Abstract
Background: Resistance training (RT) improves muscle strength and overall physical function in older adults. RT may be particularly important in the obese elderly who have compromised muscle function. Whether caloric restriction (CR) acts synergistically with RT to enhance function is unknown.
Objective: As the primary goal of the Improving Muscle for Functional Independence Trial (I’M FIT), we determined the effects of adding CR for weight loss on muscle and physical function responses to RT in older overweight and obese adults.
Design: I’M FIT was a 5-mo trial in 126 older (65–79 y) overweight and obese men and women who were randomly assigned to a progressive, 3-d/wk, moderate-intensity RT intervention with a weight-loss intervention (RT+CR) or without a weight-loss intervention (RT). The primary outcome was maximal knee extensor strength; secondary outcomes were muscle power and quality, overall physical function, and total body and thigh compositions.
Results: Body mass decreased in the RT+CR group but not in the RT group. Fat mass, percentage of fat, and all thigh fat volumes decreased in both groups, but only the RT+CR group lost lean mass. Adjusted postintervention body- and thigh-composition measures were all lower with RT+CR except intermuscular adipose tissue (IMAT). Knee strength, power, and quality and the 4-m gait speed increased similarly in both groups. Adjusted postintervention means for a 400-m walk time and self-reported disability were better with RT+CR with no group differences in other functional measures, including knee strength. Participants with a lower percentage of fat and IMAT at baseline exhibited a greater improvement in the 400-m walk and knee strength and power.
Conclusions: RT improved body composition (including reducing IMAT) and muscle strength and physical function in obese elderly, but those with higher initial adiposity experienced less improvement. The addition of CR during RT improves mobility and does not compromise other functional adaptations to RT. These findings support the incorporation of RT into obesity treatments for this population regardless of whether CR is part of the treatment. This trial was registered at clinicaltrials.gov as NCT01049698.
Keywords: body composition, caloric restriction, intermuscular thigh fat, obesity, muscle quality, muscle strength and power, older adults, resistance training, weight loss
INTRODUCTION
Aging is associated with an inherent loss of musculoskeletal strength and power that leads to increased risk of falls and mobility limitations (1–4). Aging-related declines in muscle strength and power are not entirely accounted for by a loss of muscle mass because muscle quality (strength per unit of muscle) also decreases with age and is an even stronger risk factor for mobility disability (5). Moreover, total adiposity and regional adiposity increase with age, and losses of muscle mass and function are exacerbated in the more than one-third of older adults classified as overweight or obese (6–8). Currently, participation in resistance exercise training is the only therapy known to consistently improve muscle mass, strength, power, and quality and overall physical function in older adults (9, 10). However, the majority of previous resistance training (RT)5 research was conducted in nonobese elderly, although RT may be particularly important in obese older individuals who have compromised muscle quality and function.
The obese condition is characterized by several physiologic differences from the lean state that impair muscle function including greater lipid accumulation around [subcutaneous adipose tissue (SAT)] and within [intermuscular adipose tissue (IMAT)] targeted muscle groups. Greater IMAT in the thigh region is associated with lower knee extensor strength and power (11–14) and poorer lower-extremity physical function including slower gait speed and chair rise times (15, 16). Thigh IMAT is a strong predictor of aging-related declines in gait speed (16) and the onset of mobility disability (17). Additional evidence for a role of muscle lipid storage in affecting muscle strength and quality comes from a study in younger adults that showed that the loss of lower-extremity strength during limb suspension was related to increases in thigh IMAT (18).
Some data also suggested that excess stored fat may limit the magnitude of improvement in response to RT. For example, young adults with higher BMI and greater SAT surrounding the bicep exhibited an attenuated gain in bicep strength with RT, despite a similar relative increase in muscle size as in those who were normal weight or had less SAT (19, 20). A recent study in older adults showed that the quality of knee extensor muscle in response to a combined RT, endurance, and balance training intervention only improved in subjects with low thigh IMAT at baseline (21). Whether concomitant weight and fat loss during training improves muscular adaptations to RT is not known. We reported an inverse correlation between the magnitude of total fat loss and improvement in muscle strength and power in older adults who completed a combined exercise and weight-loss intervention (22). However, there are no controlled, prospective data on whether improvements in muscle strength and overall physical function are augmented by a loss of total or regional adipose tissue induced by caloric restriction (CR) during RT.
Therefore, the primary goal of this randomized trial was to determine the effects of adding CR for weight loss on muscle strength (primary outcome), power, and quality and other physical function responses to RT in older overweight and obese adults. We hypothesized that higher initial total adiposity and thigh IMAT would blunt improvements, and fat loss, particularly from within muscle, during RT would enhance improvements in muscle and physical function.
METHODS
Study design
The Improving Muscle for Functional Independence Trial (I’M FIT) (clinicaltrials.gov; NCT01049698) was a 5-mo, randomized controlled trial designed to determine whether CR enhances improvements in skeletal muscle function in response to RT in 126 older overweight and obese men and women. Participants were randomly assigned equally to a standardized, progressive RT intervention with CR (RT+CR) or without CR (RT). The study was approved by the Wake Forest School of Medicine Institutional Review Board, and all participants provided written informed consent to participate.
Study participants
Men and women from Forsyth County, North Carolina, and surrounding areas were recruited from June 2010 to June 2013 through local advertisements. Study participants were enrolled on the basis of the following general inclusion and exclusion criteria: 1) aged 65–79 y; 2) sedentary (no RT or purposeful aerobic training in the past 6 mo); 3) BMI (in kg/m2) from 27 to 35; 4) nonsmoking ≥1 y; 5) weight stable (<5% weight change in the past 6 mo); and 6) without insulin-dependent diabetes or evidence of clinical depression, cognitive impairment, heart disease, cancer, liver or renal disease, chronic pulmonary disease, uncontrolled hypertension, physical impairment, or any contraindication for exercise or weight loss (e.g., osteoporosis).
