Abstract
Purpose
Low-grade inflammation, measured by circulating levels of cytokines, is a pathogenic mechanism for several chronic diseases of aging. Identifying factors related to inflammation among African American youths may yield insights into mechanisms underlying racial disparities in health. The purpose of the study was to determine whether (a) reported racial discrimination from ages 17 to 19 forecast heightened cytokine levels at age 22, and (b) this association is lower for youths with positive racial identities.
Methods
A longitudinal research design was used with a community sample of 160 African Americans who were 17 at the beginning of the study. Discrimination and racial identity were measured with questionnaires, and blood was drawn to measure basal cytokine levels. Ordinary least squares regression analyses were used to examine the hypotheses.
Results
After controlling for socioeconomic risk, life stress, depressive symptoms, and body mass index, racial discrimination (β = .307, p < .01), racial identity (β = −.179, p < .05), and their interaction (β = −.180, p < .05) forecast cytokine levels. Youths exposed to high levels of racial discrimination evinced elevated cytokine levels 3 years later. This association was not significant for young adults with positive racial identities.
Conclusions
High levels of interpersonal racial discrimination and the development of a positive racial identity operate jointly to determine low-grade inflammation levels that have been found to forecast chronic diseases of aging, such as coronary disease and stroke.
Keywords: African Americans, cytokines, health status disparities, inflammation, minority health, racism, self-concept
Even among young people, racial disparities across numerous health outcomes have been consistently documented. Compared with members of other racial groups, African Americans experience aging-related chronic diseases earlier in life, at greater severity, and with more serious consequences [1]. These disparities are also manifested in cardiometabolic risk. African American youths are more likely than their peers of other ethnicities to experience central adiposity, high blood pressure, insulin resistance, and subclinical atherosclerosis [2–5]. Low-grade inflammation is a common pathogenic mechanism in all of these "pre-disease states," facilitating their progression into clinical conditions such as coronary heart disease and stroke [6, 7]. For example, inflammation is known to contribute to the progression of coronary heart disease by facilitating the accumulation and detachment of atherosclerotic plaque and promoting coagulation processes involved in thrombosis [8, 9].
The health risk inequities that African Americans experience arise from more than economic hardship. Psychosocial stressors that disproportionately affect African Americans have been proposed as a mechanism that increases their vulnerability to cardiometabolic risk and poor health. Consistent with this reasoning, an emerging area of research has focused on racial discrimination, a qualitatively unique source of psychosocial stress that African Americans face [10, 11]. Racial discrimination involves routine experiences with disrespect and inferior treatment on the basis of race, which continue to pervade society [12]. Exposure to racial discrimination has been found to be associated with a range of health-relevant biological processes in adults, including glucocorticoids [10], pro-inflammatory cytokines and other markers of inflammation [13, 14], and shortened telomere length [15]. These studies provide proof-of-principle support for the link between exposure to racial discrimination and health-relevant biological measures. Despite the pivotal role that inflammation may play in the development of chronic diseases and health disparities [16], no longitudinal studies have focused on low-grade inflammation and exposure to racial discrimination during adolescence, a developmental stage during which race-related stressors may have a particularly significant impact. The present study was designed to help fill this need.
During adolescence, African American youths are continuing to explore and refine their racial identities [17]. Through this developmental process, youths come to understand the importance of their racial group membership to their thoughts, perceptions, feelings, and behaviors. Defined as the significance and positivity that individuals ascribe to membership in their racial groups [18], racial identity can condition responses to race-related stressors. For example, some studies have shown that African American youths with positive racial identities are buffered from the ill effects of discrimination on subsequent levels of depressive symptoms, perceived stress, psychological distress, and behavior problems [19].
