Abstract
Background
Blood phosphatidylethanol (PEth) is a promising biomarker of alcohol consumption. This study was conducted to evaluate its performance in patients with liver disease.
Methods
This study included 222 patients with liver disease. Patient-reported alcohol use was obtained as a reference standard, and PEth was measured by tandem mass spectrometry. Receiver operating characteristic (ROC) and contingency table analyses were used to assess the performance of PEth in detecting any drinking and averaging 4 or more drinks daily in the past 30 days.
Results
At the limit of quantitation (20 ng/mL), PEth was 73% sensitive (95% confidence interval (CI) 65–80) and 96% specific (95% CI 92–100) for any drinking in the past month. Subjects who drank but had a negative PEth result were mainly light drinkers. Subjects who reported 30-day abstinence but with quantifiable PEth either reported heavy drinking within the past 6 weeks or had data that suggested under-reported drinking. At the optimal cutoff concentration of 80 ng/mL, PEth was 91% sensitive (95% CI 82–100) and 77% specific (95% CI 70–83) for averaging at least 4 drinks daily.
Conclusion
PEth is a useful test for detecting alcohol use in liver disease patients, but cutoff concentrations for heavy drinking will result in misclassification of some moderate to heavy drinkers.
Keywords: alcohol drinking, liver disease, biomarker, phosphatidylethanol
INTRODUCTION
Liver disease is the 12th leading cause of death in the US, with alcohol serving as the primary cause or an important co-factor in approximately 50% of liver-related deaths (Yoon and Yi, 2010). Thus detecting unhealthy drinking and intervening with counseling, medication, or referral for addiction care when appropriate is an important component of treatment for patients with liver disease of any etiology. Patient self-reporting of drinking can be relied on in many instances, but, as demonstrated by a study of the ethanol metabolite ethyl glucuronide in urine (Staufer et al., 2011), it is clear that under-detection of potentially harmful levels of alcohol use is an important issue in the clinical care of liver disease patients. Because accurate classification of alcohol use is important in optimizing treatment outcomes, alcohol consumption testing may have a role in diagnosis and monitoring. Traditional alcohol consumption testing (e.g., serum liver enzymes and red cell mean corpuscular volume) is not accurate in liver disease patients, but newer biomarkers that are products of non-oxidative ethanol metabolism may be highly accurate regardless of liver function (Wurst et al., 2005). One such product is blood phosphatidylethanol (PEth), a phospholipid that results from a phospholipase-D-catalyzed reaction between phosphatidylcholine and ethanol in cell membranes (Gustavsson, 1995). Pertinent to alcohol testing, PEth is integrated into the erythrocyte membrane, and has an average half-life of approximately 10 days (Gnann et al., 2012). In a preceding preliminary study (Stewart et al., 2009) we reported PEth’s presence in most current drinkers and demonstrated its correlation to alcohol consumption in patients with liver disease. This current study was undertaken to further assess the accuracy of blood PEth levels in detecting alcohol use and harmful levels of drinking, and compare it to the heavily-validated biomarker carbohydrate-deficient transferrin.
METHODS
Subjects and determination of alcohol use
Patients presenting for care to the hepatology clinics or inpatient Liver Service at a university medical center were recruited for this study. A research assistant present in the clinics recruited subjects who had indicated to their health care provider at the time of their appointment or hospital admission that they were willing to discuss research participation involving their use of alcohol. We did not collect any information on those who did not wish to participate. Those providing written informed consent completed a timeline followback daily drinking survey that was administered by trained research assistants (Sobell and Sobell, 1992), the results of which were used to determine average daily alcohol use in the past 30 days. Particular efforts were made to include a sufficient number of current drinkers in order to adequately evaluate the sensitivity of PEth. However, in order to minimize the consequences of under-reported drinking on PEth validation, we did not recruit subjects who were suspected to engage in heavy drinking by their physician but denied alcohol use (clinical care providers did not refer such patients to the study). The rationale for this approach was to obtain a more accurate estimate of biomarker specificity, while still validly estimating the relationship between PEth and alcohol consumption based on self-report. As an additional method for detecting unreported drinking, subjects also provided urine and hair samples for measurement of ethyl glucuronide (EtG), another non-oxidative alcohol biomarker, for use in secondary analyses as discussed below. Participants received $40 for their time and effort. The study was approved by the university’s Institutional Review Board.
