Abstract
Background: Polyunsaturated fatty acids (PUFAs) may play a role in fracture, but studies have been largely confined to estimates of dietary intake.
Objective: We aimed to examine associations between fatty acids measured in late life and fish-oil consumption in early life, midlife, and late life with osteoporotic fracture risk.
Design: Osteoporotic fractures were determined from medical records over 5–9 y of follow-up in men and women aged 66–96 y. Data were analyzed from 1438 participants including 898 participants who were randomly selected from the Age, Gene/Environment Susceptibility Study, which is an observational study, and 540 participants with incident fracture. Plasma phospholipid fatty acids were assessed by using gas chromatography. Fish-oil consumption was assessed by using validated questionnaires as never (referent), less than daily, or daily. HRs and 95% CIs adjusted for age, education, height, weight, diabetes, physical activity, and medications were estimated by using Cox regression.
Results: In men, the highest tertile of PUFAs, n–3 (ω-3), and eicosapentaenoic acid were associated with decreased fracture risk [HRs (95% CIs): 0.60 (95% CI: 0.41, 0.89), 0.66 (0.45, 0.95), and 0.59 (0.41, 0.86), respectively]. In women, PUFAs tended to be inversely associated with fracture risk (P-trend = 0.06), but tertiles 2 and 3 were not independently associated with risk. Tertile 2 of n–6 and arachidonic acid was associated with fracture risk in women [HRs (95% CIs): 1.43 (1.10, 1.85) and 1.42 (1.09, 1.85), respectively]. Daily fish-oil consumption in late life was associated with lower fracture risk in men (HR: 0.64; 95% CI: 0.45, 0.91). Daily fish-oil consumption in midlife was associated with lower fracture risk in women (HR: 0.75; 95% CI: 0.58, 0.98).
Conclusions: Greater PUFA concentrations may be associated with lower osteoporotic fracture risk in older adults, particularly in men. Critical time periods for n–3 fatty acid consumption may differ by sex.
Keywords: fatty acids, fish oil, osteoporotic fracture, aging, omega-3, bone health
INTRODUCTION
Fractures are a pervasive public health problem in older adults. With the aging population, the number of fractures is expected to steadily increase (1). The consequences of a fracture are long lasting and affect the quality of life (2, 3). Older individuals who suffer from a fracture are more likely to be unable to walk independently, have difficulty with activities of daily living, to enter nursing homes, and die prematurely (2, 3). Therefore, it is important to identify potentially modifiable factors that are related to fracture risk.
Epidemiologic studies have suggested a potential role of dietary fatty acids, mainly estimated by using a food-frequency questionnaire (FFQ),6 in fractures (4–8). In the Women’s Health Initiative Study, inverse associations between the estimated consumption of MUFAs, PUFAs, n–6 fatty acids, and hip fracture and positive associations with n–3 fatty acids (9) were shown. In a study of 76,000 women and 45,000 men, inverse associations between PUFAs, n–6 fatty acids, and linoleic acid (LA) consumption and fracture risk were reported in women but not in men (6). However, weak or nonsignificant associations were reported for PUFA consumption (5) and n–3 consumption (4). Inconsistencies between studies may reflect methodologic limitations of estimating fatty acids from an FFQ, cross-sectional analysis, and lack of information on fish-oil supplementation.
Circulating fatty acids are an objective measure of fatty acids available to peripheral tissues and, therefore, less subject to errors of dietary intake estimation methods, but studies have been limited. The Framingham Osteoporosis Study showed inverse associations between plasma arachidonic acid (AA) and hip fracture risk in 765 men and women (10). A case-control study of 658 women showed lower hip-fracture risk with greater red blood cell n–3 fatty acids, EPA, and α-linolenic acid (ALA) and greater hip fracture risk with higher plasma n–6:n–3 ratios (11). Together, these studies suggested inverse associations between n–3 fatty acids and fracture risk, whereas the role of n–6 fatty acids remains unclear.
The aim of this study was to define associations between plasma fatty acids, particularly n–3 and n–6 classes, and incident osteoporotic fracture risk in a substudy of 1438 older men and women from the AGES-Reykjavik (Age, Gene/Environment Susceptibility Study). To our knowledge, this is the largest study to date with measured plasma fatty acids and fracture outcomes. In addition, this study provides novel insight by examining long-term exposure to marine n–3 fatty acids during early life, midlife, and late life.
METHODS
Study population
The AGES-Reykjavik is a random sample of 5764 men and women nested in the Reykjavik Study, which is a single-center population-based cardiovascular cohort begun in 1967 to study heart disease (12, 13). At study baseline (2002–2006), participants were aged 66–96 y. Details of the study design are provided in Harris et al. (14). All participants provided written informed consent, and the study was approved by the Institutional Review Board of the Icelandic Heart Association and the Intramural Research Program of the National Institute on Aging (VSN: 00–063).
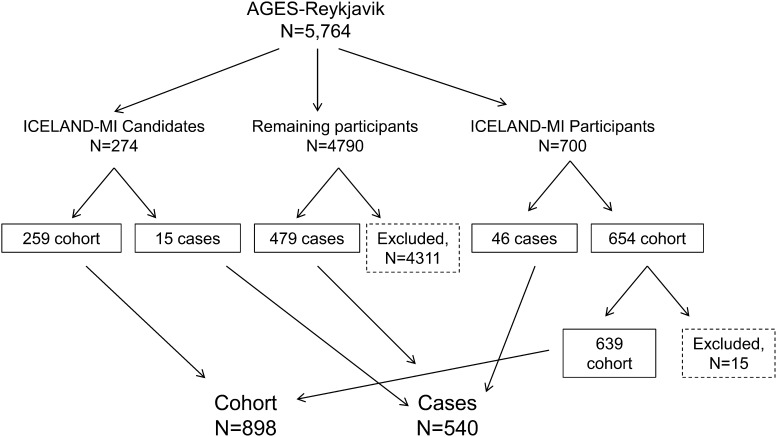
The case-cohort study design is detailed in Figure 1. Cases were defined as any AGES-Reykjavik participant without a history of osteoporotic fracture (defined in Fracture section below) at baseline and incident osteoporotic fracture during follow-up. A total of 540 cases were identified. Forty-six cases overlapped with a parallel substudy in AGES-Reykjavik of silent myocardial infarction (MI), ICELAND-MI (15, 16). Fifteen cases overlapped with individuals who were ICELAND-MI candidates (no implanted devices or severe kidney disease) but did not participate. The cohort was ICELAND-MI participants who did not develop a fracture and consented to link their data to the fracture registry (n = 639) and 259 individuals from the ICELAND-MI candidate list, which resulted in 898 cohort individuals and an overall analytic sample of 1438 subjects. Of these subjects, 1375 participants had data on fish-oil consumption in early life, midlife, and late life. Our case-cohort sample did not differ from the overall AGES-Reykjavik population with respect to demographics and covariates except for a slight difference in osteoporosis-medication use (2.09% in included participants; 3.24% in excluded participants; P = 0.03; Supplemental Table 1).
