavage of the γ subunit by a second protease distal to the furin cleavage site releases an inhibitory tract imbedded within the γ subunit, transitioning channels to a very high activity state 36. The importance of release of the inhibitory tract is shown in studies of an engineered γ subunit where the inhibitory tract has been deleted, which produces highly active channels 36,37. Furthermore, perfusion of channels with a soluble peptide corresponding to a released inhibitory tract suppresses channel activity 36,38.
Figure 1.
Two of ENaC’s three subunits (α and γ) undergo proteolytic cleavage. Furin cleaves the channel before it reaches the cell surface (closed arrowheads). Some channels escape furin cleavage and arrive at the cell surface intact. These channels exhibit very low activity, or open probability (Po). Furin cleaves the α subunit twice and the γ subunit once. Dual cleavage of the α subunit by furin releases that subunit’s inhibitory tract, resulting in channels with intermediate activity. The γ subunit harbors one furin cleavage site proximal to its inhibitory tract, and sites for various other extracellular proteases distal to the inhibitory tract (open arrowhead). Release of the γ subunit inhibitory tract occurs when the subunit has been cleaved by both furin and a second protease, resulting in a maximally activated channel.
In laboratory animals, increases in aldosterone levels, either due to dietary sodium restriction or aldosterone infusion, are associated with an increase in γ subunit cleavage 39–42. However, this observed increase in γ subunit cleavage does not necessarily denote that a γ subunit has been cleaved twice, which is a key requirement for release of the intervening γ subunit inhibitory tract. Recent studies have provided strong evidence for release of the γ subunit inhibitory tract in the settings of aldosterone administration or volume depletion. Proteolytic cleavage of the rat ENaC γ subunit by furin results in a 75 kDa C-terminal cleavage product 23. Infusion of rats with aldosterone led to a reduction in the size of this cleavage product to 70 kDa 43, consistent with a second cleavage event distal to the furin site. When aldosterone-treated rats were treated with an extracellular serine protease inhibitor, there was a shift in the size of the γ subunit from 70 kDa to 75 kDa, consistent with the protease inhibitor preventing γ subunit cleavage at a site distal to the furin cleavage site, while allowing furin cleavage.
Dual γ subunit cleavage by furin and a second protease releases the intervening peptide tract. Evidence for this comes from recent studies that employed an antibody raised against the γ subunit inhibitory tract. Sodium restriction lead to loss of the inhibitory tract from the C-terminal fragment, consistent with proteolytic removal of the inhibitory tract and channel activation 44.
Clues as to how these imbedded inhibitory tracts suppress channel activity were elucidated from a structural model of the α subunit 45. The extracellular region of each subunit is formed by multiple subdomains. Those in proximity to the membrane are comprised of β sheets, whereas those in the periphery are formed by α helices connected by loops. The inhibitory tracts lie within the periphery of the extracellular region, where they would be expected to be easily accessible to extracellular proteases 45. Kashlan et al. suggested that the inhibitory tracts are located at an interface between two extracellular subdomains, which tethers these domains and favors a low activity state 46. Consistent with this hypothesis, chemically cross-linking these extracellular subdomains stabilized the channel in a low activity state 46.
While furin cleaves the γ subunit at a site proximal to its inhibitory tract, a growing number of proteases has been identified that can cleave at sites distal to the inhibitory tract, releasing the inihibitory tract. This group of channel activating proteases includes prostasin 21,24,47, transmembrane protease serine 4 (TMPRSS4) 24,48,49, matriptase 24,50, cathepsin B 28,51, elastase 25,26,52, kallikrein 27,53 and plasmin 31,32.
