OVERVIEW
Although patent foramen ovale (PFO) is frequently encountered in health, the prevalence is increased for patients with cryptogenic stroke (CS). PFO provides a potential site of paradoxic embolism, although there is rarely mechanistic certainty for individuals with CS. This results in problems of attribution and therapeutic uncertainty for individuals with CS. Recent trials of device-based closure of PFO for patients with CS have not shown clear benefit. At the same time, the preferred medical therapy for patients with CS and PFO remains unclear. This article discusses the controversies that frame decisions for patients with CS and PFO, recent clinical trial data that inform the use of device-based therapies, and a recently developed predictive model that may aid in identifying individuals who might benefit from PFO closure.
PATENT FORAMEN OVALE: DEVELOPMENT AND PREVALENCE
During fetal development, the foramen ovale is the site where oxygenated blood is allowed to bypass the high-resistance pulmonary circulation. Throughout development, it is a critical component of the fetal circulation. Most commonly the septum primum and secundum fuse just after birth, thus closing this right-to-left communication. For approximately 20% to 25% of healthy individuals, however, the foramen ovale remains patent.1,2 For most of these patients, PFO never causes symptoms and is found only incidentally during echocardiographic investigation for other reasons. The frequency of this anatomic feature and the generally benign natural history have led some to consider PFO (in the absence of clinical significance) as a normal variant.3 Although population studies have not reliably found that PFO is associated with ischemic stroke,4-6 potential effects in these studies were undoubtedly substantially diluted by the inclusion of strokes with known causes. Despite concerns of possible ascertainment bias caused by increased attention to potential right-to-left shunting when an alternative stroke mechanism has not been identified, the prevalence of PFO for patients with CS seems higher than for matched control subjects, population samples, or autopsy studies,2 and is especially higher in patients with CS without conventional stroke risk factors. Thus for some, there is likely a mechanistic link between this site of right-to-left shunting and stroke.7,8 When PFO is found in the setting of CS, the significance (and the preferred therapy) is often unclear.
THE PROBLEM OF ATTRIBUTION
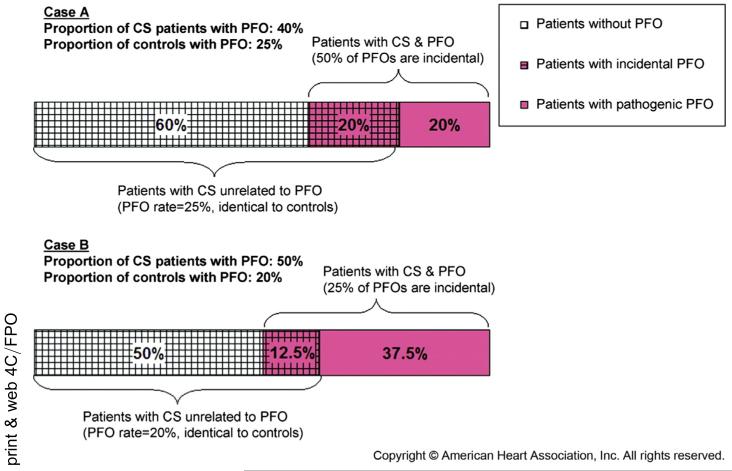
Observing a thrombus in transit through a PFO is quite rare. In the absence of this direct evidence it is not possible to know with certainty for an individual whether an observed PFO is related to stroke. Through a Bayesian transformation, however, we can estimate a PFO attributable fraction for subgroups of patients with CS. Generally, the likelihood that an observed PFO is related to an index stroke is related to the PFO prevalence in CS cases compared with control subjects. Consider the scenario described in Fig. 1.9 Assuming a control PFO prevalence rate of 25% in a healthy population, we would expect a 25% prevalence rate for patients with CS if PFO is completely incidental (and unrelated related to CS). As PFO prevalence for patients with CS rises above the control rate there is an increased likelihood than an observed PFO is related to their index stroke. The probability that an observed PFO is incidental is related directly to the control rate prevalence by the equation in Fig. 1.9
Fig. 1.