Despite its intuitive appeal, tests of the racial identity-stress buffering hypothesis have yielded mixed findings. Some studies, such as those cited previously, support the hypothesis, and others provide partial support. In a few studies, positive racial identities were associated with an intensification of the relation between discrimination and psychological distress (see Brondolo et al. [20] for a review). The absence of a consistent moderating effect of racial identity on psychological well-being, however, should not rule out the possibility that racial identity may buffer the association between racial discrimination and pro-inflammatory cytokines in African American adolescents. Indeed, a recent laboratory study [21] indicated that a positive racial identity attenuated the association between racial discrimination and autonomic nervous system reactivity among African American young adults. We predict that the association between racial discrimination and cytokine levels will be weaker among adolescents who have positive racial identities.
Finally, this study was designed to rule out some alternative explanations for the hypothesized association of racial discrimination with cytokine levels by controlling demographic and biobehavioral confounding variables, including gender, cumulative SES risk [16], perceived life stress, depressive symptoms, and body mass index (BMI). Each of these variables has been found to be associated with cytokine levels [22], rendering attributions about the unique contribution of racial discrimination somewhat ambiguous unless statistical controls are applied.
Methods
Participants
The data for this study were drawn from the Adults in the Making (AIM) prevention trial [23]. A total of 496 youths were recruited randomly from lists provided by public schools in six rural counties. Youths were enrolled in the study in 2006 when they were 17 years of age [23] and provided self-report data from 2006 to 2009 at ages 17 to 19 years. In this study, assignment to the AIM or control condition was controlled in all data analyses. Cytokine data were obtained from blood samples in 2011 and 2012 when the youths were 22 years of age. The families resided in small towns and communities in which poverty rates are among the highest in the nation and unemployment rates are above the national average [24]. At Wave 1, median household gross monthly income was $2016.00 (SD = 4353.86) and mean monthly per capita gross income was $887.54 (SD = 1578.98). The families had an average of 2.21 children, and 65.1% of the households were headed by single parents. Of the youths’ caregivers, 8.5% had a college or university degree; 68.8% had completed high school or earned a GED. Although the caregivers worked an average of 38.5 hours per week, 42% of the families lived below federal poverty standards and another 15% lived within 150% of the poverty threshold; they could be described as working poor [25].
Of the 496 youths who constituted the study sample, 304 (61.3%) agreed to the assessment of inflammation measured via blood at age 22. To minimize the influence of cytokine degradation, analyses were limited to specimens that had been processed within 24 hours of venipuncture (160/304 samples). These 160 participants constituted the sample in this study (32.2% of the Wave 1 sample). Comparisons, using independent t tests and chi-square tests, of the youths in the study sample with those not in the study sample revealed that, at study entry, they were identical in terms of socioeconomic risk, life stress, depressive symptoms, BMI, exposure to discrimination across adolescence, and racial identity.
Procedures
Families were contacted and enrolled in the study by community liaisons who resided in the counties in which the participants lived. The community liaisons were African American community members, selected on the basis of their social contacts and standing in the community, who worked with the researchers on participant recruitment and retention. At all data collection points, caregivers gave written consent to minor youths’ participation, and youths gave written assent or consent to their own participation. Each family was paid $100 after each assessment. The study was approved by the Institutional Review Board of the university at which it was conducted. To enhance rapport and cultural understanding, African American university students and community members who did not know the families served as field researchers to collect data. During each assessment, one home visit lasting 2 hours was made to each family. At each home visit, self-report questionnaires were administered to the youth on a laptop computer in a private room. The first author has used this data collection procedure for 20 years, and it has consistently yielded reliable data. When youths were 22 years of age, certified phlebotomists drew their blood into serum separator tubes.
Measures
Racial discrimination and racial identity were assessed at ages 17, 18, and 19; cytokine levels were measured at age 22. The demographic and biobehavioral confounding variables of gender, cumulative SES risk, life stress, and depressive symptoms were assessed at ages 17 to 19, and BMI was assessed at age 22. These variables were controlled in the analyses.
