Abstract
Previous studies have shown that high-fat diet (HFD) enhances adaptation if provided immediately following small bowel resection (SBR). The purpose of this study was to determine if HFD could further enhance villus growth after resection-induced adaptation had already taken place. C57/Bl6 mice underwent a 50 % proximal SBR or sham operation and were then provided a standard rodent liquid diet (LD) ad lib. After a typical period of adaptation (7 days), SBR and sham-operated mice were randomized to receive either LD or HFD (42 % kcal fat) for an additional 7 days. Mice were then harvested, and small intestine was collected for analysis. Adaptation occurred in both SBR groups; however, the SBR/HFD had significantly increased villus height compared to SBR/LD. Reverse transcription–polymerase chain reaction of villus enterocytes showed a marked increase in CD36 expression in the SBR/HFD group compared with SBR/LD mice. While exposure to increased enteral fat alone did not affect villus morphology in sham-operated mice, HFD significantly increased villus growth in the setting of resection-induced adaptation, supporting the clinical utility of enteral fat in augmenting adaptation. Increased expression of CD36 suggests a possible mechanistic role in dietary fat metabolism and villus growth in the setting of short gut syndrome.
Keywords: Short gut syndrome, Small bowel adaptation, High-fat diet, CD36
Introduction
Short gut syndrome is a morbid clinical condition that results from massive small bowel resection (SBR). In patients with less than 50 cm of small bowel, 90 % will require parenteral nutrition with a 5-year survival of approximately 40 %.1 In pediatric patients with intestinal failure due to a variety of causes, only half were able to achieve enteral autonomy while the other half required transplantation or ultimately died.2
Adaptation is a compensatory process following resection that results in villus growth and enhanced mucosal surface area. The mechanism(s) for adaptation remains an important area of research as efforts to enhance this process may benefit the short gut population and decrease the need for parenteral nutrition and its associated morbidities.
Enteral nutrition is a crucial stimulus for adaptation.3–6 The addition of a diet that has a higher fat content has particularly been shown to enhance adaptive responses. Previous studies have found increased villus height and mucosal weight after feeding rats a high-fat diet immediately after SBR.7–9 In this study, we sought to determine if a high-fat diet would augment intestinal morphology if introduced later, after an initial period of adaption.
Materials and Methods
Animals
Male C57BL/6 mice were acquired from Jackson Laboratories (Bar Harbor, ME) and were housed in WUSM animal facility. Mice were kept on a 12-h light–dark schedule and were operated on at aged 7–9 weeks. Protocols for this study were approved by the Washington University Animal Studies Committee (Protocol 20100103 and 20130308) and were in accordance with the National Institute of Health laboratory animal care and use guidelines.
Operation and Adaptation
Mice underwent 50 % proximal SBR or sham operation (transection and reanastomosis only) as previously described.10 Briefly, mice that underwent SBR had transection of the bowel at a point 12-cm proximal to the ileal–cecal junction and also at a point 1- to 2-cm distal to the ligament of Treitz. The mesentery was ligated, and the intervening bowel was removed. Intestinal continuity was restored with an end-to-end anastomosis using 9–0 monofilament suture. In mice undergoing sham operation, the bowel was transected at a point 12-cm proximal to the ileal–cecal junction, and intestinal continuity was restored with an end-to-end reanastomosis. After the operation, mice were provided free access to water for the first 24 h after surgery. Mice were then fed with standard liquid diet (LD; PMI Micro-Stabilized Rodent Liquid Diet LD 101, TestDiet; 35 % kcal fat) until postoperative day (POD) 7. We have previously demonstrated augmented villus growth by the third postoperative day, which reached a plateau by day 7.10 Mice were kept on a liquid diet during this time, as we have previously found that mice have an intestinal obstruction due to a food bezoar when given solid food in the immediate postoperative period.
Mice were then randomized and either continued with LD or switched to a high-fat diet (HFD; Harlan Teklad TD.88137; 42 % kcal fat), and were then harvested 1 week later. Thus, there were four experimental groups: mice that underwent a sham operation and either remained on LD (Sham/LD, n = 12) or were switched to HFD (Sham/HFD, n = 10) and mice that underwent 50 % proximal SBR and either remained on LD (SBR/LD, n = 6) or were switched to HFD (SBR/HFD, n = 8). In another set of experiments, SBR mice fed with HFD were further randomized to remain on HFD (n = 5) or switched back to LD (n = 6) for another 4 weeks before harvest.
On POD 14, all groups of mice were harvested and ileal tissue collected. Epithelial cells were isolated as previously described11 and were used for reverse transcription–polymerase chain reaction (RT-PCR) and Western blotting. To assess for adaptation, villus height and crypt depth were measured via H&E-stained histology. At least 20 well-oriented crypts and villi were counted per slide. Crypts were counted only if the crypt–villus junctions on both sides of the crypt were intact and if Paneth cells were present at the base of the crypt. Villi were counted only if the central lymphatic channel extended from the villus base to the tip and if the mucosal surface was in continuity with an intact crypt.
Body Composition Analysis
Mice underwent body composition analysis the day before surgery as well as on POD14. Measurements were taken on awake mice with MRI (EchoMRI 3–1, Echo Medical Systems) following manufacture’s instruction.
Food Consumption and Feces Output
On POD 7, mice were transferred to individual metabolic cages with wire inserts that permitted collection of wasted food and feces. The quantity of food ingested and feces excreted were measured everyday until harvest.