Proportion of patients with CS without PFO with incidental PFO and with pathogenic PFO. This figure shows how the proportion of incidental versus pathogenic PFO in patients with CS can be calculated based on the prevalence of PFO in patients with CS and in control. (Adapted from Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009;40(7):2350; with permission.)
PATENT FORAMEN OVALE: ANATOMIC RISK FACTORS
Transesophageal echocardiography (TEE) remains the standard tool to evaluate PFO because it allows for direct assessment of the interatrial septum and associated anatomy.1 There is increasing interest in using a multimodality approach and complementing TEE with contrast-transcranial Doppler to further define PFO in the setting of CS. Contrast-transcranial Doppler may have higher sensitivity for detecting PFO and may also offer prognostic information over TEE.10,11
Ideally, for an individual, anatomic and physiologic features would reliably distinguish benign from pathogenic PFOs. With these goals investigators have evaluated numerous candidate echocardiographic features that seem to confer risk. One associated feature, presence of an atrial septal aneurysm, has been proposed as a marker of risk.12 Atrial septal aneurysm describes a hypermobile interatrial septum and is seen more commonly in patients with PFO and recurrent CS. Another characteristic, severity of right-to-left microbubble movement across a PFO, a proxy for large physiologic shunt size, has been identified as a risk factor for stroke recurrence,13 although not consistently.14 Persistent right-to-left shunting across a PFO (throughout rest and Valsalva) has been proposed to increase risk through a similar mechanism as has persistence of a eustachian valve, an anatomic feature that preferentially shunts blood from the inferior vena cava across the PFO during fetal growth.15,16 Interestingly, in a recent analysis from the Risk of Paradoxic Embolism (RoPE) database (described later) of 1294 patients with CS evaluated for PFO with TEE, three of these proposed markers of risk (atrial septal aneurysm, shunt size, and shunt at rest) were not associated with clinical features that suggested that an observed PFO is related to CS.17 Despite the use of multiple imaging tools, it seems that anatomic and physiologic features alone cannot be used to consistently distinguish incidental from pathologic PFO.
NULL INTERVENTION TRIALS?
Because right-to-left shunting provides a potential site of paradoxic embolization, there has been significant interest in device-based PFO closure. Recent clinical trials have tested the hypothesis that stroke recurrence will decrease if a mechanistically important PFO is closed. Unfortunately, the trial results raised more questions than they answered because all three missed their primary intention-to-treat outcomes.18-20 The Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxic Embolism through a Patent Foramen Ovale trial randomized 909 patients to closure with the STARFlex (CardioSEAL, NMT Medical, Boston, MA) device versus medical therapy and followed them for 2 years. The cumulative incidence of the composite of stroke or transient ischemic attack (TIA) was 5.5% in the device-closure group and 6.8% in the medically treated arm (adjusted hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.45–1.35; P = .37). A recent observation that many of the recurrent events were not caused by paradoxic embolism but instead by alternative causes further clouds the results of this trial.21
Two intervention trials evaluated the Amplatzer PFO Occluder (AGA Medical, Golden Valley, Minnesota/St Jude, Saint Paul, MN) device. The Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) (980 patients followed for a median of 2.1 years) and the PC Trial (414 patients for an average of 4.1 years) both evaluated device closure versus medical therapy and both missed their primary end points (various composites that include stroke, TIA, and death) (HR, 0.4; 95% CI, 0.22–1.11, P = .08 for RESPECT; HR, 0.63; 95% CI, 0.24–1.62; P = .34 for PC Trial). These trials suffered from significant loss to follow-up (relative to rates of trial end points), and because outcomes in the medical arms of these trials were rare, they were likely underpowered to detect beneficial effects if any existed. It is worthwhile to note that all of these trials (especially the Amplatzer studies) show a trend toward beneficial effect of device-based closure despite a lack of statistical significance.