FIGURE 1.
Case-cohort study design of fatty acids and osteoporotic fracture risk in the AGES-Reykjavik. A total of 540 individuals developed an osteoporotic fracture; 46 cases occurred within the context of the substudy on myocardial infarction (MI), ICELAND-MI, and 15 cases occurred in individuals who were candidates for the ICELAND-MI but did not participate. A total of 639 cohort members were drawn from the ICELAND-MI (overall n = 700 minus 15 who did not consent to fracture registry linkage minus 46 cases). The remaining 259 cohort members were ICELAND-MI candidates. AGES-Reykjavik, Age, Gene/Environment Susceptibility Study.
Fatty acids
Blood samples were collected at the AGES-Reykjavik baseline after an overnight fast and stored at −80°C. Fatty acids were measured in plasma phospholipids that reflect short-term dietary consumption and fatty acids available to the periphery. Analyses for the ICELAND-MI and additional case-cohort individuals were carried out consecutively at the Biomarker Laboratory, Fred Hutchinson Cancer Research Center. Plasma lipids were extracted by using the method of Folch et al. (17). Phospholipids were separated from other lipids by using one-dimensional thin-layer chromatography (18). Fatty acid methyl esters were prepared by direct transesterification (19) and separated by using gas chromatography (Agilent Technologies 7890 Gas Chromatograph flame ionization detector; Supelco fused-silica 100-m capillary column SP-2560; initially at 160°C for 16 min, ramped up at 3.0°C/min to 240°C, and held for 15 min). The identification, precision, and accuracy were continuously evaluated by using both model mixtures of known fatty acid methyl esters and established in-house control pools. Fatty acids were expressed as the weight percentage of the total phospholipid fatty acids analyzed. Individual fatty acids were summed to calculate the major lipid classes of SFAs, MUFAs, PUFAs; n–3 and n–6 fatty acids; and the n–6:n–3 fatty acid ratio. In addition, we examined fatty acids previously associated with bone health including LA, AA, ALA, EPA, and DHA and further explored docosapentaenoic acid (DPA) because this fatty acid has seldom been reported in relation with bone health. The CV from pooled quality-control samples for LA, AA, ALA, EPA, DHA, and DPA were all <2.5%. CVs for other major fatty acids were 0.77% (palmitic), 0.47% (stearic), and 0.42% (oleic).
Fish-oil consumption
At the AGES-Reykjavik baseline, participants completed a self-administered FFQ on retrospective dietary consumption in early life (ages 14–19 y), midlife (ages 40–50 y), and late life (AGES-Reykjavik baseline ages 66–96 y) (20). The FFQ for midlife was validated with dietary data gathered from the same individuals in midlife (Reykjavik Study), and late-life intake was validated against weighed dietary intake. Correlations for fish liver oil ranged from 0.42 to 0.56 (P < 0.001) (20, 21). Early-life dietary habits have not yet been validated but are consistent with residency dependent dietary habits (i.e., higher fish consumption in coastal areas) (22). We concentrated on the consumption of fish liver oil (herein referred to as fish oil) because of the high content of n–3 fatty acids. In comparison, fish consumption in Iceland is primarily of lean species like haddock and cod that have low n–3 contents and, thus, was not considered here. Information on the composition of the supplement was not available, but the most common form of fish oil in Iceland is cod liver oil, which is rich in vitamin D, vitamin A, and n–3 fatty acids. Fish-oil consumption was categorized as never, less than daily (<1 time/mo, 1–3 times/mo, 1–2 times/mo, or 5–6 times/wk), or daily.
Fracture
The Reykjavik Study fracture registry includes fractures from enrollment in the Reykjavik Study (1967–1991) through 31 December 2011 (3). All residents in Iceland have a unique personal identification number that enables the identification and examination of hospital records. Fractures treated on an outpatient basis were always referred to the only outpatient trauma clinic in Reykjavik. Inpatient and outpatient reports from all hospitals in Reykjavik were manually examined, and fractures, including the site and date, were verified. Beginning with the introduction of computerized medical documents in 1983, all medical records for participants were manually examined and verified for fracture site and date. The same 2 orthopedic surgeons were used to confirm fractures if necessary. We focused on low-trauma fractures associated with osteoporosis (23) and from a fall from standing height or less. Our definition encompassed the following sites (International Statistical Classification of Diseases, 10th revision, codes): vertebrae (n = 69; S12.0–S12.2, S22.0, S22.1, S32.0, and T08), pelvis (n = 39; S32.1 and S32.3–S32.5), proximal humerus (n = 77; S42.2 and S42.3), distal forearm (n = 124; S52.5 and S52.6), hip (n = 190; S72.0–S72.2), proximal tibia (n = 6; S82.1), and ankle (n = 35; S82.5–82.9), for which the fracture registry has a capture rate of ∼97% (24). In the event of multiple fractures during the follow-up period, the time to the first fracture was used. Additional details of the fracture registry are provided in previous publications (24, 25).
Covariates
Covariates associated with osteoporotic fracture (26) were selected including age, education, smoking status, height, weight, physical activity, prevalent type 2 diabetes, estrogen replacement therapy, glucocorticoids, and use of osteoporosis medications raloxifen, calcitonin, and bisphosphonates. All covariates were from the AGES-Reykjavik baseline. Education was classified as primary school, high school, college, and university. Smoking was categorized as never, former, or current smoker. Physical activity was assessed by using a questionnaire as the frequency of moderate to vigorous physical activity in the year before baseline. Height and weight were measured by using standardized protocols (14). Type 2 diabetes was determined from a self-report, medication use, and clinical assessment. Medications were determined from medications brought to the clinic and from a questionnaire.
Statistical analysis
All analyses were stratified by sex because of known differences in fracture risk (25). Missing covariate data were estimated from 5 multiple imputations by using chained equations; the majority of missing data were for glucocorticoids (n = 116) followed by physical activity (n = 61). Differences between groups were assessed by using t tests for continuous variables and chi-square or Fisher’s exact test for categorical variables. Cox proportional hazards models were used to estimate HRs and 95% CIs for fatty acids in tertiles (reference group: tertile 1) and fish-oil consumption (reference group: never). Proportional hazards were confirmed from an examination of Kaplan-Meier curves and Schoenfeld residuals. Model 1 was adjusted for age and education. Model 2 was adjusted as for model 1 and for smoking status, height, weight, prevalent diabetes, physical activity, and estrogen and glucocorticoid medication use. Potential benefits of fish-oil consumption at multiple time periods were tested by comparing osteoporotic fracture risk in individuals who reported daily consumption in 1, 2, or all time periods (early life, midlife, and late life) with individuals who never consumed fish oil in all time periods. Tests for trend for fatty acids and fish-oil categories were performed by assigning the median value to each tertile or category and modeling this as a continuous variable. Sensitivity analyses were conducted by excluding participants (n = 30) who were using medication(s) for osteoporosis. All tests were 2-sided with significance set at P < 0.05. Analyses were performed with STATA version 12.1 software (StataCorp LP).