Perceived racial discrimination
At ages 17 to 19, target youths completed 9 items from a version of the Schedule of Racist Events (SRE) [26] that was designed to be developmentally appropriate for adolescents. Items in this revised SRE [27, 28] assessed the frequency during the previous year, ranging from 0 (never happened) to 2 (happened a lot), with which the respondent perceived specific discriminatory behavior events. These events included racially based slurs and insults, disrespectful treatment from community members, physical threats, and false accusations from business employees or law enforcement officials. Responses to items were summed at each wave and then were averaged across ages 17 to 19. Coefficient α was 0.94. Perceived discrimination was stable over time: The age 17 assessment was correlated with the age 18 assessment, r(151) = .62, and with the age 19 assessment, r(154) = .59. The age 18 assessment was correlated with the age 19 assessment, r(147) = .71. All p values were < .001.
Protective racial identity
At ages 17 to 19, youths reported racial identity with a scale adapted from the Centrality subscale of the Multidimensional Inventory of Black Identity [18] and the Stereotype subscale of the Multi-Construct African American Identity Questionnaire [29]. The nine-item scale assessed the importance of being Black to the youths’ identities and the extent to which they embraced positive views and rejected negative stereotypes of African Americans. Examples include “I feel close to Black people” and “I am happy that I am Black.” Possible responses ranged from 1 (strongly disagree) to 5 (strongly agree). Responses to items were summed to form a protective racial identity score at each wave and then were averaged across ages 17 to 19. Coefficient α was 0.81. Racial identity also was stable over time: The age 17 and age 18 assessments were correlated, r(150) = .65; the age 17 and age 19 assessments were correlated, r(154) = .47; and the age 18 and age 19 assessments were correlated, r(146) = .48. All p values were < .001.
Cumulative socioeconomic risk index
Six dichotomous variables formed a cumulative socioeconomic risk index, which was calculated at ages 17, 18, and 19. A score of 1 was assigned to each of the following characteristics: family poverty based on federal guidelines, primary caregiver unemployment, receipt of Temporary Assistance for Needy Families, primary caregiver single parenthood, primary caregiver education level less than high school graduation, and caregiver-reported inadequacy of family income. The scores were summed to form an index that has been found to forecast biomarkers of stress in African American adolescents [30]. The scores were then averaged across ages 17 to 19.
Intervention status and gender
Intervention status and gender were dummy coded. AIM participants were coded 1 and control participants were coded 0; male participants were coded 1 and female participants were coded 0.
Life stress
Life stress was assessed at ages 17 to 19. Youths endorsed a checklist of 12 events (e.g., acute economic stress, death of a friend, parental divorce, serious injury or illness) [31], indicating whether each had occurred during the previous 6 months. Endorsed items were summed at each wave to form the life stress scale and then were averaged across ages 17 to 19. Because this index yields count data, internal consistency was not computed.
Depressive symptoms
Youths’ depressive symptoms were measured via self-report on the Center for Epidemiologic Studies Depression scale (CES–D) [32], which is widely used with community samples. Youths rated each of 20 symptoms on a scale of 0 (rarely or none of the time), 1 (some or little of the time), 2 (occasionally or a moderate amount of time), or 3 (most or all of the time). Responses to items were summed to form the depressive symptoms scale at each wave and then were averaged across ages 17 to 19. Coefficient α was 0.89.
Youth body mass index
Each youth’s weight and height at age 22 were used to calculate BMI (weight in kilograms divided by the square of height in meters). Weight was measured by using a standard home scale, and height was measured by using a tape measure.