Real-Time PCR
RNA was prepared from harvested ileal crypts and villi as previously described8 and were homogenized in lysis buffer (RNAqueous kit, Ambion, Austin, TX). The RNA was extracted according to kit instructions and stored at −80 °C. Total RNA concentration was determined using a NanoDrop Spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE). CD36, ApoB, and microsomal triglyceride transfer protein (MTTP) primers were obtained from Life Technologies (Carlsbad, CA). β-Actin was used as the endogenous control (Applied Biosystems, Foster City, CA), and whole bowel was used as a calibrator. Real-time PCR reagents were from Applied Biosystems (Foster City, CA), and an Applied Biosystems 7500 Fast Real-Time PCR system was used (Foster City, CA).
Western Blotting
Isolated villus samples were lysed with sodium dodecyl sulfate sample buffer. The lysate was then heated for 5 min at 100 °C, and the protein concentration was determined by using the RC-DC kit (Bio-Rad, Hercules, CA). Proteins were loaded in equal amounts for Western blotting. CD36 (R&D Systems Inc., Minneapolis, MN) and actin (Cell Signaling Technology, Danvers, MA) antibodies were used.
Enterocyte Proliferation and Immunohistochemical Analysis
Formalin-fixed tissue sections were embedded in paraffin, sectioned, deparaffinized, and blocked with 3 % hydrogen peroxide in methanol. Antigen retrieval was performed using Diva Decloaking solution (Biocare Medical, Concord, CA) (120 °C for 5 min followed by 100 °C for 10min). Slides were blocked with avidin-pink and biotin-blue (Biocare Medical), treated with anti-p-Histone 3 (Cell Signaling Technology, Danvers, MA) or anti-CD36 antibodies (R&D Systems Inc., Minneapolis, MN) in DaVinci Green (Biocare Medical), stored overnight at 4 °C, and visualized with biotinylated goat anti-rat IgG (Accurate Chemical, Westbury, NY) and donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), respectively, followed by streptavidin-horseradish peroxidase (HRP; Invitrogen, Camarillo, CA) and diaminobenzidine (DAB; Sigma-Aldrich, St Louis, MO) and hematoxylin counterstaining. The number of positively-staining p-Histone-3 crypt enterocytes and the total number of cells per crypt were counted from at least 20 well-oriented crypts by blinded scoring. A proliferative index was calculated from the ratio of these measurements.
Statistics
For most experiments, means were calculated and compared using a student’s t test. For comparison of weight gain over time, ANCOVA was performed (statistiXL 1.10). Values in the text are means ± SEM. Differences were considered significant at p ≤0.05.
Results
HFD Magnifies Structural Adaptation
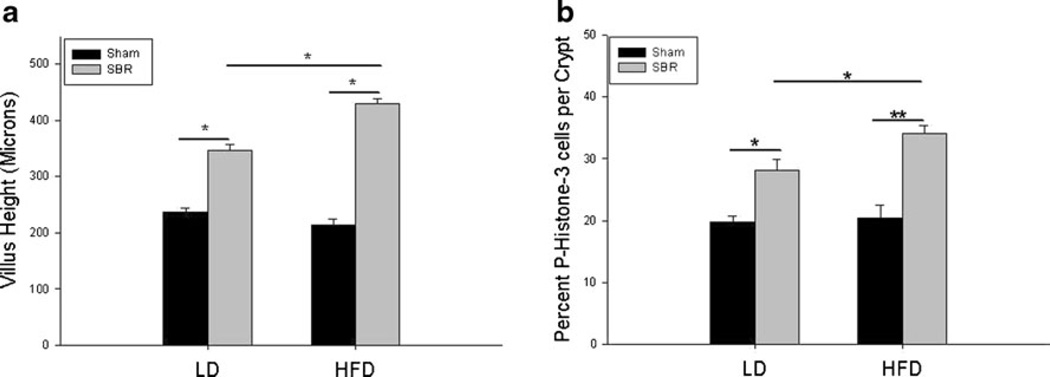
We have previously demonstrated that SBR mice fed with our standard LD for 7 days postresection achieve structural adaptation as determined by increased villus height compared to their sham counterparts. However, when HFD was given to SBR mice for 7 days, a dramatic increase of villus height was observed in addition to normal adaptation (429.2±10.2 vs 347.1±10.6 µm; Fig. 1a). HFD alone did not seem to affect villus growth as demonstrated by no differences between sham/LD and sham/HFD. However, SBR/HFD showed increased villus growth compared to SBR/LD, while the SBR/LD group demonstrated an average increase of 52.0±11.0 % in villus growth when compared to their respective intraoperative samples; the SBR/HFD group resulted in an 82.7±7.0 % increase (p =0.03).
Fig. 1.
Villus growth and enterocyte proliferation are amplified after resection with the addition of a high-fat diet. a Postoperative ileal villus height. b Proliferative rate as measured by percent of p-Histone-3 positive cells per total crypt cells. Sham operations represent transection and anastomosis without resection and small bowel resection (SBR) represents a 50 % proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet. *p <0.05; **p <0.005
Rates of proliferation in crypts followed a similar trend as villus growth (Fig. 1b). There was no difference in proliferation between sham/LD and sham/HFD. Both SBR groups had increased rates of proliferation from their sham counterparts. However, the SBR/HFD crypts demonstrated greater rates of proliferation compared to SBR/LD crypts (34.0±1.4 % p-Histone-3 positive cells per crypt vs 28.1±1.8 %, p =0.02).