RESULTS
Characteristics of participants with and without an incident osteoporotic fracture are shown in Table 1. The median (IQR) follow-up was 7.0 y (4.1–7.6 y). Compared with participants without osteoporotic fracture, participants with incident osteoporotic fracture were older, more likely to be women, less likely to be current smokers, more likely to have lower weight and height, and more likely to report less moderate to vigorous physical activity (P < 0.05 for all).
TABLE 1.
Baseline characteristics of participants with and without incident osteoporotic fracture in a case-cohort study of participants in the AGES-Reykjavik Study1
| With fracture | Without fracture | P | |
| Participants, n | 540 | 898 | |
| Women, n (%) | 350 (64.8) | 469 (52.2) | <0.001 |
| Age at study baseline,2 y | 78.5 ± 5.69 | 76.5 ± 5.48 | <0.001 |
| Age at fracture,3 y | 82.2 ± 6.02 | — | — |
| Education less than high school, n (%) | 157 (29.1) | 206 (22.9) | 0.005 |
| Current smoker, n (%) | 58 (11.2) | 103 (11.7) | 0.004 |
| Height,2 cm | 165 ± 9.20 | 168 ± 9.21 | <0.001 |
| Weight,2 kg | 72.1 ± 14.2 | 76.6 ± 13.9 | <0.001 |
| BMI,2 kg/m2 | 26.3 ± 4.17 | 26.3 ± 4.29 | <0.001 |
| Frequency of moderate-to-vigorous physical activity, n (%) | <0.001 | ||
| Never | 279 (56.0) | 378 (43.0) | |
| Rarely | 73 (14.7) | 156 (17.8) | |
| Occasionally | 30 (6.02) | 57 (6.48) | |
| Moderate | 7 (14.1) | 134 (15.2) | |
| High | 46 (9.24) | 154 (17.5) | |
| Prevalent diabetes, n (%) | 67 (12.4) | 111 (12.4) | 0.98 |
| Glucocorticoid medication, n (%) | 14 (2.82) | 22 (2.66) | 0.86 |
| Estrogen medication, n (%) | 23 (4.30) | 48 (5.35) | 0.45 |
| Osteoporosis medication,4 n (%) | 11 (2.06) | 19 (2.12) | 1.00 |
Differences between groups were tested by using 2-sided t tests for continuous variables and the chi-square test for categorical variables or Fisher's exact test for cells with frequency <5%. For the following variables, n varies because of missing data: smoking, n = 37; BMI, n = 9; height, n = 5; weight, n = 4; physical activity, n = 61; and glucocorticoid medication, n = 116. AGES-Reykjavik, Age, Gene/Environment Susceptibility Study.
Values are means ± SDs.
Values are medians ± SDs
Excludes estrogen use.
The median (IQR) of plasma fatty acids stratified by osteoporotic fracture, sex, and fish-oil consumption in late life are shown in Table 2. SFAs and PUFAs accounted for the majority of fatty acids in plasma (45% and 38%, respectively). The majority of n–6 fatty acids were LA and AA. Non-marine sources (ALA) accounted for less of total n–3 fatty acids than did marine sources (EPA and DHA). The amount of n–6, n–3, LA, AA, ALA (men only), EPA, DHA, and DPA and the n–6:n–3 ratio differed across categories of fish-oil consumption (P < 0.05 for all).
TABLE 2.
Plasma phospholipid fatty acids (%) at baseline in a case-cohort study of participants in the AGES-Reykjavik Study1
| All case-cohort participants | Fish oil never | Fish oil less than daily | Fish oil daily | P-trend | |
| Men, n | 619 | 153 | 85 | 353 | |
| SFA | 45.3 (44.7–46.0) | 45.3 (44.6–46.0) | 45.4 (44.9–46.0) | 45.4 (44.7–45.9) | 0.56 |
| MUFA | 15.3 (14.2–16.3) | 15.4 (14.5–16.3) | 15.0 (14.1–16.1) | 15.1 (14.1–16.1) | 0.08 |
| PUFA | 38.6 (37.5–39.6) | 38.4 (37.4–39.4) | 38.6 (37.3–39.6) | 38.7 (37.7–39.6) | 0.15 |
| n–6 | 28.2 (25.5–30.3) | 29.7 (28.0–31.3) | 29.3 (27.1–31.4) | 27.0 (24.5–29.0) | <0.001 |
| n–3 | 10.5 (8.41–12.8) | 8.55 (7.50–10.2) | 9.23 (8.00–11.1) | 12.0 (10.0–14.1) | <0.001 |
| n–6:n–3 ratio | 2.69 (2.00–3.57) | 3.50 (2.76–4.08) | 3.15 (2.44–3.96) | 2.29 (1.75–2.85) | <0.001 |
| LA | 17.4 (15.7–19.5) | 18.3 (16.4–19.8) | 17.5 (16.3–19.6) | 16.9 (15.3–19.1) | 0.002 |
| AA | 6.72 (5.84–7.76) | 7.45 (6.43–8.38) | 7.36 (6.17–8.21) | 6.26 (5.42–7.25) | <0.001 |
| ALA | 0.21 (0.17–0.26) | 0.22 (0.19–0.26) | 0.21 (0.16–0.26) | 0.20 (0.17–0.24) | 0.02 |
| EPA | 2.58 (1.71–3.97) | 1.74 (1.41–2.33) | 2.01 (1.59–2.84) | 3.30 (2.38–4.66) | <0.001 |
| DHA | 6.40 (5.24–7.50) | 5.37 (4.72–6.30) | 5.86 (5.02–6.71) | 7.10 (6.07–8.00) | <0.001 |
| DPA | 1.18 (1.05–1.30) | 1.14 (1.02–1.27) | 1.15 (1.04–1.27) | 1.20 (1.07–1.34) | 0.001 |
| Women, n | 819 | 204 | 86 | 494 | |
| SFA | 45.5 (45.0–46.1) | 45.5 (44.9–46.2) | 45.5 (44.9–46.2) | 45.5 (45.0–46.1) | 0.84 |
| MUFA | 15.2 (14.4–16.4) | 15.4 (14.3–16.3) | 15.0 (14.1–16.5) | 15.2 (14.4–16.2) | 0.88 |
| PUFA | 38.3 (37.3–39.1) | 38.4 (37.4–39.2) | 38.3 (37.3–39.2) | 38.3 (37.3–39.1) | 0.99 |
| n–6 | 28.6 (26.0–30.3) | 30.0 (28.8–31.4) | 29.2 (27.6–30.8) | 27.4 (24.8–29.7) | <0.001 |
| n–3 | 9.72 (8.14–12.1) | 8.32 (7.38–9.40) | 8.90 (7.59–10.5) | 10.8 (9.01–13.4) | <0.001 |
| n–6:n–3 ratio | 2.98 (2.19–3.68) | 3.62 (3.07–4.19) | 3.38 (2.68–4.07) | 2.56 (1.84–3.30) | <0.001 |
| LA | 17.8 (15.9–19.5) | 18.6 (16.9–19.9) | 18.6 (16.9–19.9) | 17.4 (15.4–19.1) | <0.001 |
| AA | 6.72 (5.72–7.75) | 7.40 (6.57–8.48) | 7.06 (6.19–7.90) | 6.33 (5.40–7.28) | <0.001 |
| ALA | 0.21 (0.17–0.26) | 0.21 (0.18–0.26) | 0.22 (0.17–0.26) | 0.21 (0.17–0.26) | 0.37 |
| EPA | 2.26 (1.63–3.41) | 1.64 (1.33–2.15) | 1.87 (1.42–2.90) | 2.79 (1.96–4.19) | <0.001 |
| DHA | 6.03 (5.10–7.12) | 5.35 (4.65–5.94) | 5.55 (4.79–6.36) | 6.60 (5.62–7.78) | <0.001 |
| DPA | 1.11 (0.99–1.24) | 1.05 (0.96–1.16) | 1.07 (0.97–1.18) | 1.14 (1.02–1.28) | <0.001 |
All values are medians; IQRs in parentheses. Data are shown separately for men and women, reflecting sex-stratified risk analyses. Data are from 1438 case-cohort participants, of whom 1375 subjects had data on fish-oil consumption in late life at study baseline (ages 66–96 y) from a food-frequency questionnaire. P-trend values are from ANOVA comparisons across categories of fish-oil consumption. AA, arachidonic fatty acid; AGES-Reykjavik, Age, Gene/Environment Susceptibility Study; ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid.