Inflammation
When youths were 22, a certified phlebotomist visited participants’ homes and collected venous blood into a Serum Separator Tube (Becton, Dickinson and Company; Franklin Lakes, NJ) through antecubital venipuncture. The specimens were couriered to a laboratory at the University of Iowa and, upon receipt, centrifuged according the manufacturer’s instructions. Serum was harvested, divided into aliquots, and then frozen at −80 degrees. To minimize the influence of cytokine degradation, analyses were further limited to specimens processed within 24 hours of venipuncture. Low-grade inflammation was subsequently measured by electrochemiluminescence on a SECTOR Imager 2400A (Meso Scale Discovery; Rockville, MD). Briefly, thawed samples were assayed in duplicate using a Human Pro-Inflammatory 7-Plex Ultra-Sensitive assay (Meso Scale Discovery), following instructions provided by the manufacturer. This assay is optimized for assessment of low-grade inflammation in peripheral blood samples from healthy subjects [33]. Its lower limits of detection range from 0.10 pg/ml (IL-8) to 0.80 pg/ml (IL-10). Circulating levels of six cytokines that orchestrate inflammation were measured: interleukins-(IL-) 1β, 6, 8, and 10, plus tumor necrosis factor-α and interferon- γ. Across runs, the median intra-assay coefficients of variation were 4.00% (IL-1β), 4.82% (IL-6), 1.78% (IL-8), 11.45% (IL-10), 4.34% (TNF-α), and 13.31 (IFN-γ). Correlations showed that concentrations of these molecules were strongly interrelated. The average inter-cytokine correlation was r = .65 and the range was r = .42–.92. Because of this clustering, an inflammation composite was formed for each subject by summing the six z-scored cytokine values. The composite showed high levels of internal consistency (Cronbach’s α = .90), and functioned as the primary outcome variable in the analyses.
Results
Plan of Analysis for the Study Hypothesis
Linear regression analysis was used in 2014 to test the study hypotheses. Two regression models were executed on cytokines at age 22. Youth gender, intervention status, cumulative SES risk, life stress, and depressive symptoms at ages 17 to 19, and BMI at age 22, were controlled in all models. The first model presented the relations of all covariates to the inflammation measure; the second model was designed to examine the main effects of perceived racial discrimination and protective racial identity; and the third model tested the two-way interaction between them.
Hypothesis Testing
Descriptive statistics and correlations among study variables are displayed in Table 1. Before investigating the study hypotheses, we executed a cross-lagged path model to explore the direction of the association between racial identity and depression: Did a positive racial identity lead to low levels of depression, or did low levels of depression lead to a positive racial identity? This is a question of interest in racial identity theory [20]. The model included racial identity and depression as measured at ages 17 and 19 (complete details of this analysis are available from the authors). The path coefficient from racial identity to depression was −.082 (p < .05), whereas the path coefficient from depression to racial identity was −.027 (ns). This supports the conjecture that racial identity serves to protect young African Americans from depression.
Table 1.
Descriptive Statistics and Correlations for the Research Variables (N = 160)
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender, male | — | |||||||||
| 2. Intervention | .068 | — | ||||||||
| 3. Family SES risk (age 17–19) | .062 | .086 | — | |||||||
| 4. Life stress (age 17–19) | −.042 | −.135 | .087 | — | ||||||
| 5. Depressive symptoms (age 17–19) | −.096 | −.074 | .187* | .397*** | — | |||||
| 6. Racial discrimination (age 17–19) | .064 | .011 | −.095 | .309*** | .331*** | — | ||||
| 7. Racial identity (age 17–19) | −.011 | .023 | −.145 | −.159* | −.334*** | .024 | — | |||
| 8. Body mass index (age 22) | −.081 | −.070 | −.131 | .024 | .047 | .103 | .047 | — | ||
| 9. Inflammation composite (age 22) | −.063 | .068 | −.027 | .046 | .033 | .235** | −.127 | .026 | — | |
| Mean | 0.36 | 0.73 | 1.92 | 2.06 | 12.16 | 4.58 | 37.48 | 30.63 | 0 | |
| SD | 0.48 | 0.44 | 1.11 | 1.18 | 7.10 | 3.36 | 4.14 | 10.08 | 5.05 | |
SES = socioeconomic status.
p < .05.
p < .01.
p < .001.
The results of the linear regression models are presented in Table 2. None of the covariates in Model 1 were associated with cytokine levels. The second model (Table 2, Model 1) was designed to determine the main effects of perceived racial discrimination and protective racial identity. Results suggested that both perceived racial discrimination (B = .436, SE = .131, β = .290, p = .001, 95% CI [.177, .695]) and protective racial identity (B = −.219, SE = .102, β = −.180, p = .033, 95% CI [−.420, −.018]) at ages 17 to 19 significantly predicted cytokine levels at age 22 beyond the effects of the covariates, F(2, 151) = 6.922, ΔR2 = .083, p = .001.