Laboratory assessment
Blood PEth and hair and urine EtG assays were performed by a contracted laboratory (US Drug Testing Laboratories, Des Plaines, IL) using liquid chromatography tandem mass spectrometry assays. The major PEth species in whole blood (18:1,16:0) was measured as described by Faller et al (Faller et al., 2011) with an initial limit of quantitation of 20 ng/mL that was lowered to 8 ng/mL during this study due to improved instrumentation. Urine EtG was measured as reported by Dresen et al (Dresen et al., 2004) with a reporting limit of 100 ng/mL, and hair EtG was measured as reported by Morini et al (Morini et al., 2006) with a limit of quantitation of 8 pg/mg. Percent disialo-carbohydrate-deficient transferrin (%dCDT) was measured using a high performance liquid chromatography assay as reported by Helander et al (Helander et al., 2003) at the Clinical Neurobiology Laboratory at the Medical University of South Carolina. The comparison between PEth and %dCDT was limited to a subset of this sample due to abnormalities in transferrin chromatography that have previously been associated with advanced liver disease, and preclude quantitation of %dCDT (Arndt et al., 2008, Stewart et al., 2010a). Neither laboratory was aware of the subjects’ alcohol use.
Determination of liver disease severity
The electronic medical record was reviewed by a physician (author SHS) to determine the etiology and severity of each subject’s liver disease. Source material included hepatology clinical notes, and radiology, laboratory, and pathology reports. The etiology of liver disease was clarified when necessary by consultation with the hepatology co-authors (DGK, IRW, and/or AR). Subjects were categorized as cirrhotic if they had biopsy-proven cirrhosis, proven esophageal varices, and/or ascites.
Data analysis
The average number of drinks per day in the 30 days preceding PEth sampling was determined from the alcohol use survey and this served as the primary reference standard for validating PEth. The 30-day horizon corresponds roughly to three PEth half-lives (Gnann et al., 2012). While self-report was our standard for validating PEth, the use of self-reported alcohol consumption introduces some uncertainty into validation studies. Therefore, in a secondary analysis, we supplemented self-report with urine and hair EtG to detect probable underreporting of alcohol use for even more accurate classification. Specifically, subjects reporting past week abstinence but having urine EtG in excess of 100 ng/ml were excluded from analysis (Stewart et al., 2013a), as were subjects reporting less than one drink per day on average over the prior 3 months, but having hair EtG in excess of 30 pg/mg, a concentration that is highly suggestive of regular heavy drinking (Pragst et al., 2010, Stewart et al., 2013b). Because not all subjects had provided urine and hair samples, we limited this secondary analysis to subjects who had results for both urine and hair EtG.
Receiver operating characteristic (ROC) analysis was used to estimate the utility of PEth in detecting any drinking in the past 30 days, or alcohol use that averaged at least 4 standard drinks per day (with a standard drink being defined as any volume of beverage containing 14 grams of ethanol). The ROC curve for at least 4 standard drinks per day (i.e., “heavier drinking”) was also compared to the corresponding curve for %dCDT. Linear regression, with log PEth as the dependent variable, was used for subjects with quantifiable PEth to estimate if liver disease severity, age or gender modified the relationship between PEth concentration and alcohol use. Finally, as the limit of quantitation for the PEth assay was lowered from 20 ng/mL to 8 ng/mL during the study due to improved mass spectrometry instrumentation, sensitivity and specificity for detecting any drinking were also estimated for the 8 ng/mL cutoff (i.e., all subjects were analyzed using the 20 ng/mL cutoff since the assay itself did not change, and subjects to which the lower cutoff applied due to improved instrumentation were analyzed again at the lower cutoff).