Several lines of evidence suggest that activation of ENaC by proteases aberrantly filtered through damaged glomeruli contributes to urinary sodium retention in nephrotic syndrome. Increased γ subunit cleavage has been observed in the setting of nephrotic syndrome54. Urinary plasminogen has been found in a growing number of disease processes associated with glomerular proteinuria 31,32,55–58. Plasminogen can be converted to its active form, plasmin, by urokinase in the tubular lumen 31,32,59–61. Resolution of nephrotic syndrome is associated with reductions in levels of urinary plasminogen and plasmin 62. ENaC’s γ subunit has a plasmin cleavage site distal to its inhibitory tract, and plasmin can directly cleave and activate the channel 31,32,57, releasing the γ subunit inhibitory tract 32,63. Plasmin may also influence ENaC activity by activating other proteases in a proteolytic cascade, analogous to the blood clotting and complement protein cascades. Svenningsen and colleagues showed that both plasmin and nephrotic urine were capable of activating ENaC currents in cultured cells, but that activation of ENaC was reduced following a knock-down of the serine protease prostasin 63. While plasmin directly cleaves and activates ENaC, they suggested plasmin, at low concentrations, may activate the channel via prostasin, a serine protease expressed in the kidney tubule. Other proteases that are activated by plasmin could be participating in a cascade leading to ENaC activation.
While prostasin-dependent cleavage of the γ subunit and channel activation have been observed in vitro 21,36, prostasin may activate ENaC by other mechanisms. Interestingly, prostasin does not require its proteolytic activity to activate ENaC 44,64. Prostasin is present within a protein complex that contains ENaC, and recent work suggests that prostasin can recruit other proteases to this complex, resulting in channel cleavage and activation 44. Svenningsen et al. have suggested that prostasin facilitates the targeting of plasmin to ENaC at the plasma membrane 63. Alternatively, plasmin could facilitate the conversion of prostasin from its inactive form to an active form.
Is there a minimum, or threshold level of urinary plasmin that is needed to activate ENaC and stimulate sodium retention by the kidney? Buhl and co-workers found that in diabetic patients with resistant hypertension, microalbuminuria is associated with sufficient plasmin to activate ENaC in cultured cells 58. Thus, even at low levels, proteases filtered by leaky glomeruli may contribute to hypertension.
If proteolytic activation of ENaC is a key contributor to sodium retention and extracellular volume expansion in the setting of proteinuria, should pharmacologic targeting of ENaC, or of specific channel activating proteases, be used as a therapeutic approach in this setting? Clinical studies are needed to address these questions. The use of ENaC inhibitors (potassium-sparing diuretics, such as amiloride) may be effective 65, but the proclivity of these agents for inducing hyperkalemia may be compounded by impaired potassium excretion in nephrotic syndrome where individuals who also have a decreased glomerular filtration rate, low aldosterone levels, or are receiving inhibitors of the renin-angiotensin-aldosterone system.
Other factors influence renal sodium handling in nephrotic syndrome
Extracellular fluid volume expansion associated with nephrotic syndrome should be opposed by the actions of atrial natriuretic peptide (ANP). Volume expansion induces release of pro-ANP from atrial myocytes 66,67. Active ANP is generated from pro-ANP by proteolytic cleavage. ANP acts on the vasculature of the kidney to increase glomerular filtration while reducing sodium reabsorption in the proximal tubule and inner medullary collecting duct 68–73. Serum ANP levels are frequently elevated in nephrotic syndrome 74–76. However, ANP-dependent natriuresis is blunted in nephrotic syndrome, reflecting a dampening of ANP-dependent signaling mechanisms 74,77,78, and contributing to the failure of ANP to correct extracellular volume overload.
An additional mechanism that may contribute to decreased ANP signaling in nephrotic syndrome is decreased conversion of pro-ANP to active ANP. Cleavage of pro-ANP is mediated by the protease corin 79, which is expressed in the kidney as well as in cardiac tissue. Corin is down-regulated in nephrotic kidneys, with an associated increase in pro-ANP and reduction in ANP 80. Corin-null mice exhibit impaired sodium excretion and salt-sensitive hypertension that is responsive to amiloride but not calcium channel blockers 81. Thus, decreased ANP activation by corin may contribute to the failure of ANP to correct hypervolemia in nephrotic syndrome.