Table 3 depicts associations between fatty acids and risk of incident osteoporotic fracture. Relative to tertile 1, tertile 3 of PUFAs, n–3, and EPA were associated with lower osteoporotic fracture risk in men with minimal (model 1) and additional adjustment for risk factors (model 2) [HRs: 0.60 (95% CI: 0.41, 0.89), 0.66 (95% CI: 0.45, 0.95), and 0.59 (95% CI: 0.41, 0.86), respectively]. The P-trend across tertiles of PUFAs, n–3, and EPA indicated significant inverse associations with incident osteoporotic fracture in models 1 and 2. MUFAs were positively associated with osteoporotic fracture risk in both models [model 2: HR, 1.60 (95% CI: 1.09, 2.33)]. In women, relative to tertile 1, tertile 2 of n–6 and AA were associated with increased osteoporotic fracture risk in minimally and fully adjusted models [model 2 n–6: HR, 1.43 (95% CI: 1.10, 1.85); model 2 AA: HR, 1.42 (95% CI: 1.09, 1.85)]. Tests for trends indicated a borderline lower risk of osteoporotic fracture risk with greater PUFAs (model 2: P = 0.06). The exclusion of participants who reported osteoporosis medication use did not change associations (data not shown).
TABLE 3.
Associations between tertiles of fatty acids and risk of osteoporotic fracture in a case-cohort study of participants in the AGES-Reykjavik Study1
| Men |
Women |
|||||||||
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||
| IQR (%) | Cohort, n | Cases, n | HR (95% CI) | HR (95% CI) | IQR (%) | Cohort, n | Cases, n | HR (95% CI) | HR (95% CI) | |
| SFA | ||||||||||
| Tertile 1 | 44.0–44.7 | 140 | 66 | 1.00 | 1.00 | 44.3–45.0 | 168 | 103 | 1.00 | 1.00 |
| Tertile 2 | 45.2–45.5 | 153 | 53 | 0.71 (0.49, 1.02) | 0.71 (0.49, 1.04) | 45.4–45.7 | 150 | 122 | 1.12 (0.86, 1.46) | 1.19 (0.91, 1.55) |
| Tertile 3 | 46.0–46.5 | 136 | 70 | 1.03 (0.73, 1.44) | 1.10 (0.77, 1.56) | 46.1–46.7 | 151 | 121 | 1.15 (0.89, 1.50) | 1.22 (0.94, 1.59) |
| P-trend | — | — | — | 0.85 | 0.56 | — | — | — | 0.29 | 0.15 |
| MUFA | ||||||||||
| Tertile 1 | 13.1–14.2 | 160 | 46 | 1.00 | 1.00 | 13.4–14.4 | 164 | 107 | 1.00 | 1.00 |
| Tertile 2 | 14.9–15.5 | 148 | 58 | 1.18 (0.80, 1.75) | 1.15 (0.77, 1.72) | 15.0–15.6 | 155 | 117 | 1.14 (0.88, 1.49) | 1.17 (0.90, 1.52) |
| Tertile 3 | 16.3–17.6 | 121 | 85 | 1.62 (1.12, 2.34) | 1.60 (1.09, 2.33) | 16.3–17.6 | 150 | 122 | 1.10 (0.84, 1.43) | 1.13 (0.86, 1.49) |
| P-trend | — | — | — | 0.008 | 0.01 | — | — | — | 0.51 | 0.37 |
| PUFA | ||||||||||
| Tertile 1 | 36.2–37.5 | 123 | 83 | 1.00 | 1.00 | 35.8–37.3 | 146 | 125 | 1.00 | 1.00 |
| Tertile 2 | 38.3–38.8 | 145 | 61 | 0.79 (0.57, 1.11) | 0.82 (0.58, 1.16) | 38.0–38.6 | 147 | 125 | 1.09 (0.85, 1.40) | 1.10 (0.85, 1.42) |
| Tertile 3 | 39.6–40.5 | 161 | 45 | 0.62 (0.43, 0.91) | 0.60 (0.41, 0.89) | 39.1–40.2 | 176 | 96 | 0.78 (0.59, 1.02) | 0.76 (0.58, 1.00) |
| P-trend | — | — | — | 0.01 | 0.01 | — | — | — | 0.08 | 0.06 |
| n–6 | ||||||||||
| Tertile 1 | 22.4–25.5 | 144 | 62 | 1.00 | 1.00 | 23.0–26.0 | 160 | 111 | 1.00 | 1.00 |
| Tertile 2 | 27.5–28.8 | 133 | 73 | 1.32 (0.94, 1.86) | 1.30 (0.92, 1.85) | 27.8–29.2 | 132 | 140 | 1.34 (1.04, 1.72) | 1.43 (1.10, 1.85) |
| Tertile 3 | 30.2–32.1 | 152 | 54 | 1.01 (0.70, 1.47) | 0.97 (0.67, 1.42) | 30.3–31.9 | 177 | 95 | 0.86 (0.65, 1.15) | 0.91 (0.68, 1.21) |
| P-trend | — | — | — | 0.92 | 0.91 | — | — | — | 0.36 | 0.56 |
| n–3 | ||||||||||
| Tertile 1 | 7.11–8.42 | 134 | 72 | 1.00 | 1.00 | 6.87–8.14 | 162 | 109 | 1.00 | 1.00 |
| Tertile 2 | 9.78–11.2 | 141 | 65 | 0.89 (0.64, 1.25) | 0.92 (0.65, 1.29) | 9.12–10.3 | 146 | 126 | 1.16 (0.90, 1.51) | 1.17 (0.90, 1.53) |
| Tertile 3 | 12.8–15.5 | 154 | 52 | 0.61 (0.43, 0.88) | 0.66 (0.45, 0.95) | 12.1–15.0 | 161 | 111 | 0.98 (0.75, 1.28) | 0.93 (0.71, 1.23) |
| P-trend | — | — | — | 0.009 | 0.03 | — | — | — | 0.87 | 0.60 |
