Abstract
Background:
To evaluate the bone mineral density in the mandible of edentulous patients at prospective intraoral implant sites. Pre-operative evaluation of bone density is essential to assist the clinician with the treatment planning of implant supported prosthesis.
Materials and Methods:
A study group of 12 edentulous subjects comprising of six male and six female between the age group of 45-55 years seeking implant supported prosthesis were selected. A radiographic stent using auto polymerizing resins incorporating the gutta-percha cones were prepared for the computed tomography scan. The bone mineral density values were recorded in various sites (trabecular and cortical) of the mandibular jaws in Hounsfield units. The data thus obtained were tabulated and statistically analyzed using Mann–Whitney U-test and Kruskal–Wallis test.
Results:
The bone mineral density in the buccal cortical region of mandible increases from incisors to molars and in the trabecular region it is more in the incisors and canines compared to the premolar and molar regions whereas in the lingual cortical region of mandible may lie on nearly the same level over the entire lingual cortex. The bone mineral density is little higher in males than females.
Conclusion:
There is variation in the bone mineral density in the buccal cortex and trabecular bone, but no significant variation in the lingual cortex when compared between male and female subjects.
Keywords: Bone mineral density, computed tomography, implant supported prosthesis
Introduction
The use of osseointegrated implants in the treatment of edentulous patients has become an accepted alternative to conventional prosthetic dentistry. Dental implants are intended to bear masticatory loads of different magnitudes. The loading capacity and success of the implant depends on load transmission at the bone to implant interface, where bone quality and architecture of bone plays a vital role. It has been demonstrated that the poorer quality of bone is associated with higher failure rates.
The strength of the bone is directly related to bone density. Factors such as the amount of bone contact, the modulus of elasticity, and axial stress contours around the implant are all affected by the bone mineral density. The initial bone density helps in mechanical immobilization of the implant during healing as well as it permits distribution and transmission of stresses from the prosthesis to the implant bone interface after healing.
Computed tomography provides cross-sectional radiographic images that facilitate proper assessment of potential recipient sites for implant placement.1 Computed tomography is a non-invasive preoperative method and has the major advantage of enabling trabecular and cortical bone density to be evaluated separately.2,3 Optimum implant orientation can be aided by the 3D radiographic data base provided by a computed tomography scan.
An in vivo study was designed to evaluate and compare the bone mineral density in different regions of the mandible in males and females for assessment of prospective implant sites.
Materials and Methods
A group of 12 edentulous subjects comprising of six males and six females between the age group of 45-55 years seeking implant supported prosthesis were selected for the purpose of this study. All the subjects were informed about the study and their written consent to participate in the study was taken. The patients selected has good general health and were deemed fit for implant therapy.
A routine screening radiograph - panoramic radiography using a standard radiographic technique was employed using a PLANMECA PM 2002 CC Proline machine (10 mA, 70 KV) and standard size radiographic cassette to rule out intra alveolar tooth remnants. A routine blood and urine analysis was performed to rule out any systemic disorders.
A diagnostic impression was made of the completely edentulous maxillary, and mandibular arches and cast were poured. Using a graphite pencil ten prospective implant sites namely at the region of central incisor, lateral incisor, canine, premolar and molar on either side of the arch were marked on the maxillary and mandibular cast. Gutta-percha cones of diameter 1 mm, height 1 mm were used in this study as markers and for standardization of sites (Figures 1 and 2). Marked stents allow more precise localization of potential fixture sites and can also serve as a surgical guide when referenced to multi planar reformatted computerized tomography.4
Figure 1.
Diagnostic maxillary cast.
Figure 2.
Diagnostic mandibular cast.
A radiographic stent using autopolymerizing resins incorporating the gutta-percha cones were prepared on the cast. Occlusal rims were prepared on these radiographic stent and adjusted for proper vertical and horizontal intermaxillary relation and were sealed in order to prevent movement of jaws during computed tomography procedure (Figure 3).
Figure 3.
Radiographic stent.