Alterations in nitric oxide signaling may also contribute to enhanced renal sodium retention in nephrotic syndrome. Rats with PAN-induced proteinuria exhibited a decrease in nitric oxide synthase expression in the kidney 82. Reduced nitric oxide signaling in nephrotic syndrome may contribute to increased sodium retention by increasing tubulo-glomerular feedback and collecting duct ENaC-mediated sodium reabsorption 83–87.
Other factors may have roles in enhancing tubular sodium absorption in nephrotic syndrome. For example, the Rho family GTPase Rac1 has been reported to activate the mineralocorticoid receptor in an aldosterone-independent manner 88. Dahl salt-sensitive rats exhibit proteinuria 89, and salt-loading of Dahl salt-sensitive rats stimulates Rac1, which activates the mineralocorticoid receptor 90 and increases ENaC expression 91. Whether Rac1 activity is up regulated in other proteinuric states remains to be seen. This mechanism could contribute to aldosterone-independent activation of mineralocorticoid receptor-dependent signaling pathways in nephrotic syndrome.
Conclusion
Edema is one of the clinical hallmarks of nephrotic syndrome. In some individuals, activation of the renin-angiotensin-aldosterone system leads to excessive sodium retention with expansion of the extracellular fluid volume and edema. However, other individuals exhibit sodium retention in spite of a suppressed renin-angiotensin-aldosterone system. Evidence suggests that mechanisms intrinsic to the kidney contribute to sodium retention in nephrotic syndrome, including activation of ENaC by aberrantly filtered proteases. One such protease, plasminogen, is found in nephrotic urine and can be activated by tubular urokinase, producing plasmin. Plasmin, either directly or as part of a protease cascade, cleaves and activates ENaC. Future studies are needed to address whether ENaC inhibitors, such as amiloride, are efficacious in treating extracellular volume expansion and edema in nephrotic syndrome. However, use of these agents may be limited by the risk of hyperkalemia.
Clinical Summary.
A number of mechanisms contribute to renal sodium retention in nephrotic syndrome. A subset of nephrotic individuals retains sodium in the setting of an activated renin-angiotensin-aldosterone system. Others exhibit sodium retention without activation of this system.
Filtered serum proteases activate the epithelial sodium channel (ENaC), providing an alternative mechanism for sodium retention in the setting of proteinuria.
Plasminogen is filtered by damaged glomeruli, and can be converted to its active form (plasmin) by tubular urokinase. Plasmin directly activates ENaC, and may also act in concert with other proteases, such as prostasin, to activate ENaC.
Homeostatic mechanisms that would normally oppose volume overload, such as atrial natriuretic peptide signaling, appear to be blunted in some individuals with nephrotic syndrome.
Inhibitors of ENaC may be beneficial in managing volume overload in the setting of nephrotic syndrome, but their use may be limited by hyperkalemia.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 DK051391, P30 DK079307 and T32 DK061296). We thank Dr. Osama Kashlan for generating Figure 1.