| n–6:n–3 ratio | ||||||||||
| Tertile 1 | 1.45–2.00 | 148 | 58 | 1.00 | 1.00 | 1.55–2.19 | 156 | 115 | 1.00 | 1.00 |
| Tertile 2 | 2.44–2.93 | 143 | 63 | 1.27 (0.88, 1.82) | 1.22 (0.85, 1.76) | 2.73–3.20 | 146 | 126 | 1.10 (0.85, 1.42) | 1.15 (0.89, 1.49) |
| Tertile 3 | 3.57–4.48 | 138 | 68 | 1.39 (0.97, 1.99) | 1.32 (0.92, 1.90) | 3.68–4.49 | 167 | 105 | 0.94 (0.71, 1.23) | 0.99 (0.75, 1.30) |
| P-trend | — | — | — | 0.07 | 0.14 | — | — | — | 0.66 | 0.95 |
| LA | ||||||||||
| Tertile 1 | 13.6–15.7 | 140 | 66 | 1.00 | 1.00 | 13.7–15.9 | 155 | 116 | 1.00 | 1.00 |
| Tertile 2 | 16.8–18.1 | 145 | 61 | 0.83 (0.59, 1.19) | 0.83 (0.58, 1.19) | 17.3–18.3 | 155 | 117 | 1.08 (0.83, 1.40) | 1.10 (0.85, 1.43) |
| Tertile 3 | 19.5–21.5 | 144 | 62 | 0.97 (0.68, 1.38) | 0.91 (0.63, 1.31) | 19.5–21.0 | 159 | 113 | 0.99 (0.76, 1.29) | 1.02 (0.78, 1.33) |
| P-trend | — | — | — | 0.83 | 0.58 | — | — | — | 0.96 | 0.90 |
| AA | ||||||||||
| Tertile 1 | 5.03–5.84 | 142 | 64 | 1.00 | 1.00 | 5.03–5.72 | 163 | 108 | 1.00 | 1.00 |
| Tertile 2 | 6.37–7.12 | 143 | 63 | 1.25 (0.87, 1.78) | 1.25 (0.87, 1.80) | 6.39–7.02 | 145 | 127 | 1.41 (1.09, 1.83) | 1.42 (1.09, 1.85) |
| Tertile 3 | 7.76–9.24 | 144 | 62 | 1.26 (0.87, 1.81) | 1.26 (0.87, 1.84) | 7.76–9.19 | 161 | 111 | 1.17 (0.89, 1.54) | 1.24 (0.94, 1.64) |
| P-trend | — | — | — | 0.22 | 0.22 | — | — | — | 0.25 | 0.12 |
| ALA | ||||||||||
| Tertile 1 | 0.14–0.17 | 145 | 61 | 1.00 | 1.00 | 0.14–0.17 | 157 | 114 | 1.00 | 1.00 |
| Tertile 2 | 0.19–0.22 | 146 | 60 | 0.89 (0.63, 1.28) | 0.86 (0.60, 1.24) | 0.20–0.22 | 148 | 124 | 1.15 (0.89, 1.49) | 1.16 (0.90, 1.51) |
| Tertile 3 | 0.26–0.34 | 138 | 68 | 1.04 (0.73, 1.48) | 0.99 (0.70, 1.42) | 0.26–0.33 | 164 | 108 | 0.93 (0.71, 1.21) | 0.91 (0.70, 1.19) |
| P-trend | — | — | — | 0.81 | 1.00 | — | — | — | 0.59 | 0.50 |
| EPA | ||||||||||
| Tertile 1 | 1.27–1.71 | 128 | 78 | 1.00 | 1.00 | 1.20–1.63 | 160 | 111 | 1.00 | 1.00 |
| Tertile 2 | 2.23–2.96 | 144 | 62 | 0.81 (0.58, 1.14) | 0.81 (0.57, 1.14) | 2.04–2.52 | 145 | 127 | 1.19 (0.92, 1.55) | 1.24 (0.95, 1.60) |
| Tertile 3 | 3.97–5.46 | 157 | 49 | 0.55 (0.39, 0.80) | 0.59 (0.41, 0.86) | 3.40–5.24 | 164 | 108 | 0.97 (0.74, 1.27) | 0.96 (0.73, 1.26) |
| P-trend | — | — | — | 0.001 | 0.005 | — | — | — | 0.82 | 0.77 |
| DHA | ||||||||||
| Tertile 1 | 4.48–5.24 | 136 | 70 | 1.00 | 1.00 | 4.32–5.10 | 164 | 107 | 1.00 | 1.00 |
| Tertile 2 | 6.02–6.83 | 146 | 60 | 0.75 (0.53, 1.06) | 0.78 (0.54, 1.11) | 5.74–6.33 | 146 | 126 | 1.28 (0.99, 1.66) | 1.28 (0.98, 1.66) |
| Tertile 3 | 7.50–8.60 | 147 | 59 | 0.75 (0.53, 1.06) | 0.80 (0.56, 1.14) | 7.12–8.48 | 159 | 113 | 1.04 (0.79, 1.36) | 1.28 (0.98, 1.66) |
| P-trend | — | — | — | 0.10 | 0.21 | — | — | — | 0.80 | 0.92 |
| DPA | ||||||||||
| Tertile 1 | 0.94–1.05 | 140 | 66 | 1.00 | 1.00 | 0.87–0.99 | 158 | 113 | 1.00 | 1.00 |
| Tertile 2 | 1.14–1.22 | 148 | 58 | 0.74 (0.52, 1.06) | 0.75 (0.52, 1.08) | 1.07–1.15 | 151 | 121 | 1.03 (0.79, 1.33) | 1.01 (0.78, 1.31) |
| Tertile 3 | 1.30–1.49 | 140 | 66 | 0.83 (0.59, 1.18) | 0.87 (0.61, 1.24) | 1.24–1.39 | 160 | 112 | 0.97 (0.74, 1.26) | 0.91 (0.70, 1.20) |
| P-trend | — | — | — | 0.33 | 0.48 | — | — | — | 0.82 | 0.51 |
Associations were tested by using multivariate Cox hazard models. Model 1 was adjusted for age at the AGES-Reykjavik Study baseline and education. Model 2 was adjusted as for model 1 and for smoking status, height, weight, prevalent diabetes, physical activity, and estrogen and glucocorticoid use. Tests for trend were performed by assigning the median value to each tertile and modeling this as a continuous variable. AA, arachidonic acid; AGES-Reykjavik, Age, Gene/Environment Susceptibility Study; ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid.