Computed tomography was done using a SIEMENS Somatrom ESPRIT PLUS fitted with a Kodak Ektascan 160 laser image was used. The patient was stabilized in a standardized position within the gantry. Computed tomography scans were obtained with the patient lying comfortably in the supine position with the plane of occlusion positioned perpendicular to the horizontal plane. For implant imaging applications, the computed tomography unit was aligned to expose tissue slices that are at right angles to the long axis of the patient’s body and ar e referred to as axially oriented images, which are parallel to the inferior border of the mandible for mandibular imaging and parallel to the hard palate for maxillary imaging. Proper positioning was verified using the scout preview images (appeared similar to a conventional radiograph of the skull, was generated on the scanner to assist in the selection of slices) and by following the manufacturers scan parameters. The axial scans were selected from and annotated on a lateral scout view to verify the areas of interest and to check for artifacts. Axial cuts were made 1.5-2 mm with 0.5 mm overlap. Individual horizontal images were displayed at 3 mm intervals from the inferior border of the mandible vertically to 1 cm above the palatal process of the maxilla. These two dimensional images are called transaxial scans. All the information obtained from computed tomography imaging was contained in this “block” of computerized data. The transaxial scans could be stored on a computer tape and rearranged or reformatted by the Dental scan software to produce cross-sectional, panographic, or three-dimensional images. The densities of bone in the various sites (trabecular and cortical) were obtained by locating a cursor at various positions on the image and using Dental scan software program to determine the density which was expressed in the Hounsfield scale (Figure 4). The bone mineral density values were recorded on both sides of the mandible in the trabecular and cortical region and mean of these was obtained. The data thus obtained were tabulated and statistically analyzed using Mann–Whitney U-test and Kruskal–Wallis test.
Figure 4.
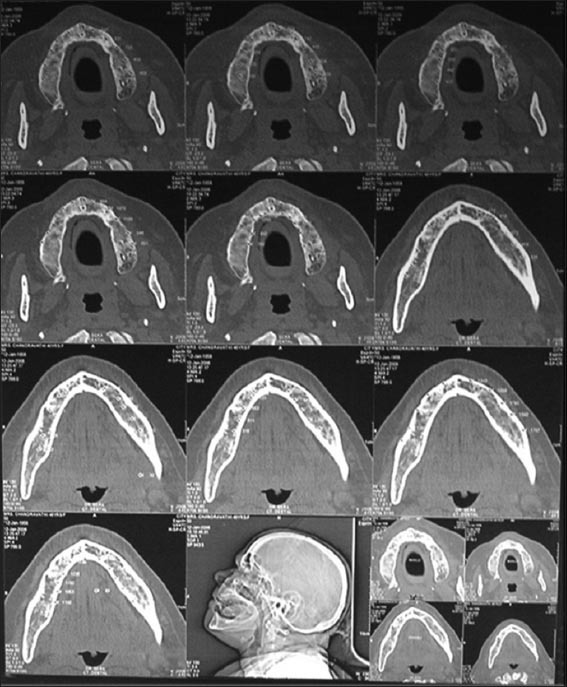
Computed tomography of the subject.
Results
The bone mineral density is compared in different regions of the mandible in male and female subjects using computed tomography. The data thus obtained were tabulated and statistically analyzed using Mann–Whitney U-test and Kruskal–Wallis test. There was a statistically significant variation in the bone mineral density in the buccal cortex and trabecular bone but no significant variation in the lingual cortex when compared between male and female subjects (Tables 1-6 and Graphs 1-3).
Table 1.
The comparison of bone mineral density in incisors, canine, premolar and molars in the buccal cortical region of mandible in the male subjects in Hounsfield units.
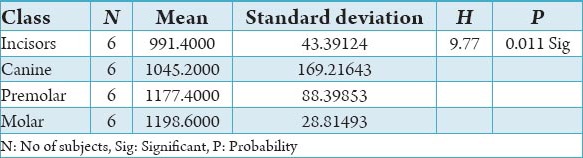
Table 2.
The comparison of bone mineral density in incisors, canine, premolar and molars in the lingual cortical region of mandible in the male subjects in Hounsfield units.
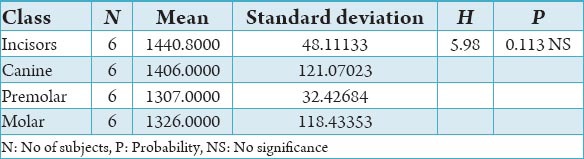
Table 3.