RESULTS
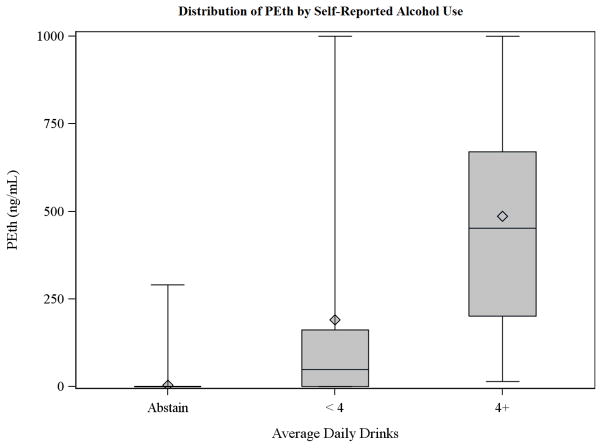
During a 32 month period 222 subjects were recruited for the study and provided a blood sample for PEth measurement. Of these, 163 (73%) also had interpretable %dCDT results. Subject characteristics are summarized by self-reported drinking status in Table 1. Heavier drinkers tended to be male, and current drinkers were less likely to be cirrhotic than abstainers. To illustrate the relationship between PEth concentration and alcohol use, the distribution of PEth within various self-reported drinking categories is illustrated in Figure 1, showing a strong positive correlation but substantial overlap between categories.
Table 1.
Subject Characteristics by Past-30-Day Drinking Status
| Characteristic | Abstinent (42%, n=94) | < 4 Drinks Daily (42%, n=93) | ≥4 Drinks Daily (16%, n=35) |
|---|---|---|---|
| % male | 50 | 57 | 71 |
| Median age (interquartile range) | 52 (47–59) | 52 (41–57) | 52 (46–58) |
| % cirrhotic* | 72 | 39 | 43 |
| Median drinks past 30 days (interquartile range) [range] | N/A | 28 (8–63) [1–119] | 266 (175–347) [120–929] |
| % Subjects with PEth ≥ 20 ng/mL | 4 | 65 | 97 |
67% of cirrhotic cases were biopsy-confirmed, and 33% were based on clinical findings as specified in the text. 57% of subjects classified as non-cirrhotic had undergone liver biopsy.
Fig. 1.
Distribution of phosphatidylethanol (PEth) by self-reported alcohol use.
As determined from the medical record, the most common clinical diagnoses included chronic Hepatitis C in which alcohol use was not felt to represent an important comorbidity (n=74, 33%), alcoholic liver disease (n=51, 23%), fatty liver diseases (n=32, 14%), chronic Hepatitis C in which alcohol use was felt to represent an important comorbidity (n=27, 12%), autoimmune liver diseases (n=18, 8%), and cryptogenic disease (n=10, 5%). The remaining 5% included subjects with Hepatitis B (n=2), granulomatous disease (n=2), medication-induced liver disease (n=3), hemochromatosis (n=1), alpha-1-antitrypsin deficiency (n=1), and congenital hepatic fibrosis (n=1). Among the cirrhotic patients, the median MELD score (Kamath et al., 2001), a measure of cirrhosis severity, was 10, with an interquartile range of 6 to 14.
ROC analyses and effects of cirrhosis, gender, and age
Using the 20 ng/mL cutoff and classifying anything less as “negative”, the area under the ROC curve (AUROC) for PEth classification of any drinking was 0.87 (95% confidence interval [CI] 0.83 to 0.91), and 0.89 (95% CI 0.85–0.94) for averaging four or more drinks daily. In subjects with quantifiable PEth, the relationship between PEth concentration and alcohol use did not depend on gender (interaction p=0.210), age (interaction p=0.438), or liver disease severity (interaction p= 0.280).