Table 2.
Perceived Racial Discrimination and Protective Racial Identity as Predictors of Cytokine Levels (N = 160)
| Cytokine levels (age 22) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Predictors | B | SE a | β | B | SE a | β | B | SE a | β |
| 1. Gender, male | −.637 | .854 | −.061 | −1.045 | .831 | −.099 | −1.247 | .824 | −.118 |
| 2. Intervention | .963 | .929 | .085 | .732 | .898 | .064 | .442 | .894 | .039 |
| 3. Family SES risk (age 17–19) b | −.165 | .379 | −.036 | .000 | .371 | .000 | .019 | .366 | .004 |
| 4. Life stress (age 17–19) | .214 | .378 | .050 | −.087 | .374 | −.020 | −.094 | .369 | −.022 |
| 5. Depressive symptoms (age 17 –19) | .014 | .064 | .020 | −.085 | .067 | −.120 | −.100 | .067 | −.140 |
| 6. Body mass index (age 22) | .010 | .041 | .020 | .004 | .040 | .007 | .003 | .039 | .005 |
| 7. Racial discrimination (age 17–19) | — | — | — | .436 | .131 | .290** | .461 | .130 | .307** |
| 8. Racial identity (age 17–19) | — | — | — | −.219 | .102 | −.180* | −.218 | .100 | −.179* |
| 9. Racial discrimination × Racial identity | — | — | — | — | — | — | −.062 | .027 | −.180* |
SE = standard error.
SES = socioeconomic status.
p < .05.
p < .01.
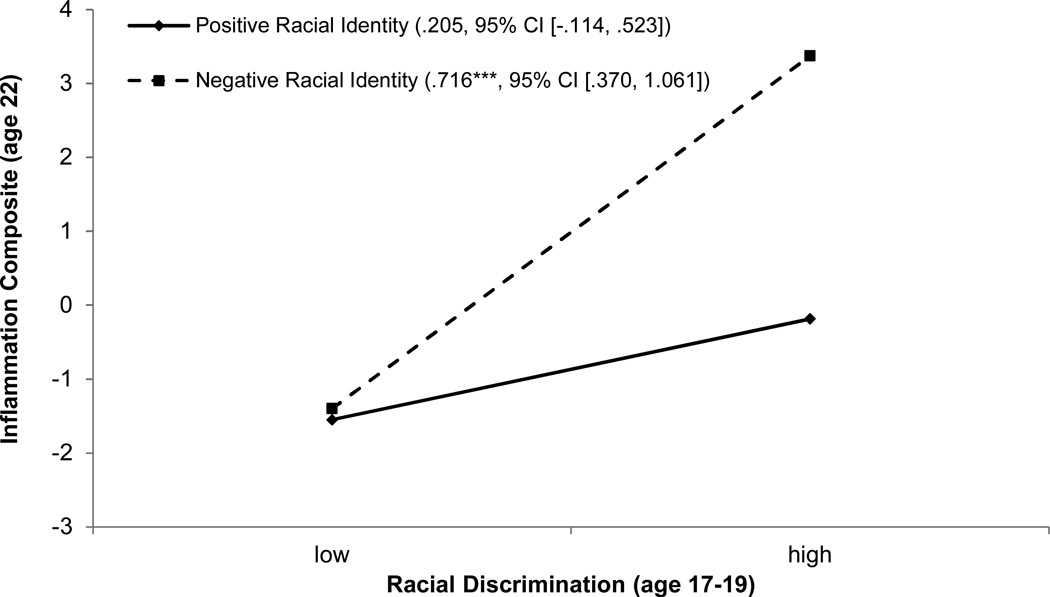
Model 3 in Table 2 presents tests for hypothesized interaction effects between perceived racial discrimination and protective racial identity on cytokines at age 22. Perceived racial discrimination and protective racial identity were centered before the interaction terms were calculated. The analysis revealed a significant interaction effect, F(1, 150) = 5.379, ΔR2 = .031, B = −.062, SE = .027, β = −.180, p = .022, 95% CI [−.114, −.009]. To interpret this finding, estimated cytokine levels were plotted at low (1 standard deviation below the mean; −1 SD) and high (1 standard deviation above the mean; +1 SD) levels of racial discrimination and racial identity; the results are presented in Figure 1. As hypothesized, protective racial identity moderated the association of racial discrimination with cytokine levels. Perceived racial discrimination at ages 17 to 19 was significantly associated with cytokine levels at age 22 among those with low protective racial identity (simple-slope = 0.716, SE = 0.175, p < .001, 95% CI [.370, 1.061]). Racial discrimination was not associated with cytokine levels among those with highly protective racial identities (simple-slope = .205, SE = 0.161, p = ns, 95% CI [−.114, .523]). Additional analyses were performed to determine whether participant gender or SES risk conditioned any of these findings; no interactions were detected.