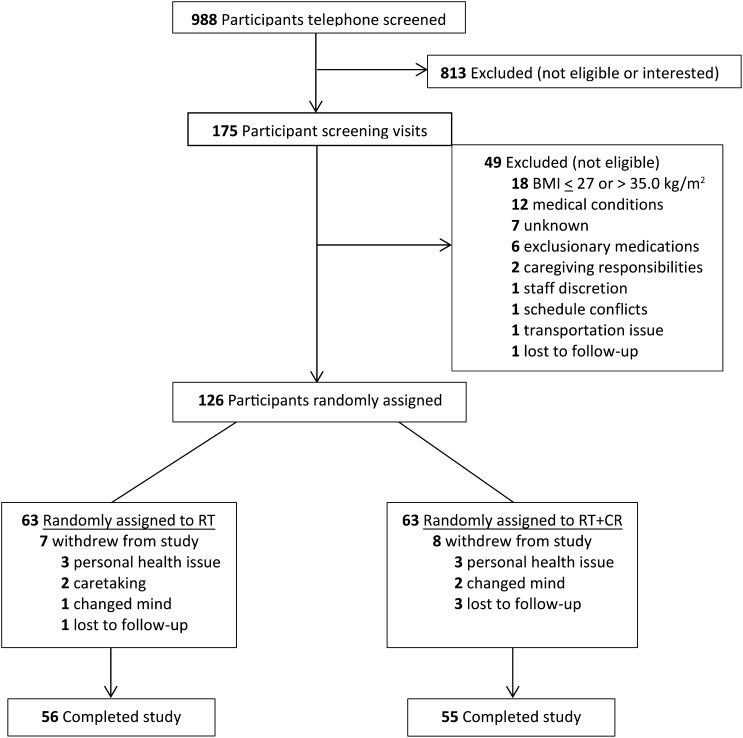
A total of 988 participants were screened by telephone to assess general eligibility criteria. Of these participants, 175 subjects were invited for a clinic screening that involved a medical history review, physical examination, cognitive and depression screening, fasting blood draw, and 12-lead resting electrocardiogram. A total of 126 participants met all study inclusion and exclusion criteria and were tested on study outcomes (by blinded assessors) before being randomly assigned (by using a computer-generated random assignment list that was stratified by sex) to one of the interventions by the study manager (Figure 1).
FIGURE 1.
Flow diagram of participant recruitment, enrollment, and retention. CR, caloric restriction; RT, resistance training.
Interventions
RT
All participants in the study underwent 5-mo of RT 3 d/wk on weight-stack resistance machines (Cybex International Inc. and Nautilus Inc.) at the Wake Forest University Clinical Research Center exercise facility. Two exercise interventionists supervised the training sessions and ensured that participants adjusted the equipment appropriately, performed the exercises safely, and maintained their training log books. Participants performed an initial 5-min warmup by walking or cycling at a slow pace followed by light stretching and concluded each session with a 5-min cool down and light stretching. The machines used were 1) leg press, 2) leg extension, 3) seated leg curl, 4) seated calf, 5) incline press, 6) compound row, 7) triceps press, and 8) bicep curl. The protocol involved a gradual progression of weight and repetitions during the first month to allow familiarization with the equipment, minimize muscle soreness, and reduce injury potential. The maximal weight that a person could lift with the correct form in a single repetition (1RM) was used to prescribe intensity. The training goal was to complete 3 sets of 10 repetitions for each exercise at 70% 1RM for that specific exercise. Participants rested ∼1 min between sets. Resistance was increased when a participant was able to complete 10 repetitions on the third set for 2 consecutive sessions. Strength testing was repeated every 4 wk, and training loads were adjusted to be consistent with the 70% 1RM goal. Each participant recorded the weight lifted, number of repetitions completed, and number of sets completed for each exercise in a training log.
CR
Participants assigned to RT only were instructed to follow a eucaloric diet, whereas those assigned to RT+CR underwent a dietary weight-loss intervention designed to elicit moderate weight loss (5–10%). This intervention incorporated meal replacements, nutrition education, and dietary behavior modification advice via weekly meetings with the study’s registered dietitian (RD) that took place either before or after one of their exercise sessions. Each participant was assigned a daily caloric intake to follow, which was derived from subtracting 600 kcal from his or her estimated daily energy needs for weight maintenance. A maximum of 2 meal replacements per day (shakes and bars; Slim-Fast Inc.) that contained ∼220 kcal with 7–10 g protein, 33–46 g carbohydrates, 1.5–5 g fat, and 2–5 g fiber were provided to participants for breakfast and lunch. Dinner and snack options were recommended by the RD in accordance with each participant’s daily caloric goals and tailored to allow for individual preferences for various food items. Participants were asked to keep a diet log of all foods consumed, and the logs were monitored weekly by the RD to verify compliance with the weight-loss intervention.
Assessments
All assessments took place in the Geriatric Research Center of the Wake Forest School of Medicine J Paul Sticht Center on Aging by examiners blinded to participant treatment assignment. All baseline assessments took place within 3 wk before the start of interventions. Physical performance postintervention assessments occurred during the last week of intervention, and body scans took place the week after interventions.
Body and thigh composition
Height and body mass were measured without shoes and outer garments removed. BMI was calculated as body mass divided by height squared. Whole-body fat mass, lean mass, and percentage of body fat were measured by using dual-energy X-ray absorptiometry (Delphi QDR; Hologic).