Despite the consistent directionality of these trials, current guidelines from the American Heart Association/American Stroke Association further downgraded their recommendation regarding device-based closure from IIb (reasonable to consider) to III (not useful, may be harmful) (Level of Clinical Efficacy 1-0, Level of Evidence T2).22 Because the potential value of this therapeutic approach is balanced by important occasional device-related complications (eg, atrial fibrillation and thrombus formation),23 this new recommendation concludes that the available data in summary clearly do not support closure. Recent analyses, however, suggest that this recommendation is perhaps premature and that closure may be beneficial for some.
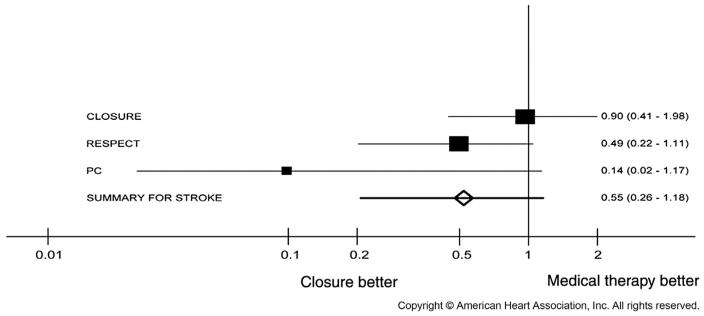
Since the publication of these major clinical trials, there have been numerous attempts by a variety of investigators to combine these trials through meta-analysis.24-30 These efforts, taken together, demonstrate a strong but not statistically significant trend toward reduction with device-based closure in the rate of the composite outcome of recurrent stroke, TIA, or death. A statistically significant beneficial effect on the outcome of stroke alone has not been consistently seen (Fig. 2), although a beneficial effect on stroke has emerged in some studies when combining only the Amplatzer trials in fixed-effect models.27,31,32 More recently a network meta-analysis supplemented the three trials described previously with the results of a randomized clinical trial that compared various device-based closure techniques (Amplatzer, STARFlex, and HELEX [W.L. Gore and Associates, Flagstaff, AZ] devices) head-to-head.33 The results of this network, which estimate comparative treatment effects by summarizing direct and indirect evidence, suggest an overall beneficial effect on recurrent stroke risk for device-based closure and, importantly, evidence that not all closure devices are similarly effective. The Amplatzer device seemed most effective in preventing recurrent stroke when compared with medical therapy (rate ratio, 0.39; 95% CI, 0.17–0.84) as compared with STARFlex (rate ratio, 1.01; 95% CI, 0.44–2.41) and HELEX devices (rate ratio, 0.71; 95% CI, 0.17–2.78) devices. As of this writing, however, the Amplatzer device remains unavailable in the United States, although PFOs may be closed using various off-label atrial septal defect devices, none of which have been designed for PFO closure.
Fig. 2.
Forest plot for the meta-analysis of hazards ratios of stroke of mechanical closure vs medical treatment from 3 randomized clinical trials. (Adapted from Kitsios GD, Thaler DE, Kent DM. Potentially large yet uncertain benefits: a meta-analysis of patent foramen ovale closure trials. Stroke 2013;44(9):2641; with permission.)
RISK OF PARADOXIC EMBOLISM DATABASE AND SCORE
Although PFO is a congenital remnant distributed randomly in the population, and not known to be associated with other observable characteristics, among the CS population it has been repeatedly and consistently noted that patients with PFO have a very different distribution of clinical variables than patients without PFO. The relationship between PFO and other clinical variables (whereby PFO seems to “protect” patients with CS from vascular risk factors, such as diabetes, hypertension, smoking, and so forth) presumably arises because patients with PFO have a stroke mechanism that does not require the same burden of vascular risk factors as do patients with CS whose stroke is caused by some other occult mechanism. This has been formally described as index event bias, because the induced correlation between risk factors (known and unknown) can confound estimates of the causal contribution of these correlated risk factors to recurrence risk.34
Nevertheless, one can use the correlation of characteristics with PFO that arise in the CS population to arrive at estimates for the PFO-attributable fraction, conditional on patient characteristics. That is, one can use the differences in characteristics in patients with CS with and without a PFO to arrive at patient-specific probabilities of finding a PFO even before a transesophageal echocardiogram, or other imaging study, is performed. In turn, through understanding the relationship between PFO prevalence and attributable fraction for patients with CS, one can estimate the likelihood an observed PFO is pathogenically related to CS. Ideally, this estimate can be used to target interventions (eg, device-based PFO closure) to those most likely to benefit.