Figure 1.
Youths’ inflammation at age 22 as a function of perceived racial discrimination and protective racial identity at age 17 to 19, with gender, intervention status, family SES index, life stress, and depressive symptoms at age 17 to 19, and BMI at age 22 controlled, presented as regression lines for different levels of racial identity (positive: 1 SD above the mean; negative: 1 SD below the mean). Numbers in parentheses refer to simple slopes with 95% confidence intervals.
***p < .001.
Discussion
During adolescence, African American youths become keenly aware of their treatment by others and are particularly cognizant of targeted rejection [34]. Against this developmental backdrop, the possibility was examined longitudinally that perceived discrimination would forecast cytokine levels during young adulthood, at age 22. The results indicated that (a) perceived racial discrimination forecast higher cytokine levels; (b) positive racial identity forecast lower cytokine levels; (c) the associations among perceived discrimination, racial identity, and cytokine levels retained their significance when confounding variables were controlled in the data analysis, and (d) the association between perceived discrimination and cytokine levels was lower among adolescents with a positive racial identity. These findings are consistent with suggestions that perceived discrimination can be a chronic social-environmental stressor that catalyzes health-relevant biological processes [10–12]. A literature search returned no prior studies that present associations between perceived racial discrimination and cytokine levels.
The present findings are also consistent with theoretical propositions that many youths are resilient and do not experience health-related biological changes despite exposure to race-related stressors [35]. Researchers, however, have not yet determined how a positive racial identity helps to attenuate the association between discrimination and health outcomes. Youths with positive racial identities may be more planful and more inclined to anticipate and to select themselves into positive, desirable situations while foreseeing and limiting their exposure to negative and demeaning circumstances. Thoits [36] found some evidence for the existence of such selection coping effects; this topic warrants further empirical consideration.
Several limitations of the study should be addressed in future research. First, because cytokines were not assessed at baseline, the study design precludes conclusions regarding their change over time. Second, usable cytokine data were obtained from only a subset of the participants. To our knowledge, participants whose specimens were processed inside and outside the 24-hour window were similar in terms of social, psychosocial, and health characteristics. Of course, they could have differed on variables that we have not considered and did not measure. In future research, therefore, it will be important to substantiate these findings in a study that is designed and executed with an a priori focus on assessing inflammation. In future studies, cytokine measures should also be collected repeatedly to assess inflammation trajectories over time. Functional tests could also be conducted to determine the extent to which immune cells are responsive to mitogen stimulation. Third, the discrimination measure assessed interpersonal discrimination only and did not assess structural discrimination; thus, it provided a limited assessment of the range of discriminatory experiences that adolescents encounter. Williams and Williams-Morris [37] called for more systematic research characterizing the multiple dimensions of racism (e.g., residential segregation). Similarly, Sue et al. [38] described a taxonomy of racial microaggressions that include microassaults and microinsults. Future research should use both chronic and daily discrimination measures, as well as measures that capture discrimination experiences across multiple domains, in probing the association between racial discrimination and health-relevant biological processes. Fourth, as Hurd et al. [39] suggest, the contribution of racial identity may vary with environmental circumstances. Future research should be designed to determine whether the buffering properties of racial identity differ with contextual factors such as levels of racial segregation and neighborhood poverty (see Brody et al. [40]). Finally, future research should also be designed to delineate the health consequences of the associations described here. As noted in the introduction, low-grade inflammation plays a role in both the earlier (obesity, insulin resistance, high blood pressure) and later (diabetes, myocardial infarction, stroke) stages of the cardiometabolic diseases that African Americans experience disproportionately. Research exploring the links among discrimination, inflammation, and the expression and persistence of these conditions would be valuable. These cautions notwithstanding, the study is among the first to show a prospective association between perceived discrimination across ages 17 to 19 and low-grade inflammation at age 22. It also highlights associations suggesting that a positive racial identity during adolescence contributes to low cytokine levels.