Risk of osteoporotic fracture by fish-oil consumption across early life, midlife, and late life is shown in Table 4. There were no associations with early-life consumption in men or women. In men, daily consumption in late life was associated with reduced osteoporotic fracture risk and remained associated after adjustment for risk factors [model 2: HR, 0.64 (95% CI: 0.45, 0.91)]. In women, daily consumption in midlife was associated with reduced osteoporotic fracture risk in minimally and fully adjusted models [model 2: HR, 0.75 (95% CI: 0.58, 0.98)]. Associations were not altered when participants who reported osteoporosis medication use were excluded (data not shown). There did not appear to be any benefit of greater exposure to fish-oil consumption across the life span on osteoporotic risk (Table 5).
TABLE 4.
Risk of osteoporotic fracture by fish-oil consumption across the life span in a case-cohort study of participants in the AGES-Reykjavik Study1
| Men |
Women |
|||||||
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||
| Cohort, n | Cases, n | HR (95% CI) | HR (95% CI) | Cohort, n | Cases, n | HR (95% CI) | HR (95% CI) | |
| Early life | ||||||||
| Never | 156 | 54 | 1.00 | 1.00 | 178 | 149 | 1.00 | 1.00 |
| <Daily | 131 | 57 | 1.25 (0.85, 1.82) | 1.31 (0.89, 1.93) | 108 | 69 | 0.94 (0.71, 1.26) | 0.90 (0.67, 1.20) |
| Daily | 134 | 61 | 1.18 (0.81, 1.71) | 1.20 (0.82, 1.76) | 174 | 110 | 0.84 (0.65, 1.07) | 0.82 (0.64, 1.06) |
| P-trend | — | — | 0.39 | 0.36 | — | — | 0.16 | 0.12 |
| Midlife | ||||||||
| Never | 111 | 47 | 1.00 | 1.00 | 125 | 114 | 1.00 | 1.00 |
| <Daily | 133 | 42 | 0.82 (0.54, 1.24) | 0.81 (0.52, 1.25) | 111 | 80 | 0.89 (0.66, 1.18) | 0.89 (0.66, 1.19) |
| Daily | 177 | 83 | 1.09 (0.75, 1.56) | 1.12 (0.77, 1.63) | 224 | 134 | 0.75 (0.58, 0.97) | 0.75 (0.58, 0.98) |
| P-trend | — | — | 0.52 | 0.33 | — | — | 0.03 | 0.03 |
| Late life | ||||||||
| Never | 98 | 56 | 1.00 | 1.00 | 114 | 90 | 1.00 | 1.00 |
| <Daily | 58 | 27 | 0.93 (0.59, 1.48) | 0.90 (0.56, 1.45) | 48 | 39 | 1.14 (0.78, 1.66) | 1.10 (0.75, 1.62) |
| Daily | 266 | 89 | 0.62 (0.44, 0.87) | 0.64 (0.45, 0.91) | 296 | 198 | 0.90 (0.70, 1.15) | 0.87 (0.67, 1.12) |
| P-trend | — | — | 0.004 | 0.01 | — | — | 0.31 | 0.22 |
Associations were tested by using multivariate Cox hazard models. Fish-oil consumption was assessed from a food-frequency questionnaire. Early life was defined as ages 14–19 y, midlife was defined as ages 40–50 y, and late life was defined as ages 66–96 y. Model 1 was adjusted for age at the AGES-Reykjavik baseline and education. Model 2 was adjusted as for model 1 and for smoking status, height, weight, prevalent diabetes, physical activity, and estrogen and glucocorticoid use. Tests for trend were performed by assigning the median value to each category and modeling this as a continuous variable. AGES-Reykjavik, Age, Gene/Environment Susceptibility Study.
TABLE 5.
Risk of osteoporotic fracture by fish-oil consumption across early life, midlife, and late life assessed by using a food-frequency questionnaire in a case-cohort study of participants in the AGES-Reykjavik Study1
| Men |
Women |
|||||
| Cohort, n | Cases, n | HR (95% CI) | Cohort, n | Cases, n | HR (95% CI) | |
| Never in all time periods | 39 | 25 | 1.00 | 52 | 46 | 1.00 |
| Daily in 1 time period | 123 | 32 | 0.55 (0.32, 0.94) | 123 | 91 | 0.91 (0.63, 1.30) |
| Daily in 2 time periods | 81 | 40 | 0.92 (0.55, 1.56) | 87 | 57 | 0.85 (0.57, 1.26) |
| Daily in all time periods | 89 | 36 | 0.70 (0.41, 1.19) | 121 | 71 | 0.78 (0.53, 1.13) |
| P-trend | — | 0.11 | — | — | 0.55 | |
Associations were tested by using multivariate Cox hazard models. Fish-oil consumption in early life, midlife, and late life was assessed by using a food-frequency questionnaire. Daily in one or 2 time periods represents any time period (e.g., daily in early life, midlife, or late life). The analysis was adjusted for age at the AGES-Reykjavik baseline, education, smoking status, height, weight, prevalent diabetes, physical activity, and estrogen and glucocorticoid use. Tests for trend were performed by assigning the median value to each category and modeling this as a continuous variable. AGES-Reykjavik, Age, Gene/Environment Susceptibility Study.
DISCUSSION
In this study of older adults, we report associations between concentrations of plasma phospholipid fatty acids in relation to incident osteoporotic fracture risk in 1438 older men and women. PUFA appears to be an important factor in fracture risk in men and women. MUFA was positively associated with osteoporotic fracture risk in men, but because of the inverse correlation between MUFA with PUFA, risk may reflect a fatty acid profile characterized by lower PUFA. In men, n–3 fatty acids and EPA appeared to be particularly associated with lower osteoporotic fracture risk. Conversely, in women, there were positive associations between intermediate concentrations of n–6 and AA and osteoporotic fracture risk. Data on fish oil suggest lower risk of osteoporotic fracture with daily fish-oil consumption in late life in men and midlife in women.