The thigh composition of the dominant leg was measured on a 64-slice computed tomography scanner (LightSpeed Plus; General Electric Medical Systems). Participants were positioned supine with arms above the head and legs positioned flat. Thigh scans were conducted at 120 KVp, 350 mA, and 10-mm helical with a pitch of 11.25 mm per rotation and a gantry speed of 0.8 s. Thigh muscle and adipose tissue volumes (in cm3) were measured by using a 5-cm section of the thigh centered at the junction of the proximal and middle-third of the femur as measured from the scout topogram. The volume of muscle and adipose tissue was segmented and measured by using the Advantage Windows 4.2 Volume Viewer (GE Healthcare). The thigh muscle area was considered the total area of nonadipose and nonbone tissue within the deep fascial plane. For the adipose tissue volume, sequences were reconstructed into the maximum 50-cm field of view to prevent the truncation of subcutaneous fat on larger individuals, and a 2.5-mm, no gap slice thickness was used. IMAT was separated from SAT by drawing a line along the deep fascial plane surrounding the thigh muscles.
Knee extensor strength, power, and quality
The prespecified primary outcome of maximal knee extensor strength [in Newton meters (Nm)] was measured with a dynamometer (Biodex Medical Systems Inc.) at speeds of 60° and 240°/s with the participant sitting and hips and knees flexed at 90°. To stabilize the hip joint and trunk, participants were restrained with straps at the chest, hip, and thigh. The seat height and depth as well as length of the lower leg were recorded to establish consistency between tests. Participants were asked to extend the knee and push as hard as possible against the resistance pad. The strength of the right leg recorded as the peak torque (in Nm) was used for analyses. Muscle power (in W) was determined as the product of the knee extensor peak torque (at the 240° speed) and the angular velocity (in radians/s) of the knee joint. We calculated muscle quality as the ratio of knee extensor peak torque to lean mass of the right leg assessed by using dual-energy X-ray absorptiometry (in Nm/kg leg lean mass).
Physical function and mobility
Mobility was measured by using a 400-m walk test for time. The participant was instructed to complete the distance (10 laps on a flat indoor surface 20 m in length) as quickly as possible without running. Lower-extremity function was assessed with the short physical performance battery (SPPB) (23), which consisted of a standing balance test, usual gait speed over a 4-m course, and time to complete 5 repeated chair rises with arms folded across the chest. Results from each of the 3 tests were scored from 0 (inability to perform the task) to 4 and summed for the total SPPB score, which ranged from 0 (lowest function) to 12 (highest function). Grip strength was measured twice on each hand to the nearest kilogram by using an isometric Hydraulic Hand Dynamometer (Jamar), and the maximal value from both hands was used in analyses. Participants were excluded from performing the test if they reported hand pain or recent wrist surgery.
Statistical analysis
The targeted sample size was predetermined to allow for a dropout rate of 15% and completion of 55 subjects/group. This sample size provided adequate statistical power (at an individual α = 0.017) to detect statistically and clinically significant group differences in sex-adjusted knee extensor strength (10 Nm) with ≥90% power and ≥80% power for meaningful group differences in secondary outcomes.
All statistical analyses were performed with SPSS software (version 21.0, IBM). An α = 0.05 was used to assess significance and all data were analyzed according to a randomly assigned group assignment. Baseline descriptive characteristics are reported as means (±SDs) or frequencies (percentages). The primary analysis was to assess statistical differences between groups for postintervention outcome values by using an ANCOVA. Besides the treatment group, each model contained age, sex, and the baseline value for the outcome. Other analyses included a 1-factor ANOVA used to assess between-group differences in baseline and change (baseline minus follow-up) values and a paired t test to assess differences between baseline and follow-up values within groups. Partial correlation analyses (adjusting for sex) were performed to examine relations between absolute changes in physical performance outcomes with baseline and change measures of body and thigh composition.
RESULTS
Retention, adherence, and baseline characteristics
Of 126 randomly assigned participants, 111 subjects (88%) completed the study (returned for final data-collection visit; see Consolidated Standards of Reporting Trials diagram in Figure 1). The retention of participants was not significantly different between groups (RT: 89%; RT+CR: 87%). The 15 participants who dropped out of the study did so reportedly because of life changes unrelated to study interventions, including relocation, family illness or caregiving responsibilities, unrelated personal health issues, change in work schedule, or new time constraints. The age, sex, race, medical status, or physical function of participants who did not complete the study did not differ from those who did complete the study. Adherence to the 3-d/wk RT protocol was very high and did not differ between groups; subjects in the RT group attended 86% of scheduled sessions, and those in the RT+CR group attended 89% of scheduled sessions. There were 2 intervention-related adverse events in the RT group and 5 intervention-related adverse events in the RT+CR group (all musculoskeletal complaints). All but one participant returned to the intervention, and training was extended if needed to complete the 20 wk.
Overall, the study sample could be considered a young-old sample (69.5 ± 3.7 y of age), overweight or obese (BMI: 30.6 ± 2.3), and mostly female (56.3%) and white (86.5%), and hypertension and osteoarthritis were the most prevalent self-reported comorbidities. These traits did not differ between study groups (Table 1).
TABLE 1.