Implications and Contribution.
African Americans’ experiences with high levels of racial discrimination across ages 17 to 19 forecast heightened cytokine levels at age 22, but this association did not emerge for youths with positive racial identities. A positive racial identity may play a role in keeping stress from “getting under the skin.”
Acknowledgements
This research was supported by National Institute on Drug Abuse Awards R01 DA019230 and P30 DA027827. The National Institute on Drug Abuse had no role in the study’s design, data collection or interpretation, the writing of the report, or the decision to submit it for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no financial relationships or conflicts of interest to report.
References
- 1.Schuster MA, Elliott MN, Kanouse DE, et al. Racial and ethnic health disparities among fifth-graders in three cities. N Engl J Med. 2012 Aug 23;367:735–745. doi: 10.1056/NEJMsa1114353. [DOI] [PubMed] [Google Scholar]
- 2.Goodman E, Daniels SR, Dolan LM. Socioeconomic disparities in insulin resistance: Results from the Princeton School District Study. Psychosom Med. 2007;69:61–67. doi: 10.1097/01.psy.0000249732.96753.8f. [DOI] [PubMed] [Google Scholar]
- 3.Flores G. Racial and ethnic disparities in the health and health care of children. Pediatrics. 2010;125:e979–e1020. doi: 10.1542/peds.2010-0188. [DOI] [PubMed] [Google Scholar]
- 4.Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68:807–813. doi: 10.1016/j.socscimed.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GK, Kogan MD, van Dyck PC. Changes in state-specific childhood obesity and overweight prevalence in the United States from 2003 to 2007. Arch Pediatr Adolesc Med. 2010;164:598–607. doi: 10.1001/archpediatrics.2010.84. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science. 2013 Jan 11;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr Rev. 2007;65:S253–S259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001 Oct 11;88:3–6. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 10.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascoe EA, Smart Richman L. Perceived discrimination and health: A meta-analytic review. Psychol Bull. 2009;135:531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. J Behav Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper DC, Mills PJ, Bardwell WA, et al. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among Blacks and Whites. Am J Hypertens. 2009;22:698–704. doi: 10.1038/ajh.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis TT, Aiello AE, Leurgans S, et al. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24:438–443. doi: 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae DH, Nuru-Jeter AM, Adler NE, et al. Discrimination, racial bias, and telomere length in African-American men. Am J Prev Med. 2014;46:103–111. doi: 10.1016/j.amepre.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer MB. Phenomenology and ecological systems theory: Development of diverse groups. In: Lerner RM, Damon W, editors. Handbook of child psychology: Vol 1 Theoretical models of human development (6th ed) Hoboken, NJ: Wiley; 2006. pp. 829–893. [Google Scholar]
- 18.Sellers RM, Rowley SAJ, Chavous TM, et al. Multidimensional Inventory of Black Identity: A preliminary investigation of reliability and construct validity. J Pers Soc Psychol. 1997;73:805–815. [Google Scholar]
- 19.Gaylord-Harden NK, Burrow AL, Cunningham JA. A cultural-asset framework for investigating successful adaptation to stress in African American youth. Child Dev Perspect. 2012;6:264–271. [Google Scholar]
- 20.Brondolo E, ver Halen NB, Pencille M, et al. Coping with racism: A selective review of the literature and a theoretical and methodological critique. J Behav Med. 2009;32:64–88. doi: 10.1007/s10865-008-9193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neblett EWJ, Roberts SO. Racial identity and autonomic responses to racial discrimination. Psychophysiology. 2013;50:943–953. doi: 10.1111/psyp.12087. [DOI] [PubMed] [Google Scholar]
