Abstract
Background:
Use of alkaline peroxide denture cleanser with different temperature of water could cause a change in surface hardness of the acrylic denture and also has a bleaching effect. The purpose of the study was to determine the effect of increased water content during thermal cycling of hot water-treated acrylic on the surface hardness of acrylic denture base when compared to warm water treated acrylic. And to compare the bleaching effect of alkaline peroxide solution on the acrylic denture base on hot water and warm water treated acrylic.
Materials and Methods:
Forty samples (10 mm × 10 mm × 2.5 mm) were prepared. After the calculation of the initial hardness 40 samples, each was randomly assigned to two groups. Group A: 20 samples were immersed in 250 ml of warm distilled water at 40°C with alkaline peroxide tablet. Group B: 20 samples were immersed in 250 ml of hot distilled water at 100°C with alkaline peroxide tablet. The surface hardness of each test sample was obtained using the digital hardness testing machine recording the Rockwell hardness number before the beginning of the soaking cycles and after completion of 30 soak cycles and compared. Values were analyzed using paired t-test. Five samples from the Group A and five samples from Group B were put side by side and photographed using a Nikon D 40 digital SLR Camera and the photographs were examined visually to assess the change in color.
Results:
Acrylic samples immersed in hot water showed a statistically significant decrease of 5.8% in surface hardness. And those immersed in warm water showed a statistically insignificant increase of 0.67% in surface hardness. Samples from the two groups showed clinically insignificant difference in color when compared to each other on examination of the photographs.
Conclusion:
Thermocycling of the acrylic resin at different water bath temperature at 40°C and 100°C showed significant changes in the surface hardness.
Keywords: Acrylic resin, surface hardness, thermocycling
Introduction
Resins are the most commonly used material in dentistry due to their satisfactory results.1,2 In human mouth dentures as an indwelling medical device, prepare an optimal environment for adhesion and multiplication of both pathogenic and non-pathogenic organisms. The wide use of removable dentures has caused a sharp increase in denture-related soft tissue infections.3,4 The rate at which deposits accumulate on dentures may vary between individuals and can be affected by factors such as saliva composition, dietary intake, surface texture, and porosity of the denture base material, duration for which the dentures are worn, and the denture-cleansing regimen adopted by the wearer. Management of these denture-related infections is challenging, and infected dentures generally need to be cleaned and disinfected. Several disinfectants have been suggested for the disinfection of such dentures. The best disinfectant should fulfill most of the requirements of the ideal agent will not cause any kind of alteration in the structural and mechanical properties the denture.5,6
The combination of mechanical and chemical methods seems to be the best choice for denture cleaning.1,7-9
In choosing a disinfectant for dental prostheses, one should be aware of possible adverse effect of the disinfecting material.
In the oral cavity, moisture contamination and thermal variation facilitate the absorption of water.9,10 Absorbed water can act as a plasticizer and affect the mechanical properties of the material causing reduction in the hardness of the denture.1 Hence, the possible effect of water absorption and thermal cycling on the hardness of the acrylic resin surface also must be considered.
Absorption of water is markedly increased at high temperatures, which causes the acrylic surface to be supersaturated with water during cooling of the specimen. The water absorption and thermal effects may combine to cause the acrylic to form zones with different optical properties. This phenomenon is distinct from crazing of acrylic, where definite cracking occurs.11
It has been shown that when patients use hot water with alkaline peroxide tablets to clean their acrylic dentures, the dentures take on a bleached appearance as these cleansers contain alkaline detergents and oxygen releasing compounds (such as sodium perborate or percarbonate).12,13 Due to an extensive surface degradation of the acrylic, there is a marked reduction in the physical properties like hardness of the acrylic resulting from the plasticizing effect of absorbed water.11
Though various studies have proved that denture cleansers cause color changes and alterations in the physical properties of acrylic resins, however, few studies have investigated the influence on mechanical properties of acrylic denture base surfaces. Furthermore, there is a lack of literature regarding the long-term effect of denture cleansers used with water at high temperature on the mechanical properties of the acrylic resin. Therefore, this in vitro study was undertaken to assess the difference in the hardness of heat-cure acrylic resin immersed in water at different temperatures along with dispersed disinfectant tablet.