There have been limited studies of circulating fatty acids and fracture with which to draw parallels. Both Orchard et al. (11) and Farina et al. (10) examined hip fracture as an outcome, whereas we used a wider definition that encompassed osteoporotic fractures at multiple sites. The fraction of blood used to assess fatty acids also differed [red blood cells in Orchard et al. (11), phosphatidylcholine in Farina et al. (10), and total phospholipids in our study, which reflect different lengths of exposure]. With these limitations taken into account, our finding of an inverse relation between n–3 fatty acids, EPA, and fracture risk in men aligns with Orchard et al. (11). Together, these results suggest that n–3 fatty acids, and particularly marine n–3 fatty acids, may be important factors for osteoporotic fracture. Our finding of a positive association between tertile 2 of AA and fracture risk in women contrasts with the inverse association reported by Farina et al. (10). Elevated risk for intermediate amounts of AA and n–6 but not higher concentrations is puzzling and additional research is needed to elucidate the role of n–6 fatty acids.
Our results extend previous trials that reported beneficial effects of fish-oil supplementation on bone mineral density (BMD) (27, 28) by suggesting that daily supplementation may be important for reducing subsequent osteoporotic fracture risk. Our results suggested a possible time dynamic with respect to fatty acids exposure in addition to a possible sex differential, whereby fish-oil consumption in late life in men and midlife in women appeared to be key time periods. Reasons for this difference are unclear but mirrored the results of associations between phospholipid n–3 fatty acids, EPA, and osteoporotic fracture in men only. Potential periodic interrelations between fish oil and skeletal health are important to explore further as these may have bearing on indications for supplementation. Note that we did not find any benefits of daily fish-oil consumption across the life span although it is possible that this reflected the low prevalence of never consuming fish oil in this population.
Relations between fatty acids and fracture risk have been hypothesized to be broadly related to bone formation and loss (29–32) including cellular effects via inflammatory modulation as well as osteoclast and osteoblast regulation, which potentially lead to effects on BMD. Serum n–3 fatty acids have been positively associated with BMD and bone accrual in healthy men (28). EPA supplementation has been shown to modestly increase femoral and lumbar spine BMD in older women with osteoporosis (27). We did not find evidence of fracture risk and daily fish-oil consumption during early life when peak BMD occurs; however, similar to previous analyses (10, 11), we did not adjust fracture estimates for BMD because of biological interrelations between BMD, fracture, and fatty acids. Therefore, we cannot comment on potential mechanisms underlying associations between fatty acids and fracture. The complex cause of fracture, which reflects a combination of reduced BMD, bone quality, and trauma from falls, is deserving of an in-depth exploration of potential mechanisms in relation to fatty acids, which we plan to undertake in a follow-up analysis.
One of the strengths of our study was the assessment of fatty acids by using different measurement domains (plasma phospholipid fatty acids, which are an objective measure of fatty acids available to the periphery, and fish-oil consumption from an FFQ). The availability of data from 3 key life stages uniquely enabled the assessment of long-term exposure to n–3 fatty acids in relation to fracture risk. However, long-term exposure assessment would have benefitted from validation of the FFQ for early life (validated for midlife and late life), and we did not have information on fatty acids during follow-up. An additional strength was data on prevalent and incident osteoporotic fracture, which were confirmed from medical records. The fracture registry contains data for the entire period of the Reykjavik study from which participants in the AGES-Reykjavik were drawn, which provided a comprehensive fracture history for participants. Therefore, we were able to minimize possible reverse causation by excluding participants with a previous fracture who may have had pre-existing risk factors for fracture that placed them at higher risk of incident fracture (33).
The worldwide consumption of fatty acids is diverse (34), which makes cross-country comparisons difficult. Iceland is typified by higher plasma n–3 fatty acids than in the United States but lower concentrations than in Asian countries (i.e., Korea and Japan) (35). Fish-oil supplementation is prevalent in Iceland, which may limit the generalizability of our results. However, national data from the United States depicted a rapid increase in fish-oil supplementation. From 2002 to 2007, the prevalence of fish-oil supplementation in individuals who use dietary supplements rose from 11.1% to 38.9% (36). Note that, in our population, cod liver oil was also a source of vitamins A and D. Thus, we could not rule out the potential confounding of our results with vitamins A and D, which have pivotal but opposing risk interrelations with bone health and fracture (37, 38). However, n–6 fatty acids and total PUFAs, which are less reflective of vitamin D, were inversely associated with osteoporotic fracture risk, suggesting that risk associations do not solely reflect vitamin D. In the future, it is important to explore interrelations between circulating fatty acids and fracture risk in additional populations with diverse characteristics to determine whether our results are generalizable. We did not correct for multiple comparisons to minimize the possibility of rejecting potentially important associations because this study is one of the few studies on circulating fatty acids and fracture, and comparisons were planned. The difference in fracture risk was >30% between low and high tertiles of PUFAs, EPA, and n–3 fatty acids and daily fish-oil supplementation in men, which suggest robust associations but more-conservative approaches such as Bonferroni adjustment may be warranted in future studies.
In conclusion, our results suggest that greater PUFA concentrations in old age are associated with reduced risk of osteoporotic fracture in men and may also be in women although associations did not reach significance. Of the PUFAs, n–3 fatty acids and EPA in men appeared to be particularly important, whereas n–6 fatty acids in women may potentially carry risk for fracture. Long-term exposure to marine n–3 fatty acids in the form of fish oil was not related to osteoporotic fracture risk, but daily fish-oil consumption during critical time periods may be associated with reduced risk of osteoporotic fracture in old age.
Supplementary Material
Acknowledgments
We thank Pho Diep for technical assistance with fatty acid analyses.
The authors’ responsibilities were as follows—RAM, TA, GE, VG, GS, and TBH: designed and conducted the research; XS, SS, KS, TFL, and IAB: provided essential data; RAM and TA: performed the statistical analyses; RAM: wrote the manuscript and took responsibility for the integrity of data and accuracy of the data analysis; IR, MEG, TFL, TBH, and LS: critically revised the manuscript; and all authors: had full access to all study data. Subsequent to the submission of the manuscript, RAM became an employee of DSM Nutritional Products. The work was not supported by DSM Nutritional Products. TBH, XS, IR, TFL, MEG, KS, SS, VG, GE, GS, LS, TA, and IAB reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: AA, arachidonic acid; AGES-Reykjavik, Age, Gene/Environment Susceptibility Study; ALA, α-linolenic acid; BMD, bone mineral density; DPA, docosapentaenoic acid; FFQ, food-frequency questionnaire, LA, linoleic acid; MI, myocardial infarction.