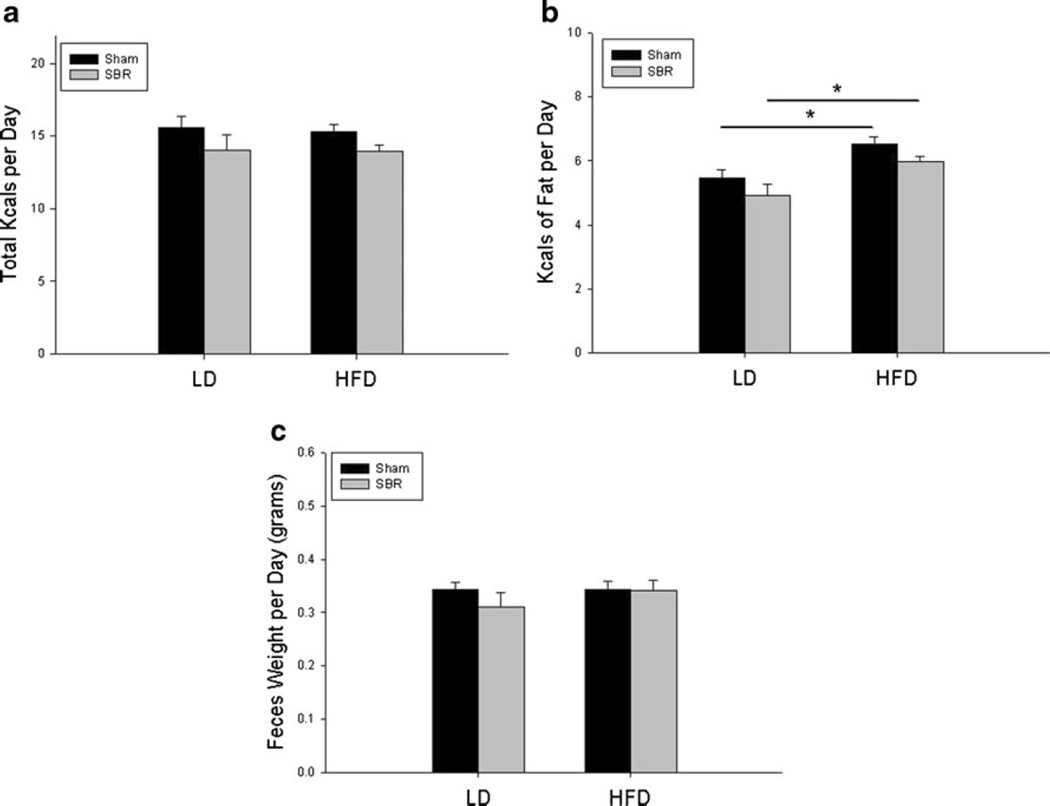
HFD Did Not Affect Calorie Intake or Feces Output
To ensure that mice did not differ in intake or output that could be a contributing factor to other results, all mice were individually caged with underlying wire platforms to accurately measure daily food consumption and feces excretion. As demonstrated in Fig. 2a, there were no differences in daily ingested food calories between different groups. As expected, the amount of kilocalories of fat ingested was higher in HFD groups (Fig. 2b). There were no differences in the amount of feces excreted per mouse group per day (Fig. 2c).
Fig. 2.
Total calorie (a) and fat calorie (b), and (c) stool output per day. Sham operations represent transection and anastomosis without resection and small bowel resection (SBR) is a 50 % proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet. *p <0.05
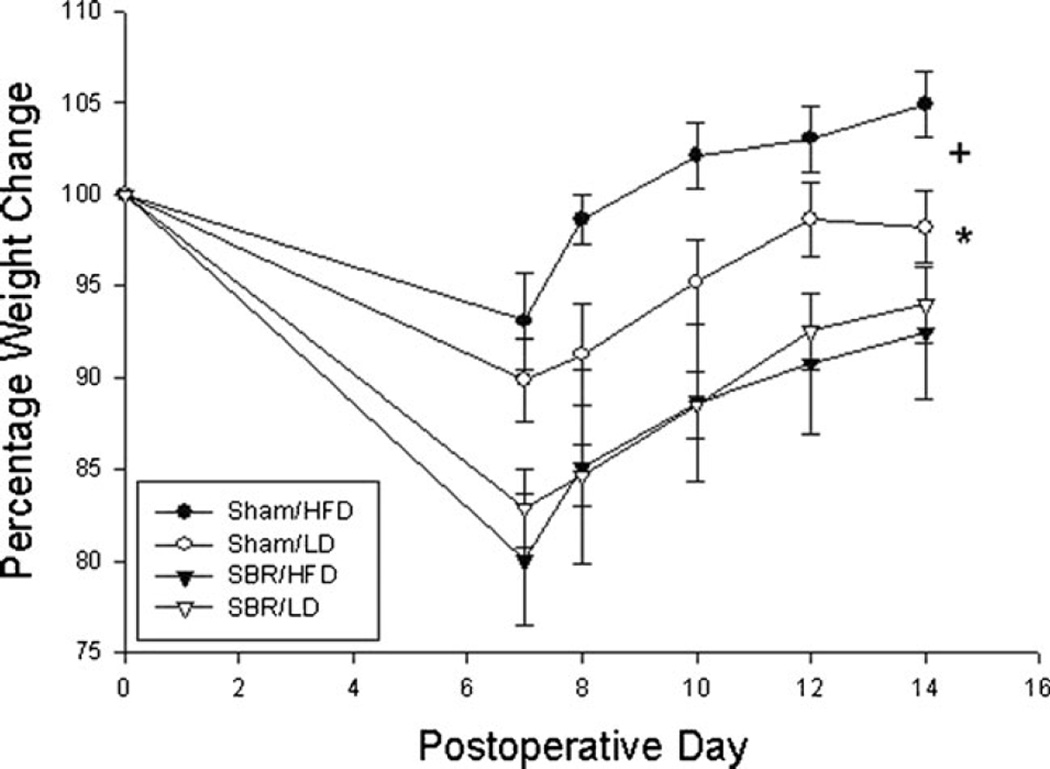
All mice experienced weight loss after surgery (Fig. 3). However, sham groups regained much more of their preoperative weight when compared with mice subjected to SBR (p < 0.005). The sham/HFD also experienced a greater weight gain than the sham/LD group (p <0.005). However, SBR/HFD mice had the same weight loss and rate of weight gain as SBR/LD mice.