In the secondary analysis, 88% of the subjects (n=196) had results for urine and hair EtG; 12 subjects were eliminated due to mismatches between self-report and EtG results. In this subset (n=184), there was no change in the AUROC for any alcohol use (0.86, 95% CI 0.81–0.90), and a small increase for averaging 4 or more drinks daily (0.92, 95% CI 0.87–0.96). This suggested that the drinking reports obtained from the study subjects were mainly accurate (with some exceptions as presented below in the description of false positive results).
Sensitivity and specificity
The sensitivity and specificity of PEth at the original limit of quantitation (20 ng/mL), the lower limit of quantitation (8 ng/mL) resulting from improved instrumentation during the study, and the cutoff concentration that maximized the sum of sensitivity and specificity for averaging at least 4 drinks daily (80 ng/mL), are listed in Table 2. A cutoff of 300 ng/mL provided 90% specificity for heavier drinking in this sample, but sensitivity was substantially reduced (71%, 95% CI 56–86). Limiting the analysis to those with hair and urine EtG results and no mismatch with self-reported drinking provided similar results, but suggested that specificity may be slightly higher than we estimated from self-report. For example, for identifying subjects consuming at least 4 drinks daily, the 80 ng/mL cutoff was 89% sensitive (95% confidence interval 78 to 100), and 82% specific (95% confidence interval 76 to 88).
Table 2.
Sensitivity and Specificity of Phosphatidylethanol (PEth) for Past-Month Drinking
| Drinking State | PEth Concentration* (ng/mL) | Sensitivity (95% confidence interval) | Specificity (95% confidence interval) |
|---|---|---|---|
| Any drinking | ≥8 | 79% (71–88) | 90% (81–98) |
| Any drinking | ≥ 20 | 73% (65–80) | 96% (92–100) |
| ≥ 4 drinks/day | ≥ 20 | 97% (92–100) | 66% (59–73) |
| ≥ 4 drinks/day | ≥80 | 91% (82–100) | 77% (70–83) |
The PEth concentrations represent the original and improved limits of PEth quantitation and the concentration that maximized sensitivity+specificity for averaging at least 4 drinks daily as described in the text.
Comparison to %dCDT
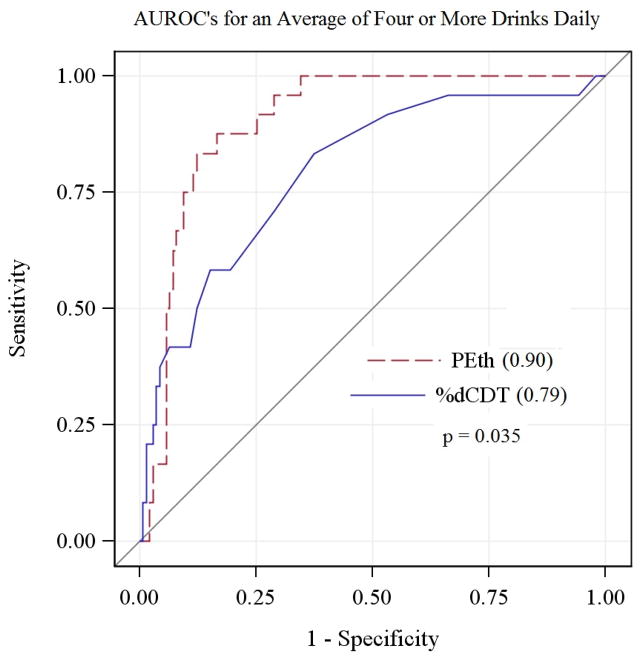
In the 163 subjects who had interpretable %dCDT results (73% of the total), the AUROC for PEth significantly exceeded that for %dCDT for averaging at least 4 drinks daily (Figure 2). The AUROC for %dCDT was 0.79 (95% CI 0.69–0.89) vs. 0.90 (95% CI 0.85–0.95) for PEth. Despite these findings, in liver disease patients with interpretable %dCDT results, the slight overlap of the ROC curves seen in Figure 2 suggests that %dCDT would have greater specificity at cutoff concentrations with very low sensitivity (i.e., < 40%).