The comparison of bone mineral density in incisors, canine, premolar and molars in the trabecular region of mandible in the male subjects in Hounsfield units.
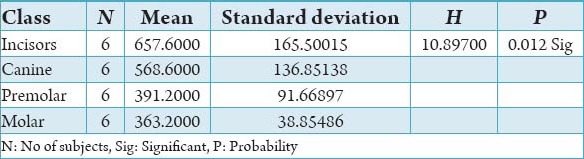
Table 4.
The comparison of bone mineral density in incisors, canine, premolar and molars in the buccal cortical region of mandible in the female subjects in Hounsfield units.
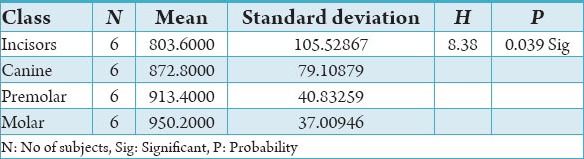
Table 5.
The comparison of bone mineral density in incisors, canine, premolar and molars in the lingual cortical region of mandible in the female subjects in Hounsfield units.
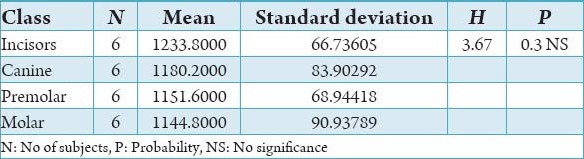
Table 6.
The comparison of bone mineral density in incisors, canine, premolar and molars in the trabecular region of mandible in the female subjects in Hounsfield units.
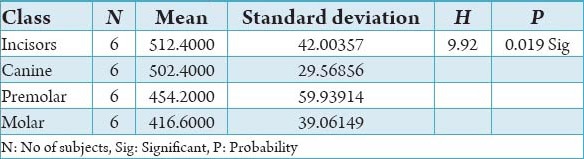
Graph 1.
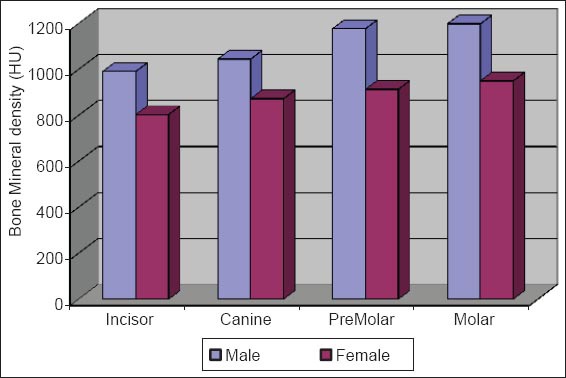
Comparision of bone mineral density of buccal cortex between male versus female.
Graph 2.
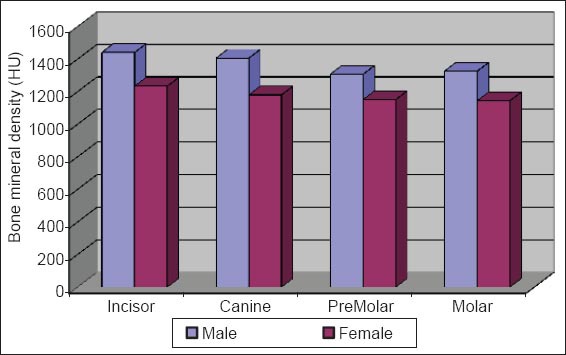
Comparision of bone mineral density of lingual cortex between male versus female.
Graph 3.
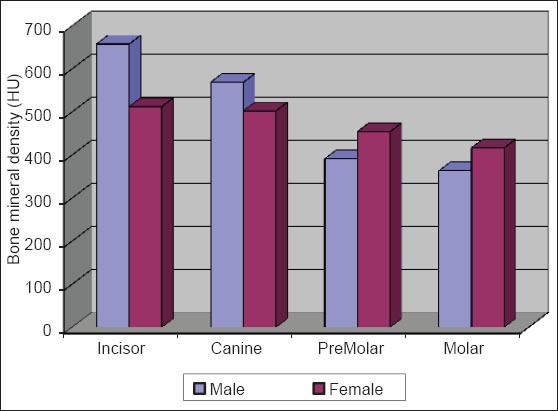
Comparision of bone mineral density of trabecular bone between male versus female.