Fig. 3.
Percent weight change after operation. Sham operations represent transection and anastomosis without resection and small bowel resection (SBR) is a 50 % proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet. *p <0.05 between SBR and sham groups. +p <0.05 between sham/HFD and sham/LD
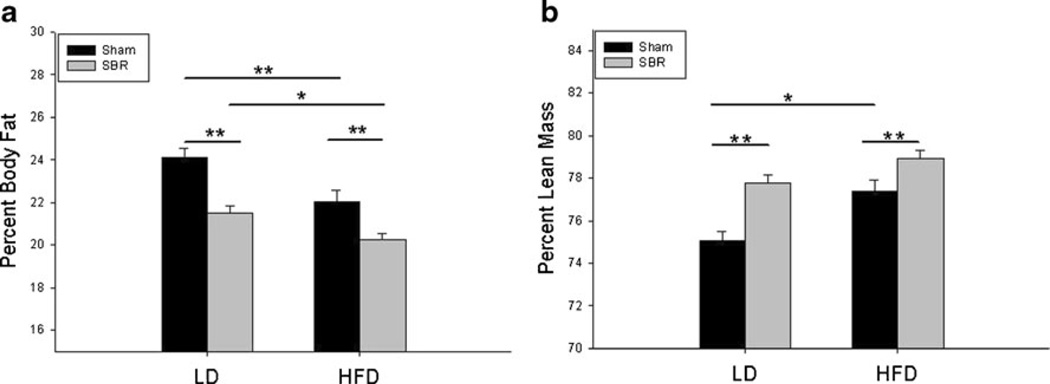
Body Composition
There were no differences in body composition between any of the groups before surgery. By POD 14, both sham groups had higher percent body fat than SBR groups (Fig. 4a). Additionally, the LD groups of both sham and SBR had increased percent body fat than their HFD counterparts. Interestingly, it was the SBR/HFD group had the lowest percent body fat of all groups. Conversely, the sham groups had the lowest percent lean mass, particularly the sham/LD (Fig. 4b). Although the SBR/HFD group had the highest percent lean mass, the difference between SBR/HFD and SBR/LD was not significant.
Fig. 4.
Percent body fat (a) and lean mass (b) measurements at postoperative day 14. Sham operations represent transection and anastomosis without resection and small bowel resection (SBR) is a 50 % proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet. *p <0.05; **p <0.005
CD36 Is Dramatically Increased in the Villus Epithelium of SBR/HFD Mice
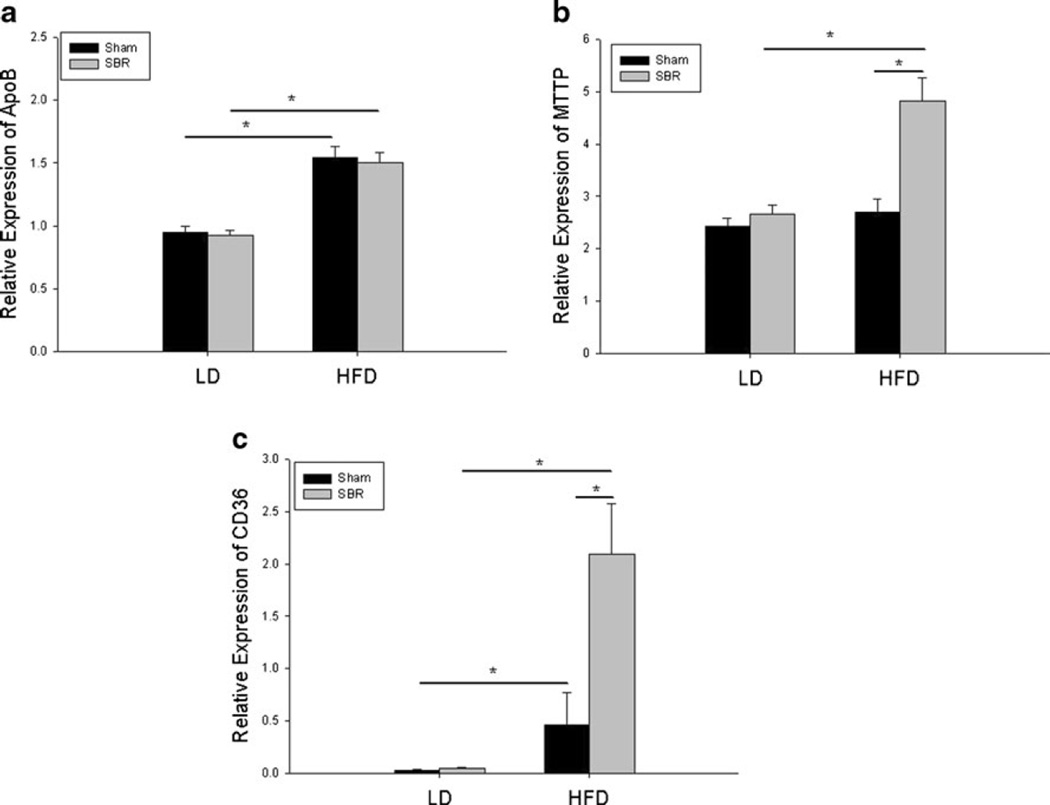
The mRNA expression of several fat transporters was examined in order to delineate cellular changes in response to enteral fat in the context of small bowel adaptation. The expression of apolipoprotein B (ApoB) was increased in both sham/HFD and SBR/HFD villus enterocytes, suggesting that the transcriptional regulation of ApoB mRNA is affected by the increased enteral fat alone independent of operation (Fig. 5a). In contrast, MTTP mRNA expression showed a modest increase in response to SBR/HFD compared to both SBR/LD and sham/HFD (Fig. 5b). This suggests that the combination of both resection and enteral fat is required to increase MTTP mRNA expression while neither resection nor enteral fat alone has any significant effect.