Fig. 2.
Area under the receiver operating characteristic curves for an average of 4 ormore drinks daily.
Alcohol use in subjects with PEth ≥ 20 ng/mL but reporting past-30-day abstinence (Potential false positives)
Four subjects who reported past-30-day abstinence had PEth ≥20 ng/mL (specifically, 38 ng/mL, 84 ng/mL, 291 ng/mL, and 422 ng/mL), and these constitute the positive results illustrated in the abstinent group in Figure 1. Two of these subjects (PEth 38 and 291 ng/mL) had been engaged in frequent heavy drinking within 40 days of study participation, and, consistent with their self-reported drinking, had negative urine EtG results and positive hair EtG results. The remaining 2 subjects who reported greater than 90 days of continuous abstinence, had mismatches between their reported alcohol use and urine and/or hair EtG results, and, even under the ideal conditions of the study, likely provided inaccurate self-reports of abstinence.
Alcohol use in subjects reporting past-30-day drinking but having undetectable PEth (Potential false negatives)
There were 34 subjects who reported some past-30-day drinking but who had PEth results < 20 ng/mL (representing 27% of past month drinkers). These subjects were mainly light drinkers, with a median total past-30-day alcohol consumption of 6.1 drinks, which ranged from <1 to 329 drinks. Only 2 subjects reported an average of more than 2 drinks daily (specifically 3 and 11 drinks/day), both of whom reported 3 weeks of abstinence, with the latter also having undergone massive red blood cell transfusion in the days preceding study participation. Drinking was significantly lower compared to the median of 86 standard drinks in past-30-day drinkers who had PEth results ≥20 ng/mL (Wilcoxon p<0.001). With the lower cutoff of 8 ng/mL (applicable to the final 126 subjects accrued for the study), 21% of past-month drinkers had negative PEth results, and all averaged less than 2 drinks daily.
DISCUSSION
Similar to findings in other populations, this study shows that PEth measured by mass spectrometry was detectable in most moderate to heavy alcohol consumers with chronic liver disease. It was not detectable in many light drinkers who would not be consuming enough alcohol to harm their liver, and rarely a heavier drinker in association with recent onset of abstinence in the past weeks. PEth outperformed %dCDT as a biomarker for heavy drinking, but is limited in screening for heavier alcohol use due to substantial overlap in PEth concentrations with moderate drinkers.
PEth was discovered in 1983 (Alling et al., 1983), and since that time has been evaluated as an alcohol consumption biomarker in a number of different study populations (e.g., Aradottir et al., 2006, Hahn et al., 2012, Hansson et al., 1997, Hartmann et al., 2007, Stewart et al., 2010b, Varga et al., 1998, Wurst et al., 2010, Stewart et al., 2010c). The current study results extend prior research findings to patients with liver disease, and tentatively identified an optimal cutoff for drinking that would exacerbate or cause liver disease (i.e., 80 ng/mL for averaging at least 4 drinks daily). However, due to overlapping PEth distributions with more moderate drinkers, misclassification will occur. A quantifiable PEth concentration rules out abstinence, although with a half-life of approximately 10 days, detectable PEth may in theory persist for many weeks depending on its concentration at the onset of abstinence. In this study population, PEth was detectable in 2 previously heavily drinking subjects who were felt to have provided reliable self-report of abstinence for approximately 5 to 6 weeks.
Results suggest that the validity of PEth remains high regardless of age, gender, and liver disease severity. However, as a number of subjects had not undergone liver biopsy, some cirrhotic subjects may have been misclassified as non-cirrhotic if they did not have signs of portal hypertension (i.e., varices or ascites). This may have diluted any true differences between subjects classified as cirrhotic vs. non-cirrhotic. In addition, the study was not highly powered for detecting differences between cirrhotic and non-cirrhotic subjects, and this finding will require confirmation.