Discussion
The initial bone density helps in mechanical immobilization of the implant during healing as well as it permits distribution and transmission of stresses from the prosthesis to the implant bone interface after healing.5 The density of the available bone is a determining factor in implant design, surgical approach, healing time and initial progressive loading during prosthetic construction.6 In a study, it was observed (66%) of implant failure in soft bone type. The reduced implant survival is more related to bone density than location.7,8 Pre-operative evaluation of bone density is essential to assist the clinician with the treatment planning of implant supported prosthesis. Thus, this study was designed to assess bone mineral density in males and females using computed tomography to find the most ideal site for implant placement. Computed tomography is a non-invasive preoperative method and widely accepted as the most precise means of evaluating the architecture of potential implant sites in the mandible and maxilla as according to Wyatt and Pharoah9 and Lindh et al.2 Computed tomography has the major advantage of enabling trabecular and cortical bone density to be evaluated separately.3,10 It allows precise three dimensional anatomic localization and furnishes direct density measurements, expressed in Hounsfield units. When the effect of mandibular status is investigated, it is important that the sample of subjects be as homogenous as possible. In this study the sample consisted of males and females of the same age and all were edentulous in the mandible. Computed tomography software program is capable of taking the axial scan information and creating a series of image slices at right angles to the curved structure of the jaws. The reported error associated with measurements with computed tomography scan is <0.5-2 mm when compared with panoramic radiograph. The bone mineral density in incisors, canine, premolar and molars in the buccal cortical region of the mandible in the males is compared (Table 1). It was seen that the bone mineral density in the incisors was (991.4), canine was (1045.2), premolar was (1177.4) and molar was (1198.6). In the females (Table 4), it was seen that in the incisors was (803.6), canine was (872.8), premolar was (913.4) and molar was (950.2) in which density increases from incisors to molars. Variation in the bone mineral density was found to be statistically significant. The results of this study are concurred with the study conducted on the intermandibular variations in the bone mass in cortices between regions of the alveolar process and mandibular body.11 These variations in the density in different regions are because the function of the mandible is different in these four regions, incisors, canine, premolar and molars. The attachment of the muscles differs from region to region. There is, therefore, great variation in shape, course of trajectories and thickness of cortices within the mandible.12 The bone mineral density in incisors, canine, premolar and molars in the lingual cortical region of the mandible in the males (Table 2). It was seen, incisors was (1440.8), canine was (1406.0), premolar was (1307.0) and molar was (1326.0). In the females (Table 5), it was seen incisors was (1233.8), canine was (1180.2), premolar was (1151.6) and molar was (1144.8) in which density is almost same in all the regions. Variation in the bone mineral density was found to be statistically not significant. The results show that the bone mineral density may lie on nearly the same level over the entire lingual cortex. The above results concurred with the study for the intermandibular variations in the bone mass in cortices between regions of the alveolar process and mandibular body. Most muscles that are attached to the lingual side of the mandible do not produce force but are related to more complicated movement of tongue and mandible.13 The bone mineral density in incisors, canine, premolar and molars in the trabecular region of the mandible in the males is compared (Table 3). It was seen that the bone mineral density in the incisors was (657.6), canine was (568.6), premolar was (391.2) and molar was (363.2). In the females (Table 6), bone mineral density in the incisors was (512.4), canine was (502.4), premolar was (454.2) and molar was (416.6) in which density is more in the incisors and canines compared to the premolar and molar regions. Variation in the bone mineral density was found to be statistically significant. The above results concurred with study the intramandibular variations in the bone mass, coarseness of the bone trabeculae between incisors, premolar and molar regions The trabecular bone is generally denser and more coarsely woven in the incisor region than in either the premolar region and is the most delicately woven in the molar region.14 This study shows that bone mineral density increases from incisors to molars in the buccal cortex and the bone mineral density may lie on nearly the same level over the entire lingual cortex. In the trabecular region bone, mineral density is more in the incisors and canines compared to the premolar and molar regions. The bone mineral density in males is little higher than females, but implant therapy can be carried out with the same degree of success. The higher bone mineral density in the anterior region of the mandible may be one of the reasons for increased success rate of osseointegration. Computed tomography is mandatory in prospective implant patient not only in assessing bone mineral density but also the height and width of the available bone.