Participant demographic and other characteristics at baseline1
| RT (n = 63) | RT+CR (n = 63) | |
| Age, y | 69.4 ± 3.62 | 69.6 ± 3.9 |
| Female, n (%) | 34 (54) | 37 (59) |
| White, n (%) | 54 (86) | 55 (87) |
| Body mass, kg | 87.3 ± 13.1 | 85.4 ± 11.7 |
| Height, cm | 168 ± 10 | 167 ± 9 |
| BMI, kg/m2 | 30.7 ± 2.4 | 30.4 ± 2.2 |
| Waist-to-hip ratio | 0.89 ± 0.09 | 0.89 ± 0.10 |
| Systolic blood pressure, mm Hg | 137 ± 22 | 134 ± 18 |
| Diastolic blood pressure, mm Hg | 76 ± 11 | 74 ± 10 |
| Self-reported comorbidity, n (%) | ||
| Hypertension | 29 (46) | 37 (59) |
| Diabetes3 | 9 (14) | 6 (10) |
| Sleep apnea | 20 (32) | 14 (22) |
| Arthritis | 37 (59) | 40 (64) |
| Chronic back pain | 15 (24) | 12 (19) |
| Medication use, n (%) | ||
| Antihypertensive | 37 (59) | 34 (54) |
| Cholesterol-lowering | 25 (40) | 34 (54) |
| Glucose control | 9 (14) | 7 (11) |
| Antidepressant/mood | 7 (11) | 13 (21) |
There were no significant differences between groups by using ANOVA at P < 0.05. CR, caloric restriction; RT, resistance training.
Mean ± SD (all such values)
Noninsulin-treated diabetes.
Treatment effects: body mass and whole-body and thigh composition
Table 2 shows baseline and mean changes in body mass and whole-body and thigh composition by study group. There were no group differences at baseline. Participants in the RT+CR group lost more body mass than did those in the RT group (−5.67% compared with −0.15% loss of initial mass, respectively). There was a large interindividual variation in the mass change in participants in the RT+CR group (range: +4.1 to −12.6 kg) with less variation in subjects in the RT group (+4.4 to −6.0 kg). In the RT+CR group, 35 participants lost ≥5% of initial body mass, and 14 subjects lost ≥10% of initial body mass, but 10 participants (18%) lost <2 kg. In the RT group, only 2 participants lost ≥5% of initial body mass, whereas 44 participants (80%) lost <2 kg.
TABLE 2.
Unadjusted whole-body and thigh composition; muscle strength, power, and quality; and physical function at baseline and changes with intervention1
| RT |
RT+CR |
||||
| Baseline (n = 61–63) | Changes relative to baseline (n = 53–56) | Baseline (n = 62–63) | Changes relative to baseline (n = 53–55) | P-between group2 | |
| Body mass, kg | 87.3 ± 13.1 | −0.1 ± 2.2 | 85.4 ± 11.7 | −4.9 ± 3.9† | <0.0001 |
| Fat mass, kg | 33.6 ± 5.5 | −0.6 ± 1.5* | 34.1 ± 5.2 | −3.6 ± 2.8† | <0.0001 |
| Lean mass, kg | 54.1 ± 11.9 | 0.3 ± 1.3 | 51.5 ± 10.5 | −1.1 ± 1.6† | <0.0001 |
| Percentage of body fat | 38.8 ± 6.5 | −0.6 ± 1.2* | 40.2 ± 6.2 | −2.2 ± 1.9† | <0.0001 |
| Total thigh volume, cm3 | 1540 ± 215 | 5.2 ± 89.8 | 1488 ± 168 | −94.2 ± 94.2† | <0.0001 |
| Thigh muscle volume, cm3 | 670 ± 164 | 20.2 ± 30.2† | 636 ± 138 | −0.9 ± 21.5 | <0.0001 |
| Thigh fat volume, cm3 | 769 ± 261 | −21.7 ± 62.5* | 759 ± 206 | −87.9 ± 71.9† | <0.0001 |
| SAT, cm3 | 737 ± 257 | −19.5 ± 61.0* | 728 ± 203 | −84.2 ± 68.5† | <0.0001 |
| IMAT, cm3 | 32.2 ± 16.2 | −2.2 ± 6.2* | 31.7 ± 16.0 | −3.8 ± 6.8† | 0.19 |
| Percentage of thigh as fat | 49.2 ± 12.6 | −1.7 ± 3.5* | 50.6 ± 10.9 | −3.1 ± 3.4† | <0.01 |
| Knee extensor strength, Nm | 112.7 ± 40.1 | 16.4 ± 21.9† | 108.5 ± 32.9 | 12.3 ± 14.8† | 0.26 |
| Knee extensor quality,3 Nm/kg | 14.29 ± 3.23 | 1.76 ± 2.40† | 14.55 ± 2.87 | 1.88 ± 1.90† | 0.77 |
| Knee extensor power, W | 200 ± 106 | 36 ± 86† | 222 ± 98 | 33 ± 75† | 0.85 |
| Grip strength, kg | 33.7 ± 11.6 | 0.9 ± 4.2 | 30.3 ± 10.9 | 2.8 ± 4.2† | 0.02 |
| Grip quality, kg/kg body mass | 0.38 ± 0.10 | 0.01 ± 0.05 | 0.35 ± 0.09 | 0.06 ± 0.05† | <0.0001 |
| Usual gait speed, m/s | 1.13 ± 0.19 | 0.08 ± 0.17* | 1.10 ± 0.18 | 0.09 ± 0.18† | 0.53 |
| Chair rise time,4 s | 11.9 ± 3.2 | −1.7 ± 3.0† | 12.7 ± 3.5 | −2.1 ± 4.0† | 0.62 |
| SPPB (0–12) | 10.9 ± 1.1 | 0.53 ± 1.20* | 10.6 ± 1.2 | 0.66 ± 1.00† | 0.55 |
| 400-m walk time, s | 308 ± 49 | 3 ± 41 | 307 ± 44 | −10 ± 29* | 0.05 |
| Self-reported disability5 (1–5) | 1.16 ± 0.21 | 0.02 ± 0.21 | 1.14 ± 0.19 | −0.05 ± 0.16* | 0.05 |
All values are means ± SDs. †,*Compared with baseline within group: †P < 0.0001, *P < 0.05. CR, caloric restriction; IMAT, thigh intermuscular adipose tissue volume; RT, resistance training; SAT, thigh subcutaneous adipose tissue volume; SPPB, short physical performance battery.