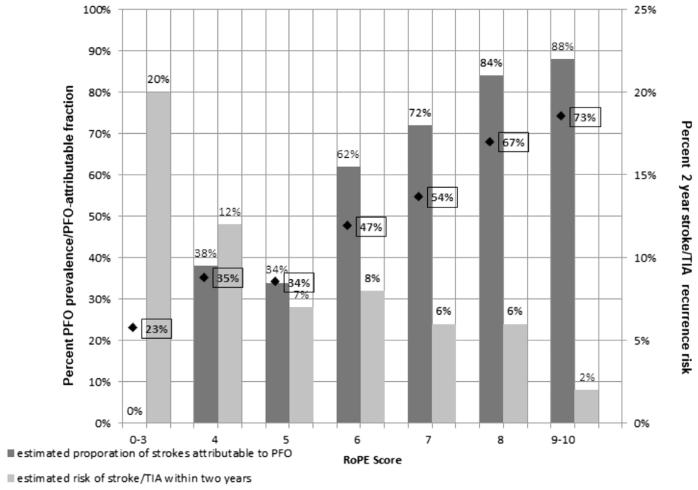
Recently, investigators have derived and begun to apply a risk score to improve clinical decision making for patients with CS and PFO. The RoPE study was designed to examine the ideas that only patients with a high risk of recurrent paradoxic embolism will benefit from device-based PFO closure, and that the attributable recurrence risk can be estimated based on the likelihood an observed PFO is related to CS and risk of stroke recurrence.35 This research group combined 12 studies of patients with CS investigated for PFO (N = 3674) to create a multivariate regression model predicting the presence of PFO for patients with CS. This model, based on easily identifiable clinical characteristics, was converted into an easy to use point score (RoPE score, Table 1), which allows estimation of a stratum-specific PFO attributable fraction for patients with CS (Fig. 3).
Table 1. The RoPE score.
| Characteristic | Points | RoPE Score |
|---|---|---|
| No history of hypertension | 1 | |
| No history of diabetes | 1 | |
| No history of stroke or TIA | 1 | |
| Nonsmoker | 1 | |
| Cortical infarct on imaging | 1 | |
| Age | ||
| 18–29y | 5 | |
| 30–39 y | 4 | |
| 40–49 y | 3 | |
| 50–59 y | 2 | |
| 60–69 y | 1 | |
| ≥70 y | 0 | |
| Total Score (sum of individual points) | ||
| Maximum Score (A patient <30 y with no hypertension, no diabetes, no history of stroke or TIA, nonsmoker, and cortical infarct) |
10 | |
| Minimum Score (A patient ≥70 y with hypertension, diabetes, prior stroke, current smoker, and no cortical infarct) |
0 | |
Clinical risk score based on easily identifiable characteristics to predict the probability that an observed PFO is related to CS. Higher point score indicates an increased likelihood that a PFO is related to an index CS.
Adapted from Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related versus incidental patent foramen ovale in cryptogenic stroke. Neurology 2013;81(7):623; with permission.
Fig. 3.
Estimated proportion of strokes attributable to PFO for patients with CS and estimated risk of stroke/TIA recurrence within 2 years as a function of RoPE score (x-axis). ◆ and % in boxes represent stratum-specific PFO prevalence.