Coupled with our previously reported findings on urine EtG and hair EtG in this sample, these results show that negative mass spectrometry assays for urine and hair EtG (Stewart et al., 2013a, Stewart et al., 2013b), together with blood PEth, can generally eliminate concerns over recurring moderate to heavy drinking in the days (urine EtG), weeks (blood PEth), and months (hair EtG) preceding a clinical encounter. However, the absence of these ethanol metabolites does not rule out lighter drinking. Biomarker assessment is not subject to real or potential biases, and, when resources are available to measure PEth and other alcohol elimination products, they can serve as a valuable supplement to a clinical assessment of alcohol use in liver disease patients (Allen et al., 2013). Similar use of PEth has been shown for example in an impaired professionals program (Skipper et al., 2013), and as a method for relapse detection among problem drinkers (Helander et al., 2012). In clinical research settings focusing on alcohol-related liver disease, biomarkers have the potential to reduce misclassification of alcohol use, which would typically be an important covariate or treatment outcome. If used in this manner, treatment effects might be estimated with greater efficiency and accuracy.
Several limitations merit discussion. We relied on self-report as our reference standard for alcohol use, and the accuracy of our sensitivity and specificity estimates depend on the validity of self-reported drinking. Inaccurate self-report would likely tend toward an underestimation of alcohol use, which could cause an underestimation of biomarker specificity. Our secondary analyses excluding subjects with mismatches between self-reported drinking and hair or urine EtG supported the validity of self-report, but did suggest that PEth specificity may be slightly higher than we have estimated. There will be situations where the PEth concentration does not reflect typical consumption in the prior weeks (e.g., prolonged elimination in a person with particularly high concentrations prior to drinking reduction or cessation, or quantifiable levels resulting from a recent but isolated binge (Gnann et al., 2012)). Large volume blood transfusions could result in false positive or false negative results for PEth testing, as may have been the case for one of our study participants. It is important to note that this was not necessarily a representative sample of patients with liver disease, as recruitment was completed in a regional referral center for liver diseases. As a result, sensitivity for any past-month drinking may differ from our results in the general population of patients with liver disease, depending on the actual distribution of alcohol use in the target population (e.g., sensitivity would be lower if the population included a higher proportion of light drinkers, in whom PEth is often undetectable). However, our specificity estimates would not differ in the general population of patients with liver disease, nor would the sensitivity estimate for heavier drinking. Although mass spectrometry continues to make inroads into clinical testing (Shushan, 2010), the cost-effectiveness of PEth will likely be low in an unselected clinical population due to the expense of current assay methods. Conversely, PEth may be highly cost-effective if the consequences for misclassification are high, e.g., in liver transplantation evaluations or patients in whom alcohol use may be complicating their liver disease, or research studies examining treatment efficacy for alcohol-related liver disease.
In conclusion, in patients with liver disease, blood PEth measurement is a useful test for detecting moderate to heavy alcohol consumption in the past weeks, and quantifiable levels rule out abstinence in the past weeks to perhaps months given an approximately 10-day half-life. Results show that the detection of drinking in general is an unambiguous benefit of PEth. The use of a cutoff concentration to detect heavy drinking has some potential to supplement alcohol assessment, but cannot stand alone due to misclassification of some moderate to heavy drinkers. Our results can be used to aid interpretation of PEth in liver disease patients. However, PEth remains a relatively new biomarker, and additional study is needed to further refine interpretation and determine optimal clinical applications.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA017911 and K05AA017435). Dr. Koch was also supported by a Junior Faculty Development Award from the American College of Gastroenterology.