Fig. 5.
Fat related mRNA expression of apolipoprotein B (a), microsomal triglyceride transfer protein (b), and CD36 (c) as determined by RT-PCR. β-Actin was used as the endogenous control, and whole bowel was used as a calibrator. Sham operations represent transection and anastomosis without resection and small bowel resection (SBR) is a 50 % proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet. *p <0.05; **p <0.005
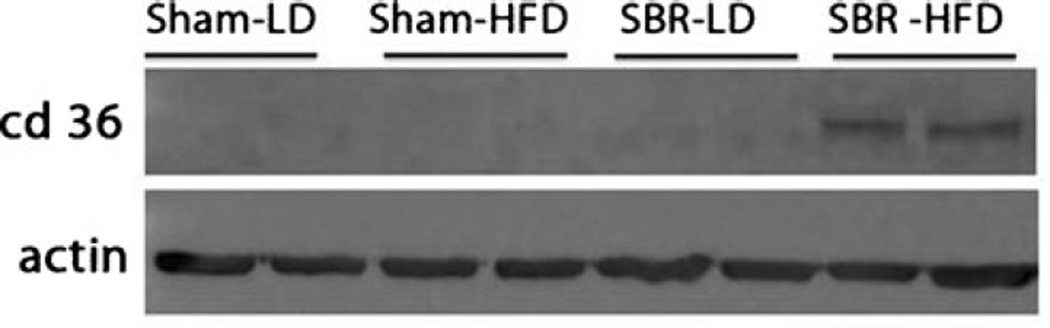
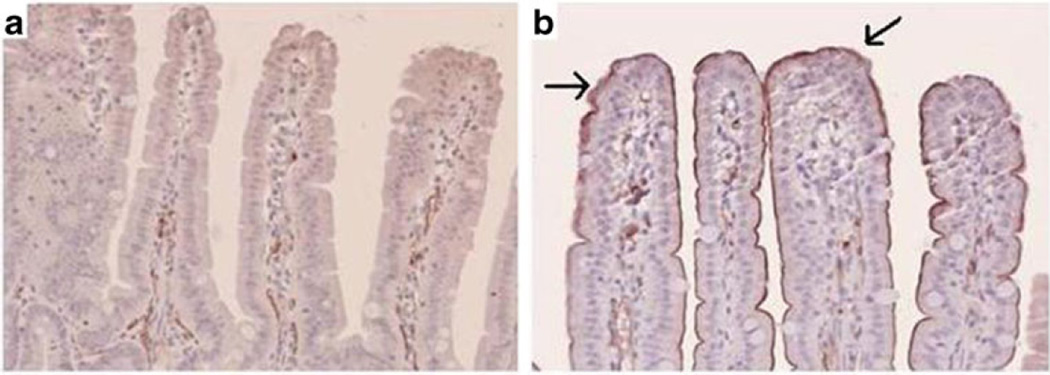
The most dramatic change occurred in CD36 mRNA expression (Fig. 5c). Almost negligible amounts of CD36 were detected in sham/LD and SBR/LD groups. The addition of HFD alone increased CD36 expression 15-fold from sham/LD to sham/HFD. However, the provision of HFD following SBR resulted in a significant increase inCD36 expression when compared with SBR/LD (50-fold). These findings were also confirmed by Western blot for CD36 protein expression (Fig. 6), and enterocyte expression was verified by immunohistochemical staining (Fig. 7).
Fig. 6.
CD36 protein expression. Western blot of protein from ileal villus enterocytes probed for CD36. Actin was used as protein binding control. Sham operations represent transection and anastomosis without resection and small bowel resection (SBR) is a 50 % proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet
Fig. 7.