In general, for patients with CS and traditional stroke risk factors (eg, older age, smoking, hypertension, diabetes) and the absence of cortical infarct location on neuroimaging, it is less likely that an identified PFO is related to their index CS. In contrast, for younger patients with CS with few of these traditional stroke risk factors and a cortical infarct location, it is more likely that an identified PFO is related to CS. One of the key observations from this large database was that stroke recurrence risk was highest for patients with the lowest PFO attributable fraction (see Fig. 3). This finding underscores the difficulty of patient selection for PFO closure. Although the clinical intuition supporting this score is compelling, whether this approach can be used to identify a more treatment-responsive population for clinical trials, or to ultimately target PFO-specific therapy, remains unclear.
OPTIMAL ANTITHROMBOTIC APPROACH
Although ischemic stroke is an arterial event, venous thromboembolism is likely the most appropriate therapeutic target in the setting of paradoxic embolism through a PFO. Theoretically, treatment aimed at venous thromboembolism (anticoagulation) might be more effective than antiplatelet therapy for minimizing the risk of recurrent stroke. Ambiguity surrounding this issue dominates the current standard of care because recent clinical trials have left decisions about medical therapy to the discretion of treating physicians.18-20 The only relevant randomized trial of comparing antithrombotic therapies, The PFO in Cryptogenic Stroke Study, included all patients in the Warfarin-Aspirin Recurrent Stroke Study who had a TEE.36 Of the subset of patients with CS and PFO (N = 98), there was no significant difference in the rate of stroke, TIA, or death at 2 years for those treated with warfarin or aspirin (P = .48; HR, 0.72; 95% CI, 0.29–1.81). A recent meta-analysis that included PFO in Cryptogenic Stroke Study along with observational studies of patients with CS and PFO suggests a similarly sized relative benefit for anticoagulation compared with antiplatelet therapy for patients with CS and PFO.37 The component studies in this meta-analysis, however, did not control for confounding by indication, an important issue because patients treated with anticoagulation are different from those treated with antiplatelet therapy with respect to the likelihood of having purported high-risk echocardiographic or neuroradiologic features.38
Currently, guidelines recommend only antiplatelet therapy for ischemic strokes unless a clear (or presumed) embolic source in the heart is identified (eg, mechanical heart valves or atrial fibrillation) (Level of Clinical Efficacy 2-1, Level of Evidence T1-T3).22 These guidelines may lead to undertreatment of an important potential therapeutic target for patients with CS that is caused by paradoxic embolism. In the absence of clear data it may be reasonable to consider anticoagulant therapy, in essence directed toward the likely pathophysiologic cause of stroke, for patients with likely PFO-related CS with high recurrence risk and lower risk of bleeding. Those with CS in the setting of PFO most accurately represent a subgroup of patients with embolic strokes of undetermined source39; an ongoing clinical trial is attempting to understand the medical treatment that is most appropriate for these patients.40 For now there is no consensus on the preferred antiplatelet or anticoagulant medical regimes for these patients and treatment decisions are often made, in the setting of pathogenic uncertainty, with a weak evidentiary base.
SUMMARY
PFO is common in the general population and as a result it is frequently encountered in the search for cardioembolic sources of ischemic stroke. It is unclear if PFO-specific anatomic or physiologic features can identify high-risk PFOs. Recent clinical trials of device-based closure for patients with CS and PFO have not shown benefit, although they have been limited by low stroke recurrence rates. Subsequent meta-analyses have shown trends toward benefit for some, although these have generally not been statistically significant for the composite end points that emulate the primary trial analyses. Fortunately, investigators have recently developed a risk score to identify the likelihood an observed PFO is related to CS. However, more work is needed to determine whether device-based closure targeted toward a subgroup of patients might be beneficial. In the meantime optimal medical therapy for patients with CS and PFO is unknown and studies continue to better define and treat patients with likely paradoxic embolism to minimize the risk of stroke recurrence.