Footnotes
Conflict of interest:
All authors report no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown EA, Markandu ND, Sagnella GA, Squires M, Jones BE, MacGregor GA. Evidence that some mechanism other than the renin system causes sodium retention in nephrotic syndrome. Lancet. 1982;2:1237–40. doi: 10.1016/s0140-6736(82)90102-7. [DOI] [PubMed] [Google Scholar]
- 2.Cumming AD, Jeffrey S, Lambie AT, Robson JS. The kallikrein-kinin and renin-angiotensin systems in nephrotic syndrome. Nephron. 1989;51:185–91. doi: 10.1159/000185283. [DOI] [PubMed] [Google Scholar]
- 3.Usberti M, Gazzotti RM, Poiesi C, D’Avanzo L, Ghielmi S. Considerations on the sodium retention in nephrotic syndrome. Am J Nephrol. 1995;15:38–47. doi: 10.1159/000168800. [DOI] [PubMed] [Google Scholar]
- 4.Noddeland H, Riisnes SM, Fadnes HO. Interstitial fluid colloid osmotic and hydrostatic pressures in subcutaneous tissue of patients with nephrotic syndrome. Scand J Clin Lab Invest. 1982;42:139–46. [PubMed] [Google Scholar]
- 5.Beach RE, Walden C, Boudreaux JP, DuBose TD., Jr The role of lymphatic flow in edema formation of nephrotic syndrome. Am J Med Sci. 1989;297:339–42. doi: 10.1097/00000441-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer JI, Keim HJ, Laragh JH, Sealey JE, Jan KM, Chien S. Nephrotic syndrome: vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Ann Intern Med. 1979;91:688–96. doi: 10.7326/0003-4819-91-5-688. [DOI] [PubMed] [Google Scholar]
- 7.Koot BG, Houwen R, Pot DJ, Nauta J. Congenital analbuminaemia: biochemical and clinical implications. A case report and literature review. Eur J Pediatr. 2004;163:664–70. doi: 10.1007/s00431-004-1492-z. [DOI] [PubMed] [Google Scholar]
- 8.Oliver WJ. Physiologic Responses Associated with Steroid-Induced Diuresis in the Nephrotic Syndrome. J Lab Clin Med. 1963;62:449–64. [PubMed] [Google Scholar]
- 9.Koomans HA, Geers AB, vd Meiracker AH, Roos JC, Boer P, Dorhout Mees EJ. Effects of plasma volume expansion on renal salt handling in patients with the nephrotic syndrome. Am J Nephrol. 1984;4:227–34. doi: 10.1159/000166814. [DOI] [PubMed] [Google Scholar]
- 10.Geers AB, Koomans HA, Boer P, Dorhout Mees EJ. Plasma and blood volumes in patients with the nephrotic syndrome. Nephron. 1984;38:170–3. doi: 10.1159/000183302. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro MD, Hasbargen J, Hensen J, Schrier RW. Role of aldosterone in the sodium retention of patients with nephrotic syndrome. Am J Nephrol. 1990;10:44–8. doi: 10.1159/000168052. [DOI] [PubMed] [Google Scholar]
- 12.Brown EA, Markandu ND, Sagnella GA, Jones BE, MacGregor GA. Lack of effect of captopril on the sodium retention of the nephrotic syndrome. Nephron. 1984;37:43–8. doi: 10.1159/000183206. [DOI] [PubMed] [Google Scholar]
- 13.Usberti M, Gazzotti RM. Hyporeninemic hypoaldosteronism in patients with nephrotic syndrome. Am J Nephrol. 1998;18:251–5. doi: 10.1159/000013347. [DOI] [PubMed] [Google Scholar]
- 14.de Seigneux S, Kim SW, Hemmingsen SC, Frokiaer J, Nielsen S. Increased expression but not targeting of ENaC in adrenalectomized rats with PAN-induced nephrotic syndrome. Am J Physiol Renal Physiol. 2006;291:F208–17. doi: 10.1152/ajprenal.00399.2005. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa I, Rennke HG, Hoyer JR, et al. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschenes G, Wittner M, Stefano A, Jounier S, Doucet A. Collecting duct is a site of sodium retention in PAN nephrosis: a rationale for amiloride therapy. J Am Soc Nephrol. 2001;12:598–601. doi: 10.1681/ASN.V123598. [DOI] [PubMed] [Google Scholar]
- 17.Lourdel S, Loffing J, Favre G, et al. Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J Am Soc Nephrol. 2005;16:3642–50. doi: 10.1681/ASN.2005040363. [DOI] [PubMed] [Google Scholar]
- 18.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–54. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 19.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–51. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orce GG, Castillo GA, Margolius HS. Inhibition of short-circuit current in toad urinary bladder by inhibitors of glandular kallikrein. Am J Physiol. 1980;239:F459–65. doi: 10.1152/ajprenal.1980.239.5.F459. [DOI] [PubMed] [Google Scholar]