Between-group differences for baseline and change values were analyzed using a 1-factor ANOVA with significance at P < 0.05. There were no significant differences between groups at baseline. Within-group differences between baseline and follow-up values were determined by using a paired t test.
Per kilogram of leg lean mass.
Time for 5 consecutive chair rises as fast as possible.
Scale of 1 (usually did with no difficulty) to 5 (unable to do).
Decreases in total body fat mass, lean mass, and percentage of fat were all greater in the RT+CR group than in the RT group. Within the RT group, there were small but significant declines in total fat mass and percentage of fat but no mean change in total mass or lean mass. Within the RT+CR group, there was a significant loss of both fat and lean mass. The amount of lean mass lost was 26.9% of the total body mass lost and correlated positively with the amount of total (R = 0.64, P < 0.0001) and fat (R = 0.44) mass lost.
Changes in total thigh fat, SAT, and thigh muscle volume were greater in the RT+CR group than in the RT group, but decreases in IMAT were similar between groups (Table 2). The percentage of fat in the thigh decreased more in the RT+CR group than in the RT group. Within the RT group, total thigh fat, SAT, and IMAT volumes decreased significantly (by 3.2%, 3.0%, and 5.1%, respectively), and thigh muscle volume increased by 3.1%. Within the RT+CR group, all thigh fat volumes decreased significantly (by ∼12%), but thigh muscle volume did not change.
Model-adjusted (age, sex, and baseline value) least-squares means and 95% CIs for postintervention measures of body and thigh composition are shown in Table 3. Except for the IMAT volume, all values were significantly lower in the RT+CR group than in the RT group.
TABLE 3.
Model-adjusted postintervention body and thigh composition; muscle strength, power, and quality; and physical function least-squares means and adjusted group differences (95% CIs)1
| Postintervention values | RT | RT+CR | RT – RT+CR differences | P |
| Body mass, kg | 86.7 | 81.9 | 4.8 (3.7, 6.0) | <0.0001 |
| Fat mass, kg | 33.4 | 30.3 | 3.0 (2.2, 3.9) | <0.0001 |
| Lean mass, kg | 53.6 | 52.2 | 1.5 (0.9, 2.0) | <0.0001 |
| Percentage of body fat | 38.8 | 37.1 | 1.7 (1.1, 2.3) | <0.0001 |
| Total thigh volume, cm3 | 1513 | 1413 | 100 (66, 134) | <0.0001 |
| Thigh muscle volume, cm3 | 680 | 658 | 21 (11, 31) | <0.0001 |
| Thigh fat volume, cm3 | 737 | 672 | 64 (39, 90) | <0.0001 |
| Thigh SAT, cm3 | 707 | 644 | 63 (38, 88) | <0.0001 |
| Thigh IMAT, cm3 | 29.3 | 27.8 | 1.4 (−0.9, 3.7) | 0.23 |
| Percentage of thigh as fat | 48.0 | 46.7 | 1.4 (0.0, 2.7) | 0.04 |
| Knee extensor strength, Nm | 128.0 | 124.9 | 3.16 (−3.2, 9.5) | 0.46 |
| Knee extensor quality,2 Nm/kg | 15.37 | 15.62 | −0.25 (−0.93, 0.43) | 0.52 |
| Knee extensor power, W | 250 | 254 | −4.0 (−34.7, 26.7) | 0.79 |
| Grip strength, kg | 33.6 | 35.3 | −1.7 (−3.3, −0.03) | 0.05 |
| Grip quality, kg/kg body mass | 0.38 | 0.43 | −0.45 (−0.65, −0.02) | <0.0001 |
| Usual gait speed, m/s | 1.22 | 1.22 | 0.00 (−0.07, 0.06) | 0.84 |
| Chair rise time,3 s | 10.4 | 10.2 | 0.2 (−0.7, 1.1) | 0.68 |
| SPPB (0–12) | 11.3 | 11.4 | −0.1 (−0.5, 0.3) | 0.59 |
| 400-m walk time, s | 311 | 298 | 13 (0.35, 27) | 0.05 |
| Self-reported disability4 (1–5) | 1.17 | 1.09 | 0.08 (0.01, 0.14) | 0.03 |
n = 53–55. Values were adjusted for age, sex, and the baseline value of each outcome. P values were determined by using an ANCOVA between-group test of adjusted postintervention values. CR, caloric restriction; IMAT, thigh intermuscular adipose tissue volume; Nm, Newton meter; RT, resistance training; SAT, thigh subcutaneous adipose tissue volume; SPPB, short physical performance battery.
Per kilogram of leg lean mass.
Time for 5 consecutive chair rises as fast as possible.
Scale of 1 (usually did with no difficulty) to 5 (unable to do).
Treatment effects: muscle strength and power, muscle quality, and physical function
Table 2 shows baseline and mean changes in muscle strength and power, muscle quality, and physical function by study group. There were no group differences at baseline. Except for grip strength, changes in muscle and physical function did not differ between groups. Within each group, knee extensor strength and power as well as knee extensor muscle quality (calculated as knee strength per kilogram of leg lean mass) increased significantly (strength: RT, 15.3%; RT+CR, 13.0%; power: RT, 45.9%; RT+CT, 36.3%; and quality: RT, 12.1%; RT+CR, 13.6%). Both groups also increased in usual gait speed (RT: 7.9%; RT+CR: 9.5%) and SPPB score and improved the chair rise time (RT: 10.4%; RT+CR: 15.7%). The RT+CR group also significantly improved grip strength, 400-m walk time, and self-reported disability by 17.9%, 3.1%, and 3.4%, respectively, whereas these outcomes did not change in the RT group (Table 2).