CD36 immunostaining from ileal tissue samples from SBR/LD (a) and SBR/HFD (b) mice at ×20 magnification. Arrows represent positive staining for CD36. Small bowel resection (SBR) represents a 50% proximal resection with primary anastomosis. LD liquid diet, HFD high-fat diet
Villus Height and CD36 Levels Decrease after High Fat Diet Is Removed
While both groups of mice had been on HFD for at least 7 days, mice that had kept on HFD for an additional 28 days showed a 76.9±30.9%increase in villus morphology, similar to the 82.7± 7.0 % increase in SBR/HFD mice. However, mice that been placed back on LD had significantly attenuated villus height, with a growth of only 25.7±19.0 %, significantly lower than the mice that had been maintained on HFD (p <0.05). CD36 expression matched this pattern as mice on a prolonged HFD had elevated CD36 gene expression, while mice that returned to LD had CD36 levels similar to SBR/LD (data not shown). These results suggest that continued provision of an HFD is required to maintain the hyperplastic mucosal phenotype. Although mice that remained on HFD tended to have a higher percent change in body weight at the time of harvest compared to mice that were switched to a low-fat diet (111.2±3.2 vs 101.0±4.3 %), this was not statistically significant (p =0.09).
Discussion
We have found that supplementation of HFD after resection led to increased structural and proliferative changes with enhanced expression of fat transporters, especially CD36. We have shown that these changes take place despite equal amounts of total kilocalories ingested and stool output. Return to an LD from an HFD resulted in a regression of villus height and downregulation of CD36. These data would suggest that fat is a preferred substrate to enhance structural features of adaptation and may be a useful therapeutic intervention for patients with short gut syndrome.
Prior studies of enteral fat after SBR used menhaden oil, which is high in polyunsaturated long-chain fatty acids (LCFA), particularly the omega-3 fatty acid docosahexaenoic acid (DHA).8, 9 These reports demonstrated that the rats treated with menhaden before and after a 70 % SBR had increased mucosal weight, DNA, and protein content in the duodenum and ileum. Another study from the same group involved diets with the same proportion of fat (all 30 %) but different compositions. Rats underwent SBR and were postoperatively treated with safflower oil or arachidonic acid/DHA. While both diets were composed of polyunsaturated LCFA, the AA/DHA diet had increased mucosal mass, DNA, and protein content of the intestinal wall when compared with rats receiving safflower oil.8 Lard, a different type of fat composed of saturated LCFA and monounsaturated LCFA, has also been found to be associated with a significant intestinal phenotype after SBR. Comparing rats undergoing 75 % SBR on normal chow (10 % kcal fat) versus lard-based high-fat diet (50 % kcal fat), the rats on HFD had significantly increased jejunal and ileal villus height.7
In contrast with the above studies, low-fat diets have been found to have a negative impact on adaptation. Specifically, rats given a low-fat diet postresection were found to have decreased body weight, mucosal weight, reduced CD36 mRNA levels in duodenum/jejunum/ileum, and attenuated villus height/proliferation/apoptosis in the ileum.12, 13
Our study used two different diets—our standard liquid diet (LD) and a high-fat Western diet (HFD), which were composed of 35 and 42 % kcal of fat, respectively. Additionally, the composition of fat in our diets was different; LD contained a combination of olive oil, corn oil, and safflower oil (of which the largest majority was oleic acid, a monounsaturated LCFA), while the HFD contained anhydrous milk fat (the majority of which is saturated LCFA). We used this particular LD as it had been our laboratory standard throughout our studies of SBR and adaptation in mice. Similarly, our preliminary studies had suggested that this particular HFD was associated with substantial postresection villus growth in a line of genetically altered mice. As such, we were eager to further explore the possibility of HFD-induced enhanced adaptation in normal mice. We had previously determined that the majority of adaptive growth in mice occurs within the first 7 days after surgery and is generally a critical period of cell-signaling and plasticity.10 Thus, we questioned if adding an HFD after this period would still produce a significant change.
Although the actual difference in percent kilocalorie of fat was small between the two diets, the effects were significant. There were significant phenotypic alterations in both villus morphology and mRNA expression of fat transporters seen in the SBR/HFD group that were not seen in the SBR/LD group. It is possible that the specific type of fat in the HFD (saturated LCFA) is responsible for the effects. However, monounsaturated LCFA, which are found in high concentrations in lard, have produced a similar phenotype in resection models.7
Despite enhanced structural adaptation in the SBR/HFD group, we did not find any metabolic advantages based on weight gain or body composition analysis. The body composition data revealed that mice fed LD in both sham and SBR groups had greater percent body fat than HFD groups. Sham/HFD had greater percent lean mass than sham/LD as well as greater weight gain, suggesting that HFD may encourage increased protein production and lean mass growth compared to LD. There were no differences in percentage body weights between SBR/LD and SBR/HFD mice. While the SBR/HFD mice demonstrated a trend toward a higher percent lean mass than its LD counterpart, this difference was not significant. Additionally, although there was a trend of increased percentage weight gain in mice that had remained on HFD for an additional 4 weeks compared to those that had been switched to LD, this was also not significant. These findings suggest that the body composition alterations within the HFD mice may be revealed over a more prolonged experimental interval, or that structural adaptation may not coincide with function. The latter may be due to immature intestinal epithelia resulting in impaired fat absorption or processing, as we did not measure the downstream effects of fat metabolism beyond transporter mRNA expression. However, a previous study measuring the effects of HFD in a short bowel rat model found that fat absorption was actually increased in resected rats on HFD compared to resected rats on normal chow and sham rats, although no changes in body weight were observed on POD 14.14
Our proximal small bowel resection model allows for the animal to retain the part of the bowel that has the greatest capacity for fat absorption, as fat and bile acids are best absorbed in the ileum. A distal resection (i.e., an ileocecectomy) would be expected to result in impaired absorption of fat. While the mechanism for high-fat-diet-induced hyperplasia was not determined in this study, we found that different fat transporters demonstrate different gene expression changes in response to both diet and operation. A previous study had shown that the gene expression of ApoB and MTTP, both involved in lipoprotein synthesis, were elevated in mice who were given a high-fat diet for 7 days.15 Similarly, our study showed that ApoB mRNA expression increased in the setting of HFD regardless of operation. However, MTTP mRNA expression is elevated only in the SBR/HFD group with a relative increase of roughly twofold.