REFERENCES
- 1.Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res 1990;252:163–6. [PubMed] [Google Scholar]
- 2.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med 1997;103:12S–7S; discussion 17S–9S. [DOI] [PubMed]
- 3.Siggeirsdottir K, Aspelund T, Jonsson BY, Mogensen B, Launer LJ, Harris TB, Sigurdsson G, Gudnason V. Effect of vertebral fractures on function, quality of life and hospitalisation the AGES-Reykjavik study. Age Ageing 2012;41:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr 2011;141:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetou V, Orfanos P, Zylis D, Sieri S, Contiero P, Tumino R, Giurdanella MC, Peeters PH, Linseisen J, Nieters A, et al. Diet and hip fractures among elderly Europeans in the EPIC cohort. Eur J Clin Nutr 2011;65:132–9. [DOI] [PubMed] [Google Scholar]
- 6.Virtanen JK, Mozaffarian D, Willett WC, Feskanich D. Dietary intake of polyunsaturated fatty acids and risk of hip fracture in men and women. Osteoporos Int 2012;23:2615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtanen JK, Mozaffarian D, Cauley JA, Mukamal KJ, Robbins J, Siscovick DS. Fish consumption, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Bone Miner Res 2010;25:1972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin V, Reeves KW, Bertone-Johnson E. Fatty acid consumption and the risk of osteoporotic fracture. Nutr Rev 2013;71:600–10. [DOI] [PubMed] [Google Scholar]
- 9.Orchard TS, Cauley JA, Frank GC, Neuhouser ML, Robinson JG, Snetselaar L, Tylavsky F, Wactawski-Wende J, Young AM, Lu B, et al. Fatty acid consumption and risk of fracture in the Women's Health Initiative. Am J Clin Nutr 2010;92:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: the Framingham Osteoporosis Study. J Bone Miner Res 2012;27:1222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orchard TS, Ing SW, Lu B, Belury MA, Johnson K, Wactawski-Wende J, Jackson RD. The association of red blood cell n–3 and n–6 fatty acids with bone mineral density and hip fracture risk in the women's health initiative. J Bone Miner Res 2013;28:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornsson OJ, Davidsson D, Olafsson H, Olafsson O, Sigfusson N, Thorsteinsson T. Report XVIII. Health survey in the Reykjavik area. -Men. Stages I-II, 1967-1968, 1970-1971 and 1974-1975. Participants, invitation, response etc. Reykjavik, Iceland: The Icelandic Heart Association; 1979.
- 13.Bjornsson G, Bjornsson OJ, Davidsson D, Kristjansson BT, Olafsson O, Sigfusson N, Thorsteinsson T. Report abc XXIV. Health survey in the Rekjavik area. -Women. Stages I-III, 1968-1969, 1971-1972 and 1976-1978. Participants, invitation, response etc. Reykjavik, Iceland: The Icelandic Heart Association; 1982. [Google Scholar]
- 14.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Ballegooijen AJ, Visser M, Cotch MF, Arai AE, Garcia M, Harris TB, Launer LJ, Eiriksdottir G, Gudnason V, Brouwer IA. Serum vitamin D and parathyroid hormone in relation to cardiac structure and function: the ICELAND-MI substudy of AGES-Reykjavik. J Clin Endocrinol Metab 2013;98:2544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Schlierf G, Wood P. Quantitative determination of plasma free fatty acids and triglycerides by thin–layer chromatography. J Lipid Res 1965;6:317–9. [PubMed] [Google Scholar]
- 19.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20. [PubMed] [Google Scholar]
- 20.Eysteinsdottir T, Thorsdottir I, Gunnarsdottir I, Steingrimsdottir L. Assessing validity of a short food frequency questionnaire on present dietary intake of elderly Icelanders. Nutr J 2012;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eysteinsdottir T, Gunnarsdottir I, Thorsdottir I, Harris T, Launer LJ, Gudnason V, Steingrimsdottir L. Validity of retrospective diet history: assessing recall of midlife diet using food frequency questionnaire in later life. J Nutr Health Aging 2011;15:809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigurjonsson J. Survey on diet and health in Iceland (1939-1940). Reykjavik, Iceland: Icelandic Nutrition Council; 1943. [Google Scholar]
- 23.Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, Cummings SR. Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med 1991;115:837–42. [DOI] [PubMed] [Google Scholar]
- 24.Siggeirsdottir K, Aspelund T, Sigurdsson G, Mogensen B, Chang M, Jonsdottir B, Eiriksdottir G, Launer LJ, Harris TB, Jonsson BY, et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol 2007;22:631–9. [DOI] [PubMed] [Google Scholar]
- 25.Siggeirsdottir K, Aspelund T, Jonsson BY, Mogensen B, Gudmundsson EF, Gudnason V, Sigurdsson G. Epidemiology of fractures in Iceland and secular trends in major osteoporotic fractures 1989-2008. Osteoporos Int 2014;25:211–9. [DOI] [PubMed] [Google Scholar]
- 26.Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, LeBoff MS, Lewis CE, Chen Z, Stefanick ML, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA 2007;298:2389–98. [DOI] [PubMed] [Google Scholar]
- 27.Kruger MC, Coetzer H, de Winter R, Gericke G, van Papendorp DH. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milano) 1998;10:385–94. [DOI] [PubMed] [Google Scholar]
- 28.Högström M, Nordstrom P, Nordstrom A. n–3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 Study. Am J Clin Nutr 2007;85:803–7. [DOI] [PubMed] [Google Scholar]
- 29.Watkins BA, Lippman HE, Le Bouteiller L, Li Y, Seifert MF. Bioactive fatty acids: role in bone biology and bone cell function. Prog Lipid Res 2001;40:125–48. [DOI] [PubMed] [Google Scholar]
- 30.Watkins BA, Li Y, Seifert MF. Nutraceutical fatty acids as biochemical and molecular modulators of skeletal biology. J Am Coll Nutr 2001;20(5 Suppl):410S–S; discussion 417S–20S. [DOI] [PubMed]
- 31.Kruger MC, Coetzee M, Haag M, Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res 2010;49:438–49. [DOI] [PubMed] [Google Scholar]
- 32.Salari P, Rezaie A, Larijani B, Abdollahi M. A systematic review of the impact of n–3 fatty acids in bone health and osteoporosis. Med Sci Monit 2008;14:RA37–44. [PubMed] [Google Scholar]
- 33.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721–39. [DOI] [PubMed] [Google Scholar]
- 34.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n–3 and n–6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr 2006;83(6 Suppl):1483S–93S. [DOI] [PubMed] [Google Scholar]
- 35.Sekikawa A, Steingrimsdottir L, Ueshima H, Shin C, David Curb J, Evans RW, Hauksdottir AM, Kadota A, Choo J, Masaki K, et al. Serum levels of marine-derived n–3 fatty acids in Icelanders, Japanese, Koreans, and Americans-a descriptive epidemiologic study. Prostaglandins Leukot Essent Fatty Acids 2012;87:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CH, Wang CC, Kennedy J. Changes in herb and dietary supplement use in the U.S. adult population: a comparison of the 2002 and 2007 National Health Interview Surveys. Clin Ther 2011;33:1749–58. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257–64. [DOI] [PubMed] [Google Scholar]
- 38.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA 2002;287:47–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.