The model-adjusted least-squares means and 95% CIs for postintervention measures of physical performance are shown in Table 3. Compared with the RT intervention, the RT+CR intervention resulted in greater grip strength, a faster 400-m walk time, and less self-reported disability. There were no group differences in knee extensor strength, power, or quality, usual gait speed, chair rise time, or SPPB.
Relation of changes in muscle function with initial body and thigh composition
Because we hypothesized that baseline adiposity would blunt the magnitude of improvement in muscle function in response to RT, we analyzed whether changes in muscle function in the RT group were related to baseline body and thigh composition by using a partial correlation analysis adjusted for sex. Percentage of changes in 400-m walk and chair rise times were positively related to initial fat mass (r = 0.32 and r = 0.32, P < 0.05), percentage of body fat (r = 0.42, P < 0.01; r = 0.24, P = 0.08), and IMAT volume (r = 0.28 and r = 0.27, P < 0.05) but not initial lean mass or thigh muscle, thigh fat, or SAT volumes. Changes in usual gait speed were not related to initial body or thigh composition. Changes in knee extensor strength and quality were negatively related to the initial percentage of body fat (r = −0.28 and r = −0.30, P < 0.05) and IMAT volume (r = −0.41 and r = −0.44, P < 0.01) but did not correlate with initial total fat or lean mass or thigh muscle, thigh fat, or SAT volumes. Changes in knee extensor power were related only to the initial percentage of body fat (r = −0.31, P < 0.05). None of the changes in muscle-function measures correlated with initial lean mass or muscle volume. To summarize, individuals with a lower percentage of total body fat and less IMAT at baseline exhibited greater improvement in 400-m walk time, chair rise time, and muscle strength and power in response to the RT intervention.
Relation of changes in muscle function with changes in body and thigh composition
We also analyzed whether the magnitude of improvement in performance outcomes was related to the degree of total or thigh fat loss or muscle gain by using a partial correlation analysis adjusting for sex. In both groups combined, the percentage of change in 400-m walk time correlated positively with the percentage of change in total fat mass (r = 0.25, P < 0.01) and IMAT volume (r = 0.22, P < 0.05) and the absolute change in the percentage of body fat (r = 0.28, P < 0.01) but not changes in total lean mass or thigh muscle volume. Grip strength per kilogram of body weight improved more in subjects who lost more fat mass (r = −0.39, P < 0.01) and percentage of body fat (r = −0.34, P < 0.01). Changes in usual gait speed, chair rise time, or knee extensor strength, power, and quality were not related to changes in whole-body or thigh composition.
DISCUSSION
Resistance exercise training improves the functional abilities of older persons (10); thus, the current physical activity guidelines recommend that all older adults perform muscle-strengthening activities ≥2 nonconsecutive days per week (9). These guidelines do not differ for the more than one-third of older adults with obesity whose muscle function is compromised (8) nor do they include a recommendation to reduce body weight and fat to enhance physical function or mobility in these patients. This randomized controlled trial tested the hypothesis that individuals with greater initial adiposity (particularly within skeletal muscle) would experience blunted functional responses to a standardized RT intervention and that the combination of CR for weight loss with RT would enhance responses more than would RT alone in a sample of older overweight and obese adults. We showed that RT alone improved body composition, including a reduced IMAT, but individuals with a higher total percentage of body fat and IMAT volume exhibited less improvement in knee extensor strength and power, muscle quality, and 400-m walk and chair rise times. The addition of the CR intervention to RT resulted in an ∼5.5% decrease in total body mass and a greater decline in total fat mass, total lean mass, percentage of body fat, and thigh total and SAT volumes than did RT alone, but there was no significant group difference in the magnitude of IMAT lost (although RT+CR lost ∼12% of their initial IMAT volume compared with ∼5% for RT only). Both RT alone and RT+CR significantly improved knee extensor strength, power, muscle quality, usual gait speed, chair rise time, and SPPB to a similar degree, whereas only RT+CR improved grip strength, 400-m walk time, and self-reported disability. RT and RT+CR interventions were safe and well tolerated in this population as evidenced by the very high overall compliance to the exercise protocol.
The majority of published RT studies in older adults (≥65 y of age) have been conducted in normal-weight or nonobese individuals (8, 24). Our data showed that, on average, RT alone improved knee extensor muscle strength (by 15%), power (by 46%), and quality (by 12%) as well as general lower-extremity physical function assessed by gait speed, chair rise time, and SPPB score in overweight and obese adults aged 65–79 y, which indicated that this intervention was effective for improving muscle and physical function in this subset of the older population. This finding is in line with the few previous studies of RT conducted in middle-aged to older adults with obesity (25–28) as well as a number of studies that showed RT increased gait speed and other functional measures in nonobese elderly (10). However, in our study, RT alone did not improve the long-distance walking ability (400-m walk time) despite strength gains, which suggested that weight loss may be necessary to elicit mobility improvements in this population. This result is notable because this measure of mobility is a very strong predictor of mortality and loss of independence (29, 30). Furthermore, even within participants in our study whose baseline BMI spanned from 27 to 35, the large interindividual range in the percentage of body fat (28–48%) and IMAT volume (7–74 cm3) at baseline was predictive of individual responses to RT. After adjustment for sex because of its large effect on body composition, subjects with a higher percentage of fat and IMAT volume had blunted responses to RT. This finding is similar to that of 3 previous studies [2 studies in younger adults (19, 20) and another study in older adults but with a combined RT, endurance, and balance training intervention (21)]. These data suggest that individuals with more total and intermuscular adiposity are at a physiologic disadvantage with respect to muscular adaptations in response to RT.