CD36 is a transmembrane glycoprotein found in heart, skeletal muscle, adipose tissue, capillary endothelium, and the intestine.16 CD36 is found on the brush border membrane of duodenal and jejunal villi and has been implicated as a facilitator of fatty acid uptake.17–19 Previous studies have underscored the complexity of the CD36 system. While CD36-null mice have not been found to have evidence of net lipid malabsorption, intestinal lipid secretion into the lymphatic system was impaired thus suggesting a role for CD36 in chylomicron formation. Additionally, fat absorption was increased in the distal intestine in CD36 null mice.20–22
The expression of CD36 is not only dependent on ingestion of fat but also the duration and amount of fat. CD36 was found to be increased in the jejunum of mice after being fed an HFD (45 % kcal fat) for 5 weeks compared to the jejunum of mice fed a lower-fat diet (15 % kcal fat).23 However, rats that were given lipid boluses were found to have a rapid and significant decrease in CD36 in jejunal enterocytes.24
The role of CD36 in small bowel adaptation with HFD has also been explored in a previous study. In rats given a 50%kcal fat diet after a mid-75 % SBR, CD36 mRNA expression was significantly decreased in the ileum compared to resected rats who had been given a 10 % kcal fat diet despite an overall increase in measured fat absorption.14 It was theorized that CD36 was downregulated due to higher concentrations of LCFA and that increased overall mucosal weight would dilute individual cells LCFA absorptive capacity. We found contrasting results in that CD36 levels were dramatically elevated in villus enterocytes of mice fed HFD after surgery. There are a number of differences in procedure that may have been contributing to these disparate results including a different operation (mid- vs proximal SBR), diverse high-fat and control diets, and enterocyte isolation protocol that we used for the study of gene expression. Additionally, our mice were only on an HFD for 1 week as opposed to 2 weeks.
Our findings suggest that CD36 may play a role in postresection HFD-induced villus growth. The pattern of CD36 expression that we have found links closely to the pattern of villus morphology. Additionally, when HFD diet is removed, villus morphology and CD36 expression are both attenuated. Past research has suggested a mechanistic link between CD36 and the glucagon-like peptide-2/insulin-like growth factor-1 receptor (GLP-2/IGF-1R) axis.
GLP-2 is an intestinal trophic factor that has been of great interest recently as prospective studies found that TPN-dependant patients required lower volumes of parental support when teduglutide, a GLP-2 analog, was administered subcutaneously once daily.25, 26 Exogenous GLP-2 has been demonstrated to stimulate intestinal lipid absorption and chylomicron production via a CD36 pathway.27, 28 The trophic effects of GLP-2 are also mediated by IGF-1/IGF-1R.29 Small bowel resection and the ingestion of nutrients, especially fatty acids, have been known to stimulate GLP-2 secretion.30
Taken together, it is possible that postresection HFD-induced GLP-2 secretion could be responsible for both increased villus morphology and elevated CD36 expression. Because IGF-1 is the trophic factor directly responsible for villus growth in response to GLP-2 secretion, then deletion of IGF-1R would subsequently negate any trophic effects of GLP-2. If GLP-2 is in fact responsible for postresection HFD-induced “super-adaptation”, then we would expect to see a loss of this phenotype if this experiment were to replicated with intestinal IGF-1R KO mice.
Conclusion
Our data shows that the addition of an HFD after small bowel resection enhances structural adaptation as well as increases the expression of the fat transporters, MTTP and CD36, while removal of an HFD reverses these results. We did not find any advantages in weight gain or lean mass within the limited time frame of this experiment. However, the structural augmentation produced by an HFD should not be ignored and may still have the potential to be a useful adjunct to the short gut population over longer periods of time.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK 059288 (Warner), T32 GM008795 (Choi) P30DK52574—Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine, and the Children’s Surgical Sciences Institute of the St. Louis Children’s Hospital Foundation. Dr. Choi is also supported by The Marion and Van Black Endowed Pediatric Surgical Fellowship.
Footnotes
This paper was presented as a plenary presentation at the 54th Annual Meeting of the Society for Surgery of the Alimentary Tract, May 18–21, 2013.
Contributor Information
Pamela M. Choi, Division of Pediatric Surgery, St Louis Children’s Hospital, One Children’s Place, Suite 5S40, St. Louis, MO 63110, USA Department of Surgery, Washington University School of Medicine, St. Louis, MO 63110, USA.
Raphael C. Sun, Division of Pediatric Surgery, St Louis Children’s Hospital, One Children’s Place, Suite 5S40, St. Louis, MO 63110, USA Department of Surgery, Washington University School of Medicine, St. Louis, MO 63110, USA.
Jun Guo, Division of Pediatric Surgery, St Louis Children’s Hospital, One Children’s Place, Suite 5S40, St. Louis, MO 63110, USA; Department of Surgery, Washington University School of Medicine, St. Louis, MO 63110, USA.