- 21.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–10. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 22.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–38. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughey RP, Bruns JB, Kinlough CL, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–4. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 24.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus Oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–9. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 26.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–29. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picard N, Eladari D, El Moghrabi S, et al. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem. 2008;283:4602–11. doi: 10.1074/jbc.M705664200. [DOI] [PubMed] [Google Scholar]
- 28.Alli AA, Song JZ, Al-Khalili O, et al. Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity. J Biol Chem. 2012;287:30073–83. doi: 10.1074/jbc.M111.338574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butterworth MB, Zhang L, Heidrich EM, Myerburg MM, Thibodeau PH. Activation of the epithelial sodium channel (ENaC) by the alkaline protease from Pseudomonas aeruginosa. J Biol Chem. 2012;287:32556–65. doi: 10.1074/jbc.M112.369520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Caballero A, Ishmael SS, Dang Y, et al. Activation of the epithelial sodium channel by the metalloprotease meprin beta subunit. Channels (Austin) 2011;5:14–22. doi: 10.4161/chan.5.1.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–91. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svenningsen P, Bistrup C, Friis UG, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299–310. doi: 10.1681/ASN.2008040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canessa CM, Schild L, Buell G, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–7. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 34.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem. 2006;281:18901–7. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- 35.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–96. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- 36.Bruns JB, Carattino MD, Sheng S, et al. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–60. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 37.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–5. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel. Am J Physiol Renal Physiol. 2010;299:F854–61. doi: 10.1152/ajprenal.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol. 2001;280:F112–8. doi: 10.1152/ajprenal.2001.280.1.F112. [DOI] [PubMed] [Google Scholar]
- 40.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol. 2009;297:F1249–55. doi: 10.1152/ajprenal.00401.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–93. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- 43.Uchimura K, Kakizoe Y, Onoue T, et al. In vivo contribution of serine proteases to the proteolytic activation of gammaENaC in aldosterone-infused rats. Am J Physiol Renal Physiol. 2012;303:F939–43. doi: 10.1152/ajprenal.00705.2011. [DOI] [PubMed] [Google Scholar]
- 44.Carattino MD, Mueller GM, Palmer LG, et al. Prostasin interacts with the epithelial sodium channel and facilitates cleavage of the gamma subunit by a second protease. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00157.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kashlan OB, Boyd CR, Argyropoulos C, et al. Allosteric inhibition of the epithelial Na+ channel through peptide binding at peripheral finger and thumb domains. J Biol Chem. 2010;285:35216–23. doi: 10.1074/jbc.M110.167064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashlan OB, Blobner BM, Zuzek Z, Carattino MD, Kleyman TR. Inhibitory tract traps the epithelial Na+ channel in a low activity conformation. J Biol Chem. 2012;287:20720–6. doi: 10.1074/jbc.M112.358218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malsure S, Wang Q, Charles RP, et al. Colon-Specific Deletion of Epithelial Sodium Channel Causes Sodium Loss and Aldosterone Resistance. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol. 2008;132:521–35. doi: 10.1085/jgp.200810030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passero CJ, Mueller GM, Myerburg MM, Carattino MD, Hughey RP, Kleyman TR. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the gamma-subunit distal to the furin cleavage site. Am J Physiol Renal Physiol. 2012;302:F1–8. doi: 10.1152/ajprenal.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kota P, Garcia-Caballero A, Dang H, Gentzsch M, Stutts MJ, Dokholyan NV. Energetic and structural basis for activation of the epithelial sodium channel by matriptase. Biochemistry. 2012;51:3460–9. doi: 10.1021/bi2014773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan CD, Hobbs C, Sameni M, Sloane BF, Stutts MJ, Tarran R. Cathepsin B contributes to Na+ hyperabsorption in cystic fibrosis airway epithelial cultures. J Physiol. 2014 doi: 10.1113/jphysiol.2013.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem. 2007;282:58–64. doi: 10.1074/jbc.M605125200. [DOI] [PubMed] [Google Scholar]
- 53.Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol. 2012;303:F540–50. doi: 10.1152/ajprenal.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SW, Wang W, Nielsen J, et al. Increased expression and apical targeting of renal ENaC subunits in puromycin aminonucleoside-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol. 2004;286:F922–35. doi: 10.1152/ajprenal.00277.2003. [DOI] [PubMed] [Google Scholar]
- 55.Lau SO, Tkachuck JY, Hasegawa DK, Edson JR. Plasminogen and antithrombin III deficiencies in the childhood nephrotic syndrome associated with plasminogenuria and antithrombinuria. J Pediatr. 1980;96:390–2. doi: 10.1016/s0022-3476(80)80678-0. [DOI] [PubMed] [Google Scholar]
- 56.Vaziri ND, Gonzales EC, Shayestehfar B, Barton CH. Plasma levels and urinary excretion of fibrinolytic and protease inhibitory proteins in nephrotic syndrome. J Lab Clin Med. 1994;124:118–24. [PubMed] [Google Scholar]
- 57.Buhl KB, Friis UG, Svenningsen P, et al. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension. 2012;60:1346–51. doi: 10.1161/HYPERTENSIONAHA.112.198879. [DOI] [PubMed] [Google Scholar]
- 58.Buhl KB, Oxlund CS, Friis UG, et al. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J Hypertens. 2014;32:1672–7. doi: 10.1097/HJH.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 59.Kristensen P, Eriksen J, Dano K. Localization of urokinase-type plasminogen activator messenger RNA in the normal mouse by in situ hybridization. J Histochem Cytochem. 1991;39:341–9. doi: 10.1177/39.3.1899685. [DOI] [PubMed] [Google Scholar]
- 60.Piedagnel R, Tiger Y, Lelongt B, Ronco PM. Urokinase (u-PA) is produced by collecting duct principal cells and is post-transcriptionally regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Cell Physiol. 2006;206:394–401. doi: 10.1002/jcp.20485. [DOI] [PubMed] [Google Scholar]
- 61.Wagner SN, Atkinson MJ, Wagner C, Hofler H, Schmitt M, Wilhelm O. Sites of urokinase-type plasminogen activator expression and distribution of its receptor in the normal human kidney. Histochem Cell Biol. 1996;105:53–60. doi: 10.1007/BF01450878. [DOI] [PubMed] [Google Scholar]
- 62.Andersen RF, Buhl KB, Jensen BL, et al. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol. 2013;28:1227–34. doi: 10.1007/s00467-013-2439-2. [DOI] [PubMed] [Google Scholar]
- 63.Svenningsen P, Uhrenholt TR, Palarasah Y, Skjodt K, Jensen BL, Skott O. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1733–41. doi: 10.1152/ajpregu.00321.2009. [DOI] [PubMed] [Google Scholar]
- 64.Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol. 2006;17:968–76. doi: 10.1681/ASN.2005060637. [DOI] [PubMed] [Google Scholar]
- 65.Deschenes G, Guigonis V, Doucet A. Molecular mechanism of edema formation in nephrotic syndrome. Arch Pediatr. 2004;11:1084–94. doi: 10.1016/j.arcped.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 66.Lang RE, Tholken H, Ganten D, Luft FC, Ruskoaho H, Unger T. Atrial natriuretic factor--a circulating hormone stimulated by volume loading. Nature. 1985;314:264–6. doi: 10.1038/314264a0. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka I, Misono KS, Inagami T. Atrial natriuretic factor in rat hypothalamus, atria and plasma: determination by specific radioimmunoassay. Biochem Biophys Res Commun. 1984;124:663–8. doi: 10.1016/0006-291x(84)91606-1. [DOI] [PubMed] [Google Scholar]