The finding that baseline adiposity exerts a negative influence on the magnitude of improvement in response to RT was not surprising because of known adverse consequences of excess total fat and muscle lipid content on muscle function. Obesity is consistently associated with a lower strength-to-mass ratio (muscle quality) in older adults (7, 31, 32), and a higher muscle lipid content is associated with compromised muscle strength and power (11–13). The mechanisms by which greater total and intermuscular fat impair muscle function in older adults are not definitively known but could be due to reduced adaptation to increased loading (33), reduced mitochondrial function and capillary density (34–37), or interference with contractile proteins perhaps secondary to local release and elevated concentrations of fatty acids or cytokines by fat (38–41).
We had hypothesized that the addition of CR to elicit weight loss during RT would augment responses to RT and that fat loss, particularly from within muscle, would enhance RT-induced improvements in muscle function. An additive beneficial effect of CR was evident only for grip strength, 400-m walk time, and self-reported disability. Contrary to our expectations, there was not enhanced improvement in knee extensor strength, power, or quality in subjects assigned to RT+CR. This result may have been due to the similar absolute loss of IMAT between groups. A decrease in muscle fat infiltration was shown in some (42, 43) but not all (44) previous studies of RT alone in nonobese persons. Thus to our knowledge, our finding that RT resulted in a significant decrease in IMAT as well as decrease in total fat mass, percentage of body fat, and thigh SAT in the absence of significant weight loss in obese older adults is a novel finding that supports the incorporation of RT into obesity treatments for this population.
The absence of an additive benefit from CR may also have been due to the modest amount of weight loss achieved (average of 5.5%); however, subgroup analyses limited to subjects who lost ≥6% or even >10% of body weight did not affect results (data not shown). In addition, correlation analyses in all participants combined confirmed the group analysis in that only changes in grip strength and 400-m walk time correlated with changes in total and thigh adiposity, whereas changes in usual gait speed, chair rise time, and strength, power, and quality were not related to changes in whole-body or thigh composition. Thus, because significant improvements in body composition and most measures of function were evident in both groups, the greatest clinical benefit to function could be attributed to the RT. However, metabolic, physiologic, quality-of-life, and other health variables related to obesity that were not assessed in this study may benefit from a combined intervention of CR and RT.
Because of justifiable concerns regarding the balance of risks and benefits of prescribing CR for intentional weight loss to overweight and obese older adults, it is important to point out that all physical performance measures improved in the RT+CR group despite the loss of total lean mass. Although the relative amount of total mass lost as lean tissue approached 27%, the absolute loss of lean mass was relatively small (mean loss: 1.1 kg), and thigh muscle volume did not decrease with RT+CR. Moreover, as we previously showed, any effects of the loss or blunted increase of lean mass with weight loss on physical performance is offset by improvements in total and regional adiposity (22, 45). Fat mass loss is a stronger predictor of functional improvements with weight loss than is lean mass loss (46). These findings, along with the observation in this study [and by other authors (47)] that strength and power increase substantially more than muscle mass or myofiber size, indicate that gains in muscle strength and physical function with RT are not compromised by a concomitant loss of lean tissue resulting from CR.
Primary strengths of this study were the randomized controlled trial design, large sample size of older adults within a fairly homogenous age range (65–79 y), and the tightly controlled and supervised RT intervention with high compliance. In addition, we used state-of-the-art and comprehensive measures of body and thigh composition and muscle strength and power and included several functional assessments that were important prognostic indicators. However, our data should be interpreted within the context of the study limitations. First, although the sample of older adults were all overweight or obese, they were also relatively high-functioning as evidenced by an average usual gait speed ≥1.1 m/s, high SPPB scores (only 6 subjects were ≤8), and little self-reported disability at baseline. A frailer, lower-functioning population may have responded differently to the interventions. Despite the large sample size, our study was not powered for stratified analyses on the basis of, for example, sex, race, age, and body composition. The study was also not designed to account for potential behavior or dietary nutrient changes that may have occurred in conjunction with the diet intervention. Thus, direct effects of CR and resultant weight loss could not be teased apart from whether the greater improvement in mobility and grip strength resulted from CR per se to a lowered fat mass (or body weight) resulting from the CR or a combination of both.
In conclusion, to our knowledge, this study showed the following several novel and clinically relevant findings that can guide treatment of the prevention of disability in obese older adults: 1) the performance of RT 3 d/wk at a moderately high intensity for 5 mo is safe and effective for improving body composition (including reducing intermuscular fat) and muscle strength and lower-extremity function; 2) when combined with RT, CR improves mobility more than does RT alone, and CR does not compromise other functional adaptations to RT; and 3) individuals with higher initial amounts of adiposity experienced less improvement in response to RT. Data are needed to understand the biological mechanisms by which fat and lipid stores affect muscle function and determine the legacy effects of these short-term interventions as well as effects of longer-term (2–3 y) interventions.