- 22.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Nat Acad Sci U S A. 2009 Aug 25;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brody GH, Yu T, Chen Y-f, et al. The Adults in the Making program: Long-term protective stabilizing effects on alcohol use and substance use problems for rural African American emerging adults. J Consult Clin Psychol. 2012;80:17–28. doi: 10.1037/a0026592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proctor BD, Dalaker J. Poverty in the United States: 2002 (Current Population Reports, P60-222) Washington, DC: US Census Bureau; 2003. [Google Scholar]
- 25.Boatright SR. The Georgia county guide (28th ed.) Athens, GA: Center for Agribusiness and Economic Development; 2009. [Google Scholar]
- 26.Landrine H, Klonoff EA. The Schedule of Racist Events: A measure of racial discrimination and a study of its negative physical and mental health consequences. J Black Psychol. 1996;22:144–168. [Google Scholar]
- 27.Simons RL, Simons LG, Burt CH, et al. Supportive parenting moderates the effect of discrimination upon anger, hostile view of relationships, and violence among African American boys. J Health Soc Behav. 2006;47:373–389. doi: 10.1177/002214650604700405. [DOI] [PubMed] [Google Scholar]
- 28.Brody GH, Chen Y-f, Murry VM, et al. Perceived discrimination and the adjustment of African American youths: A five-year longitudinal analysis with contextual moderation effects. Child Dev. 2006;77:1170–1189. doi: 10.1111/j.1467-8624.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith EP, Brookins CC. Toward the development of an ethnic identity measure for African American youth. J Black Psychol. 1997;23:358–377. [Google Scholar]
- 30.Brody GH, Yu T, Chen E, et al. Is resilience only skin deep? Rural African Americans’ preadolescent socioeconomic status-related risk and competence and age 19 psychological adjustment and allostatic load. Psychol Sci. 2013;24:1285–1293. doi: 10.1177/0956797612471954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brody GH, Chen Y-f, Kogan SM. A cascade model connecting life stress to risk behavior among rural African American emerging adults. Dev Psychopathol. 2010;22:667–678. doi: 10.1017/S0954579410000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES–D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 33.Fu Q, Zhu J, Van Eyk JE. Comparison of multiplex immunoassay platforms. Clin Chem. 2010;56:314–318. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson HC. Racial socialization. In: Jones RL, editor. Black psychology, 4th edition. Hampton, VA: Cobb and Henry; 2004. pp. 176–189. [Google Scholar]
- 35.Chen E, Miller GE. “Shift-and-persist” strategies: Why low socioeconomic status isn’t always bad for health. Perspect Psychol Sci. 2012;7:135–158. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thoits PA. Stressors and problem-solving: The individual as psychological activist. J Health Soc Behav. 1994;35:143–160. [PubMed] [Google Scholar]
- 37.Williams DR, Williams-Morris R. Racism and mental health: The African American experience. Ethn Health. 2000;5:243–268. doi: 10.1080/713667453. [DOI] [PubMed] [Google Scholar]
- 38.Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: Implications for clinical practice. Am Psychol. 2007;62:271–286. doi: 10.1037/0003-066X.62.4.271. [DOI] [PubMed] [Google Scholar]
- 39.Hurd NM, Sellers RM, Cogburn CD, et al. Racial identity and depressive symptoms among Black emerging adults: The moderating effects of neighborhood racial composition. Dev Psychol. 2013;49:938–950. doi: 10.1037/a0028826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brody GH, Lei M-K, Chen E, et al. Neighborhood poverty and allostatic load in African American youth. Pediatrics. 2014;134:1–8. doi: 10.1542/peds.2014-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]