Christopher R. Erwin, Division of Pediatric Surgery, St Louis Children’s Hospital, One Children’s Place, Suite 5S40, St. Louis, MO 63110, USA Department of Surgery, Washington University School of Medicine, St. Louis, MO 63110, USA.
Brad W. Warner, Division of Pediatric Surgery, St Louis Children’s Hospital, One Children’s Place, Suite 5S40, St. Louis, MO 63110, USA Department of Surgery, Washington University School of Medicine, St. Louis, MO 63110, USA.
References
- 1.Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043–1050. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 2.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723–728. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menge H, Grafe M, Lorenz-Meyer H, et al. The influence of food intake on the development of structural and functional adaptation following ileal resection in the rat. Gut. 1975;16:468–472. doi: 10.1136/gut.16.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buts JP, Morin CL, Ling V. Influence of dietary components on intestinal adaptation after small bowel resection in rats. Clin Invest Med. 1979;2:59–66. [PubMed] [Google Scholar]
- 5.Dodge ME, Bertolo RF, Brunton JA. Enteral feeding induces early intestinal adaptation in a parenterally fed neonatal piglet model of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2012;36:205–212. doi: 10.1177/0148607111417447. [DOI] [PubMed] [Google Scholar]
- 6.Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130:S93–S99. doi: 10.1053/j.gastro.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Sukhotnik I, Mor-Vaknin N, Drongowski RA, et al. Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatr Surg Int. 2004;20:419–424. doi: 10.1007/s00383-004-1168-9. [DOI] [PubMed] [Google Scholar]
- 8.Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr. 1999;28:41–45. doi: 10.1097/00005176-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Vanderhoof JA, Park JH, Herrington MK, et al. Effects of dietary menhaden oil on mucosal adaptation after small bowel resection in rats. Gastroenterology. 1994;106:94–99. doi: 10.1016/s0016-5085(94)94589-6. [DOI] [PubMed] [Google Scholar]
- 10.Helmrath MA, VanderKolk WE, Can G, et al. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 11.Guo J, Longshore S, Nair R, et al. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem. 2009;284:134–140. doi: 10.1074/jbc.M806133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhotnik I, Gork AS, Chen M, et al. Effect of low fat diet on lipid absorption and fatty-acid transport following bowel resection. Pediatr Surg Int. 2001;17:259–264. doi: 10.1007/s003830100590. [DOI] [PubMed] [Google Scholar]
- 13.Sukhotnik I, Shiloni E, Krausz MM, et al. Low-fat diet impairs postresection intestinal adaptation in a rat model of short bowel syndrome. J Pediatr Surg. 2003;38:1182–1187. doi: 10.1016/s0022-3468(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 14.Sukhotnik I, Mor-Vaknin N, Drongowski RA, et al. Effect of dietary fat on fat absorption and concomitant plasma and tissue fat composition in a rat model of short bowel syndrome. Pediatr Surg Int. 2004;20:185–191. doi: 10.1007/s00383-004-1143-5. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez Vallejo SJ, Alqub M, Luquet S, et al. Short-term adaptation of postprandial lipoprotein secretion and intestinal gene expression to a high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2009;296:G782–G792. doi: 10.1152/ajpgi.90324.2008. [DOI] [PubMed] [Google Scholar]
- 16.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassir F, Wilson B, Han X, et al. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 18.Lobo MV, Huerta L, Ruiz-Velasco N, et al. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem. 2001;49:1253–1260. doi: 10.1177/002215540104901007. [DOI] [PubMed] [Google Scholar]
- 19.Poirier H, Degrace P, Niot I, et al. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP) Eur J Biochem. 1996;238:368–373. doi: 10.1111/j.1432-1033.1996.0368z.x. [DOI] [PubMed] [Google Scholar]
- 20.Nauli AM, Nassir F, Zheng S, et al. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dur S, Krause K, Pluntke N, et al. Gene structure and expression of the mouse APOBEC-1 complementation factor: multiple transcriptional initiation sites and a spliced variant with a premature stop translation codon. Biochim Biophys Acta. 2004;1680:11–23. doi: 10.1016/j.bbaexp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Goudriaan JR, Dahlmans VE, Febbraio M, et al. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol Cell Biochem. 2002;239:199–202. [PubMed] [Google Scholar]
- 23.Lynes M, Narisawa S, Millan JL, et al. Interactions between CD36 and global intestinal alkaline phosphatase inmouse small intestine and effects of high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1738–R1747. doi: 10.1152/ajpregu.00235.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran TT, Poirier H, Clement L, et al. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem. 2011;286:25201–25210. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012 Dec;143(6):1473–1481. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 26.O’Keefe SJ, Jeppesen PB, Gilroy R, et al. Safety and Efficacy of Teduglutide After 52Weeks of Treatment in Patients With Short Bowel Intestinal Failure. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh J, Longuet C, Maida A, et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. 1005. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 28.Newberry EP, Davidson NO. Intestinal lipid absorption, GLP-2, and CD36: still more mysteries to moving fat. Gastroenterology. 2009;137:775–778. doi: 10.1053/j.gastro.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowland KJ, Trivedi S, Lee D, et al. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology. 2011;141:2166–2175. doi: 10.1053/j.gastro.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Martin GR, Wallace LE, Hartmann B, et al. Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2005;288:G431–G438. doi: 10.1152/ajpgi.00242.2004. [DOI] [PubMed] [Google Scholar]