Acknowledgments
The authors’ responsibilities were as follows—BJN: was responsible for the study concept, design, and funding; BJN, EC, MFL, and APM: provided study oversight; EC, JJC, MFL, and APM: conducted the research and collected data; BJN, OD, and APM: analyzed and interpreted data; BJN and APM: drafted the manuscript and had primary responsibility for the final content of the manuscript; EC, OD, JJC, and MFL: critically revised the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CR, caloric restriction; IMAT, intermuscular adipose tissue; I’M FIT, Improving Muscle for Functional Independence Trial; RD, registered dietitian; RT, resistance training; SAT, subcutaneous adipose tissue; SPPB, short physical performance battery; 1RM, one-repetition maximum.
REFERENCES
- 1.Berger MJ, Doherty TJ. Sarcopenia: prevalence, mechanisms, and functional consequences. Interdiscip Top Gerontol 2010;37:94–114. [DOI] [PubMed] [Google Scholar]
- 2.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care 2010;13:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med 2011;27:387–99. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP, Visser M; Health Aging and Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc 2003;51:323–30. [DOI] [PubMed] [Google Scholar]
- 6.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev 2010;11:671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci 2011;66:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent HK, Raiser SN, Vincent KR. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev 2012;11:361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1435–45. [DOI] [PubMed] [Google Scholar]
- 10.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009;3:CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 2001;90:2157–65. [DOI] [PubMed] [Google Scholar]
- 12.Sipilä S, Koskinen SO, Taaffe DR, Takala TE, Cheng S, Rantanen T, Toivanen J, Suominen H. Determinants of lower-body muscle power in early postmenopausal women. J Am Geriatr Soc 2004;52:939–44. [DOI] [PubMed] [Google Scholar]
- 13.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 2008;88:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014;2014:309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 16.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, Newman AB, Simonsick EM, Studenski SA, Nicklas BJ, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr 2013;97:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–33. [DOI] [PubMed] [Google Scholar]
- 18.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007;85:377–84. [DOI] [PubMed] [Google Scholar]
- 19.Pescatello LS, Kelsey BK, Price TB, Seip RL, Angelopoulos TJ, Clarkson PM, Gordon PM, Moyna NM, Visich PS, Zoeller RF, et al. The muscle strength and size response to upper arm, unilateral resistance training among adults who are overweight and obese. J Strength Cond Res 2007;21:307–13. [DOI] [PubMed] [Google Scholar]
- 20.Peterson MD, Liu D, Gordish-Dressman H, Hubal MJ, Pistilli E, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Seip RL, et al. Adiposity attenuates muscle quality and the adaptive response to resistance exercise in non-obese, healthy adults. Int J Obes (Lond) 2011;35:1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus RL, Addison O, Lastayo PC. Intramuscular adipose tissue attenuates gains in muscle quality in older adults at high risk for falling. A brief report. J Nutr Health Aging 2013;17:215–8. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci 2007;62:866–71. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 24.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp Gerontol 2013;48:888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent KR, Braith RW, Vincent HK. Influence of resistance exercise on lumbar strength in older, overweight adults. Arch Phys Med Rehabil 2006;87:383–9. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci 2006;61:480–7. [DOI] [PubMed] [Google Scholar]
- 27.Bouchard DR, Soucy L, Senechal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause 2009;16:66–72. [DOI] [PubMed] [Google Scholar]
- 28.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, Lee S, Lam M, Ross R. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009;169:122–31. [DOI] [PubMed] [Google Scholar]
- 29.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med 2011;26:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006;295:2018–26. [DOI] [PubMed] [Google Scholar]
- 31.Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, Sergi G, Bosello O, Zamboni M.. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord 2004;28:234–41. [DOI] [PubMed]
- 32.Vilaça KH, Carneiro JA, Ferriolli E, Lima NK, de Paula FJ, Moriguti JC. Body composition, physical performance and muscle quality of active elderly women. Arch Gerontol Geriatr 2014;59:44–8. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson DJ, Erskine RM, Winwood K, Morse CI, Onambele GL. The impact of obesity on skeletal muscle architecture in untrained young vs. old women. J Anat 2014;225:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen KF, Befroy D, Dufour S, Dziiura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr 2007;85:662–77. [DOI] [PubMed] [Google Scholar]
- 36.van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch 2006;451:606–16. [DOI] [PubMed] [Google Scholar]
- 37.Gavin TP, Stallings HW III, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol (1985) 2005;98:315–21. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, You T, Yang R, Lyles MR, Demons J, Gong DW, Nicklas GJ. Muscle strength is associated with adipose tissue gene expression of inflammatory adipokines in postmenopausal women. Age Ageing 2010;39:656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 2001;50:1612–7. [DOI] [PubMed] [Google Scholar]
- 40.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005–11. [DOI] [PubMed] [Google Scholar]
- 41.Argilés JM, Lopez-Soriano J, Almendro V, Busquets S, Lopez-Soriano FJ. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev 2005;25:49–65. [DOI] [PubMed] [Google Scholar]
- 42.Sipilä S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol 1995;78:334–40. [DOI] [PubMed] [Google Scholar]
- 43.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 2010;14:362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs JL, Marcus RL, Morrell G, Lastayo P. Resistance exercise with older fallers: its impact on intermuscular adipose tissue. Biomed Res Int 2014;2014:398960. [DOI] [PMC free article] [PubMed]
- 45.Marsh AP, Shea MK, Vance Locke RM, Miller ME, Isom S, Miller GD, Nicklas BJ, Lyles MF, Carr JJ, Kritchevsky SB. Resistance training and pioglitazone lead to improvements in muscle power during voluntary weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013;68:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013;68:80–6. Erratum in: J Gerontol A Biol Sci Med Sci 2014;69:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degens H, Erskine RM, Morse CI. Disproportionate changes in skeletal muscle strength and size with resistance training and ageing. J Musculoskelet Neuronal Interact 2009;9:123–9. [PubMed] [Google Scholar]