References
- ALLEN JP, WURST FM, THON N, LITTEN RZ. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transplantation. 2013;19:369–76. doi: 10.1002/lt.23596. [DOI] [PubMed] [Google Scholar]
- ALLING C, GUSTAVSSON L, ANGGARD E. An abnormal phospholipid in rat organs after ethanol treatment. FEBS Lett. 1983;152:24–8. doi: 10.1016/0014-5793(83)80474-8. [DOI] [PubMed] [Google Scholar]
- ARADOTTIR S, ASANOVSKA G, GJERSS S, HANSSON P, ALLING C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol & Alcoholism. 2006;41:431–7. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- ARNDT T, VAN DER MEIJDEN BB, WIELDERS JP. Atypical serum transferrin isoform distribution in liver cirrhosis studied by HPLC, capillary electrophoresis and transferrin genotyping. Clin Chim Acta. 2008;394:42–6. doi: 10.1016/j.cca.2008.03.033. [DOI] [PubMed] [Google Scholar]
- DRESEN S, WEINMANN W, WURST FM. Forensic confirmatory analysis of ethyl sulfate--a new marker for alcohol consumption--by liquid-chromatography/electrospray ionization/tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1644–8. doi: 10.1016/j.jasms.2004.08.004. [DOI] [PubMed] [Google Scholar]
- FALLER A, RICHTER B, KLUGE M, KOENIG P, SEITZ HK, THIERAUF A, GNANN H, WINKLER M, MATTERN R, SKOPP G. LC-MS/MS analysis of phosphatidylethanol in dried blood spots versus conventional blood specimens. Anal Bioanal Chem. 2011;401:1163–6. doi: 10.1007/s00216-011-5221-y. [DOI] [PubMed] [Google Scholar]
- GNANN H, WEINMANN W, THIERAUF A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcoholism: Clinical & Experimental Research. 2012;36:1507–11. doi: 10.1111/j.1530-0277.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- GUSTAVSSON L. Phosphatidylethanol formation: specific effects of ethanol mediated via phospholipase D. Alcohol & Alcoholism. 1995;30:391–406. [PubMed] [Google Scholar]
- HAHN JA, DOBKIN LM, MAYANJA B, EMENYONU NI, KIGOZI IM, SHIBOSKI S, BANGSBERG DR, GNANN H, WEINMANN W, WURST FM. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcoholism: Clinical & Experimental Research. 2012;36:854–62. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSSON P, CARON M, JOHNSON G, GUSTAVSSON L, ALLING C. Blood phosphatidylethanol as a marker of alcohol abuse: levels in alcoholic males during withdrawal. Alcoholism: Clinical & Experimental Research. 1997;21:108–10. [PubMed] [Google Scholar]
- HARTMANN S, ARADOTTIR S, GRAF M, WIESBECK G, LESCH O, RAMSKOGLER K, WOLFERSDORF M, ALLING C, WURST FM. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addiction Biology. 2007;12:81–4. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- HELANDER A, PETER O, ZHENG Y. Monitoring of the alcohol biomarkers PEth, CDT, and EtG/EtS in an outpatient treatment setting. Alcohol & Alcoholism. 2012;47:552–7. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- HELANDER A, HUSA A, JEPPSSON JO. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem. 2003;49:1881–90. doi: 10.1373/clinchem.2003.023341. [DOI] [PubMed] [Google Scholar]
- KAMATH PS, WIESNER RH, MALINCHOC M, KREMERS W, THERNEAU TM, KOSBERG CL, D’AMICO G, DICKSON ER, KIM WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- MORINI L, POLITI L, GROPPI A, STRAMESI C, POLETTINI A. Determination of ethyl glucuronide in hair samples by liquid chromatography/electrospray tandem mass spectrometry. Journal of Mass Spectrometry. 2006;41:34–42. doi: 10.1002/jms.943. [DOI] [PubMed] [Google Scholar]
- PRAGST F, ROTHE M, MOENCH B, HASTEDT M, HERRE S, SIMMERT D. Combined use of fatty acid ethyl esters and ethyl glucuronide in hair for diagnosis of alcohol abuse: interpretation and advantages. Forensic Science International. 2010;196:101–10. doi: 10.1016/j.forsciint.2009.12.028. [DOI] [PubMed] [Google Scholar]