- 68.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 69.Fried TA, Osgood RW, Stein JH. Tubular site(s) of action of atrial natriuretic peptide in the rat. Am J Physiol. 1988;255:F313–6. doi: 10.1152/ajprenal.1988.255.2.F313. [DOI] [PubMed] [Google Scholar]
- 70.Sonnenberg H, Honrath U, Chong CK, Wilson DR. Atrial natriuretic factor inhibits sodium transport in medullary collecting duct. Am J Physiol. 1986;250:F963–6. doi: 10.1152/ajprenal.1986.250.6.F963. [DOI] [PubMed] [Google Scholar]
- 71.Dunn BR, Ichikawa I, Pfeffer JM, Troy JL, Brenner BM. Renal and systemic hemodynamic effects of synthetic atrial natriuretic peptide in the anesthetized rat. Circ Res. 1986;59:237–46. doi: 10.1161/01.res.59.3.237. [DOI] [PubMed] [Google Scholar]
- 72.Zeidel ML, Kikeri D, Silva P, Burrowes M, Brenner BM. Atrial natriuretic peptides inhibit conductive sodium uptake by rabbit inner medullary collecting duct cells. J Clin Invest. 1988;82:1067–74. doi: 10.1172/JCI113663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature. 1987;326:697–8. doi: 10.1038/326697a0. [DOI] [PubMed] [Google Scholar]
- 74.Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. 1992;90:1302–12. doi: 10.1172/JCI115995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perico N, Delaini F, Lupini C, et al. Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int. 1989;36:57–64. doi: 10.1038/ki.1989.161. [DOI] [PubMed] [Google Scholar]
- 76.Koepke JP, DiBona GF. Blunted natriuresis to atrial natriuretic peptide in chronic sodiumretaining disorders. Am J Physiol. 1987;252:F865–71. doi: 10.1152/ajprenal.1987.252.5.F865. [DOI] [PubMed] [Google Scholar]
- 77.Peterson C, Madsen B, Perlman A, Chan AY, Myers BD. Atrial natriuretic peptide and the renal response to hypervolemia in nephrotic humans. Kidney Int. 1988;34:825–31. doi: 10.1038/ki.1988.256. [DOI] [PubMed] [Google Scholar]
- 78.Lee EY, Humphreys MH. Phosphodiesterase activity as a mediator of renal resistance to ANP in pathological salt retention. Am J Physiol. 1996;271:F3–6. doi: 10.1152/ajprenal.1996.271.1.F3. [DOI] [PubMed] [Google Scholar]
- 79.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polzin D, Kaminski HJ, Kastner C, et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–9. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Shen J, Cui Y, et al. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ni Z, Vaziri ND. Downregulation of nitric oxide synthase in nephrotic syndrome: role of proteinuria. Biochim Biophys Acta. 2003;1638:129–37. doi: 10.1016/s0925-4439(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 83.Ichihara A, Inscho EW, Imig JD, Navar LG. Neuronal nitric oxide synthase modulates rat renal microvascular function. Am J Physiol. 1998;274:F516–24. doi: 10.1152/ajprenal.1998.274.3.F516. [DOI] [PubMed] [Google Scholar]
- 84.Thorup C, Persson AE. Inhibition of locally produced nitric oxide resets tubuloglomerular feedback mechanism. Am J Physiol. 1994;267:F606–11. doi: 10.1152/ajprenal.1994.267.4.F606. [DOI] [PubMed] [Google Scholar]
- 85.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995;6:89–94. doi: 10.1681/ASN.V6189. [DOI] [PubMed] [Google Scholar]
- 86.Helms MN, Yu L, Malik B, Kleinhenz DJ, Hart CM, Eaton DC. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am J Physiol Cell Physiol. 2005;289:C717–26. doi: 10.1152/ajpcell.00006.2005. [DOI] [PubMed] [Google Scholar]
- 87.Guo LJ, Alli AA, Eaton DC, Bao HF. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol. 2013;304:F930–7. doi: 10.1152/ajprenal.00638.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shibata S, Nagase M, Yoshida S, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 89.Sterzel RB, Luft FC, Gao Y, et al. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int. 1988;33:1119–29. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- 90.Shibata S, Mu S, Kawarazaki H, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kakizoe Y, Kitamura K, Ko T, et al. Aberrant ENaC activation in Dahl salt-sensitive rats. J Hypertens. 2009;27:1679–89. doi: 10.1097/HJH.0b013e32832c7d23. [DOI] [PubMed] [Google Scholar]