Conclusion
An in vivo study was conducted to compare the bone mineral density in different regions of the mandible in male and female subjects using computed tomography. The bone mineral density values were recorded in the trabecular and cortical region of the mandible in incisor, canine, premolar and molar areas of all the subjects. The bone mineral density in the incisor and canine areas is more compared to premolar and molar areas. The bone mineral density is relatively more in the males as compared to females. It was thus concluded that intra oral implant therapy can be carried out with the same degree of success in both male and female subjects provided there is no other systemic contraindication using computed tomography
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Iplikçioglu H, Akça K, Cehreli MC. The use of computerized tomography for diagnosis and treatment planning in implant dentistry. J Oral Implantol. 2002;28(1):29–36. doi: 10.1563/1548-1336(2002)028<0029:TUOCTF>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Lindh C, Nilsson M, Klinge B, Petersson A. Quantitative computed tomography of trabecular bone in the mandible. Dentomaxillofac Radiol. 1996;25(3):146–50. doi: 10.1259/dmfr.25.3.9084263. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi A, Tanimoto K, Akagawa Y, Suei Y, Wada T, Rohlin M. Trabecular bone pattern of the mandible. Comparison of panoramic radiography with computed tomography. Dentomaxillofac Radiol. 1997;26(2):85–9. doi: 10.1038/sj.dmfr.4600209. [DOI] [PubMed] [Google Scholar]
- 4.Israelson H, Plemons JM, Watkins P, Sory C. Barium-coated surgical stents and computer-assisted tomography in the preoperative assessment of dental implant patients. Int J Periodontics Restorative Dent. 1992;12(1):52–61. [PubMed] [Google Scholar]
- 5.Misch CE. Contemporary Implant Dentistry. 2nd ed. St Louis: Mosby; 1999. pp. 109–18. [Google Scholar]
- 6.Kribbs PJ, Smith DE, Chesnut CH., 3rd Oral findings in osteoporosis. Part II: Relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent. 1983;50(5):719–24. doi: 10.1016/0022-3913(83)90215-9. [DOI] [PubMed] [Google Scholar]
- 7.Friberg B, Jemt T, Lekholm U. Early failures in 4,641 consecutively placed Brånemark dental implants: A study from stage 1 surgery to the connection of completed prostheses. Int J Oral Maxillofac Implants. 1991;6(2):142–6. [PubMed] [Google Scholar]
- 8.Engquist B, Bergendal T, Kallus T, Linden U. A retrospective multicenter evaluation of osseointegrated implants supporting overdentures. Int J Oral Maxillofac Implants. 1988;3(2):129–34. [PubMed] [Google Scholar]
- 9.Wyatt CC, Pharoah MJ. Imaging techniques and image interpretation for dental implant treatment. Int J Prosthodont. 1998;11(5):442–52. [PubMed] [Google Scholar]
- 10.Von Wowern N, Klausen B, Kollerup G. Osteoporosis: A risk factor in periodontal disease. J Periodontal. 1994;65(12):1134–8. doi: 10.1902/jop.1994.65.12.1134. [DOI] [PubMed] [Google Scholar]
- 11.von Wowern N. Variations in bone mass within the cortices of the mandible. Scand J Dent Res. 1977;85(6):444–55. doi: 10.1111/j.1600-0722.1977.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 12.Devlin H, Horner K, Ledgerton D. A comparison of maxillary and mandibular bone mineral densities. J Prosthet Dent. 1998;79(3):323–7. doi: 10.1016/s0022-3913(98)70245-8. [DOI] [PubMed] [Google Scholar]
- 13.Roberts WE, Gonsalves M. Aging of bone tissue. In: Holm Pedersen P, Loe H., editors. Geriatric Dentistry: A Textbook of Oral Gerentology. 1st ed. Copenhagen: Munksgaard; 1986. pp. 83–93. [Google Scholar]
- 14.Von Wowern N. Variations in bone mass within the trabecule of the mandible. Scand J Dent Res. 1977;85(7):613–22. doi: 10.1111/j.1600-0722.1977.tb02123.x. [DOI] [PubMed] [Google Scholar]