Treatment decisions for patients with PFO and CS should be individualized because many observed PFOs are incidental and are unrelated to an index CS. Patients with CS and PFO represent a heterogeneous group where the likelihood an observed PFO is related to CS and also the risk of stroke recurrence are highly variable, although they can be estimated. Device-based PFO closure holds promise as an effective therapy if it can be directed toward patients with a likely paradoxic embolism and a reasonably high risk of recurrence. Further research is needed to establish a firmer evidentiary basis for the optimal medical approach, and the value of device-based PFO closure.
KEY POINTS.
Three recent clinical trials of device-based PFO closure were negative with respect to their intention-to-treat primary outcomes, although meta-analyses showed promising beneficial trends.
Many believe that anticoagulation therapy is indicated in these patients, whose stroke may be caused by venothromboembolic disease, but reliable evidence for this is lacking.
The RoPE score can stratify patients with CS based on easily identifiable clinical characteristics according to the likelihood a CS may be attributable to a discovered PFO.
For patients with CS and PFO, recurrent stroke risk is lowest in those with clinical features most suggestive of a PFO-attributable stroke.
It is unknown whether risk scores can identify patients who are likely to benefit from device-based PFO closure.
REFERENCES
- 1.Di Tullio MR. Patent foramen ovale: echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr. 2010;23(2):144–55. doi: 10.1016/j.echo.2009.12.008. [quiz: 220] [DOI] [PubMed] [Google Scholar]
- 2.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112(7):1063–72. doi: 10.1161/CIRCULATIONAHA.104.524371. [DOI] [PubMed] [Google Scholar]
- 3.Kutty S, Sengupta PP, Khandheria BK. Patent foramen ovale. J Am Coll Cardiol. 2012;59(19):1665–71. doi: 10.1016/j.jacc.2011.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Di Tullio MR, Sacco RL, Sciacca RR, et al. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. 2007;49(7):797–802. doi: 10.1016/j.jacc.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 5.Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47(2):440–5. doi: 10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Petty GW, Khandheria BK, Meissner I, et al. Population-based study of the relationship between patent foramen ovale and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81(5):602–8. doi: 10.4065/81.5.602. [DOI] [PubMed] [Google Scholar]
- 7.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318(18):1148–52. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 8.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55(8):1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 9.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40(7):2349–55. doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mojadidi MK, Roberts SC, Winoker JS, et al. Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: a bivariate meta-analysis of prospective studies. JACC Cardiovasc Imaging. 2014;7(3):236–50. doi: 10.1016/j.jcmg.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Serena J, Jiménez-Nieto M, Silva Y, et al. Patent foramen ovale in cerebral infarction. Curr Cardiol Rev. 2010;6(3):162–74. doi: 10.2174/157340310791658794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agmon Y, Khandheria BK, Meissner I, et al. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation. 1999;99(15):1942–4. doi: 10.1161/01.cir.99.15.1942. [DOI] [PubMed] [Google Scholar]
- 13.Lee J-Y, Song J-K, Song J-M, et al. Association between anatomic features of atrial septal abnormalities obtained by omni-plane transesophageal echocardiography and stroke recurrence in cryptogenic stroke patients with patent foramen ovale. Am J Cardiol. 2010;106(1):129–34. doi: 10.1016/j.amjcard.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Thaler DE, Ruthazer R, Weimar C, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs other PFOs. Neurology. 2014;83(3):221–6. doi: 10.1212/WNL.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigatelli G, Dell’Avvocata F, Cardaioli P, et al. Permanent right-to-left shunt is the key factor in managing patent foramen ovale. J Am Coll Cardiol. 2011;58(21):2257–61. doi: 10.1016/j.jacc.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 16.Schuchlenz HW, Saurer G, Weihs W, et al. Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004;17(3):231–3. doi: 10.1016/j.echo.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging. 2014;7(1):125–31. doi: 10.1161/CIRCIMAGING.113.000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368(12):1083–91. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 19.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368(12):1092–100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- 20.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366(11):991–9. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 21.Elmariah S, Furlan AJ, Reisman M, et al. Predictors of recurrent events in patients with cryptogenic stroke and patent foramen ovale within the CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale) trial. JACC Cardiovasc Interv. 2014;7(8):913–20. doi: 10.1016/j.jcin.2014.01.170. [DOI] [PubMed] [Google Scholar]