- SHUSHAN B. A review of clinical diagnostic applications of liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev. 2010;29:930–44. doi: 10.1002/mas.20295. [DOI] [PubMed] [Google Scholar]
- SKIPPER GE, THON N, DUPONT RL, BAXTER L, WURST FM. Phosphatidylethanol: the potential role in further evaluating low positive urinary ethyl glucuronide and ethyl sulfate results. Alcoholism: Clinical & Experimental Research. 2013;37:1582–6. doi: 10.1111/acer.12121. [DOI] [PubMed] [Google Scholar]
- SOBELL L, SOBELL M. Timeline follow-back: A technique for assessing self-reported ethanol comsumption. In: LITTEN RZ, ALLEN JP, editors. Measuring alcohol consumption: Psychological and biological methods. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- STAUFER K, ANDRESEN H, VETTORAZZI E, TOBIAS N, NASHAN B, STERNECK M. Urinary ethyl glucuronide as a novel screening tool in patients pre and post liver transplantation improves detection of alcohol consumption. Hepatology. 2011;54:1640–1649. doi: 10.1002/hep.24596. [DOI] [PubMed] [Google Scholar]
- STEWART SH, BOWEN E, COMTE-WALTERS S, ANTON RF. Liver Disease and HPLC-Quantification of Disialotransferrin for Heavy Alcohol Use: A Case Series. Alcoholism: Clinical & Experimental Research. 2010a;34:1956–1960. doi: 10.1111/j.1530-0277.2010.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, KOCH DG, BURGESS DM, WILLNER IR, REUBEN A. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcoholism: Clinical & Experimental Research. 2013a;37:150–5. doi: 10.1111/j.1530-0277.2012.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, KOCH DG, WILLNER IR, RANDALL PK, REUBEN A. Hair ethyl glucuronide is highly sensitive and specific for detecting moderate-to-heavy drinking in patients with liver disease. Alcohol & Alcoholism. 2013b;48:83–7. doi: 10.1093/alcalc/ags109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, LAW TL, RANDALL PK, NEWMAN R. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcoholism: Clinical & Experimental Research. 2010c;34:488–92. doi: 10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, REUBEN A, BRZEZINSKI WA, KOCH DG, BASILE J, RANDALL PK, MILLER PM. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol. 2009;44:464–7. doi: 10.1093/alcalc/agp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA A, HANSSON P, LUNDQVIST C, ALLING C. Phosphatidylethanol in blood as a marker of ethanol consumption in healthy volunteers: comparison with other markers. Alcoholism: Clinical & Experimental Research. 1998;22:1832–7. [PubMed] [Google Scholar]
- WURST FM, ALLING C, ARADOTTIR S, PRAGST F, ALLEN JP, WEINMANN W, MARMILLOT P, GHOSH P, LAKSHMAN R, SKIPPER GE, NEUMANN T, SPIES C, JAVORS M, JOHNSON BA, AIT-DAOUD N, AKHTAR F, ROACHE JD, LITTEN R. Emerging biomarkers: new directions and clinical applications. Alcoholism: Clinical & Experimental Research. 2005;29:465–73. doi: 10.1097/01.alc.0000156082.08248.ab. [DOI] [PubMed] [Google Scholar]
- WURST FM, THON N, ARADOTTIR S, HARTMANN S, WIESBECK GA, LESCH O, SKALA K, WOLFERSDORF M, WEINMANN W, ALLING C. Phosphatidylethanol: normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addict Biol. 2010;15:88–95. doi: 10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- YOON Y, YI H. Surveillance report # 88- Liver cirrhosis mortality in the United States, 1970–2007. US Department of Health and Human Services, Public Health Service, National Institutes of Health; 2010. [Google Scholar]