- 22.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 23.Meier B. Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart. 2005;91(4):444–8. doi: 10.1136/hrt.2004.052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaraja V, Raval J, Eslick GD, et al. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ. 2013;22(11):903–9. doi: 10.1016/j.hlc.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Spencer FA, Lopes LC, Kennedy SA, et al. Systematic review of percutaneous closure versus medical therapy in patients with cryptogenic stroke and patent foramen ovale. BMJ Open. 2014;4(3):e004282. doi: 10.1136/bmjopen-2013-004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riaz IB, Dhoble A, Mizyed A, et al. Transcatheter patent foramen ovale closure versus medical therapy for cryptogenic stroke: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. 2013;13:116. doi: 10.1186/1471-2261-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capodanno D, Milazzo G, Vitale L, et al. Updating the evidence on patent foramen ovale closure versus medical therapy in patients with cryptogenic stroke: a systematic review and comprehensive meta-analysis of 2,303 patients from three randomised trials and 2,231 patients from 11 observational s. EuroIntervention. 2014;9(11):1342–9. doi: 10.4244/EIJV9I11A225. [DOI] [PubMed] [Google Scholar]
- 28.Hakeem A, Marmagkiolis K, Hacioglu Y, et al. Safety and efficacy of device closure for patent foramen ovale for secondary prevention of neurological events: Comprehensive systematic review and meta-analysis of randomized controlled trials. Cardiovasc Revasc Med. 2013;14(6):349–55. doi: 10.1016/j.carrev.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Kitsios GD, Thaler DE, Kent DM. Potentially large yet uncertain benefits: a meta-analysis of patent foramen ovale closure trials. Stroke. 2013;44(9):2640–3. doi: 10.1161/STROKEAHA.113.001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rengifo-Moreno P, Palacios IF, Junpaparp P, et al. Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2013;34(43):3342–52. doi: 10.1093/eurheartj/eht285. [DOI] [PubMed] [Google Scholar]
- 31.Pandit A, Aryal MR, Pandit AA, et al. Amplatzer PFO occluder device may prevent recurrent stroke in patients with patent foramen ovale and cryptogenic stroke: a meta-analysis of randomised trials. Heart Lung Circ. 2014;23(4):303–8. doi: 10.1016/j.hlc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Ntaios G, Papavasileiou V, Makaritsis K, et al. PFO closure vs. medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol. 2013;169(2):101–5. doi: 10.1016/j.ijcard.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 33.Stortecky S, da Costa BR, Mattle HP, et al. Percutaneous closure of patent foramen ovale in patients with cryptogenic embolism: a network meta-analysis. Eur Heart J. 2015;36(2):120–8. doi: 10.1093/eurheartj/ehu292. [DOI] [PubMed] [Google Scholar]
- 34.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305(8):822–3. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81(7):619–25. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105(22):2625–31. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 37.Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43(2):422–31. doi: 10.1161/STROKEAHA.111.631648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaler DE, Ruthazer R, Weimar C, et al. Determinants of antithrombotic choice for patent foramen ovale in cryptogenic stroke. Neurology. 2014;83(21):1954–7. doi: 10.1212/WNL.0000000000001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429–38. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 40.Diener HC, Easton J, Granger C, et al. Rationale, objectives and design of a secondary stroke prevention study of dabigatran etexilate versus acetylsalicylic acid in patients with embolic stroke of undetermined source (RE-SPECT-ESUS); Presented at the European Stroke Conference; Nice, France. May 7, 2014. [Google Scholar]