Aims and objectives
The present study was undertaken to fulfill the following objectives:
To determine and compare the long-term effect of the water bath with alkaline peroxide denture cleanser tablet on the surface hardness of acrylic denture base after thermocycling at two different temperatures.
To compare the long-term effect of the water bath with alkaline peroxide denture cleanser on the color changes of acrylic denture base after thermocycling at two different temperatures.
Materials and Methods
A total of 40 heat cure acrylic (LUCITONE® 199-Dentsply® Trubyte-NEWYORK) samples of the dimension 10 mm × 10 mm × 2.5 mm were fabricated to test the effects of water immersion and heat on surface hardness. All the samples are of a standard thickness of 2.5 mm to mimic the clinical conditions. The standard length and width of 10 mm were kept according to the specifications for testing on the Rockwell hardness tester (Model RASN, FUEL Instruments and Engineers Pvt. Ltd).
To achieve acrylic samples of standardized dimensions ten customized stainless steel metal dies of dimension 10 mm × 10 mm × 2.5 mm were fabricated at Central Institute of Plastic Engineering and Technology, Bhopal. This was done to ensure that all acrylic resin samples had same dimensions (Figure 1).
Figure 1.
Fabrication of stainless steel dies
Preparation of acrylic samples
The lower member of the universal dental flask was filled with dental stone, and all the 10 dies were placed on the wet stone maintaining enough distance between each other. After setting of the stone, a thin layer of petroleum jelly was applied all over the set plaster as well as the dies. The counter of the flask was then poured with dental stone under vibration and finally the lid was placed over the counter and the entire assembly was secured using a dental clamp. After the dental stone had set according to manufactures instructions plus 10-15 min, the flask was opened, and the dies were gently teased out. The mold spaces thus created were packed with conventional heat-cure acrylic resin LUCITONE® 199 (Dentsply® Trubyte)using a mixture of monomer and polymer in the ratio of 21 g (32 cc)/10 ml by weight. The trial closures were carried out using the hydropress and cellophane sheets until no flash was obtained. Later, the flask was clamped and left for 30 min. before curing. Curing was done in the digitally controlled acryliser (Apex Dental Equipments Ltd. India) using a long curing cycle of 9 h at 73°C and a half hour of terminal boiling temperature at 100°C. To avoid distortions, the clamped flask was bench cooled at room temperature for 30 min. Then immersed in cool water (16-27°C) for 15 min before deflasking. After deflasking the samples were recovered, and excess acrylic in all of the samples was trimmed away using a tungsten carbide bur. The samples were then subsequently finished using 200 grit sand papers 79 mounted on a mandrill, with a micromotor at 2000 rpm for 2 min (Figure 2).
Figure 2.
Prepared acrylic specimen.
Testing of the samples
Surface hardness measurements were made for all samples with a digital Rockwell hardness tester ([Model RASN], Manufactured by FUEL Instruments and Engineers Pvt. Ltd.). Rockwell hardness tests consist of forcing an indentor (Ball)into the surface of a test piece with two loads (Figure 3).
Figure 3.

Testing of specimen for Rockwell hardness.
Pre immersion testing
All the samples were first numbered from 1 to 40 before subjecting them to the hardness test. A ¼ inch diameter ball indenter and with a load of 60 kg force was used for testing. 23 Indentation hardness number was calculated for each sample, and the values generated were tabulated thus forming the control group.
Preparation of samples for experimental Group A and Group B: After the calculation of the Rockwell hardness of the 40 samples, they were divided into two groups of 20 samples each.
Experimental Group A: 20 samples (Sample No. 1 to Sample No. 20)
Experimental Group B: 20 samples (Sample No. 21 to Sample No. 40)
Group A samples were immersed in 250 ml of warm deionized distilled water at 40°C. Alkaline peroxide (Clanden tablet 85 DENT AIDS - New Delhi, India) used for cleaning dentures was also added to this water bath. The water in the bath was changed after every 24 h, and fresh water at 40°C was poured in with fresh clanden tablet. This cycle was repeated for 30 days.
Group B samples were immersed in 250 ml of hot deionized distilled water at 100°C. Alkaline peroxide (Clanden tablet 85)used for cleaning dentures was also added to this water bath. The water in the bath was changed after every 24 h, and fresh water at 100°C was poured in with fresh clanden tablet. This cycle was repeated for 30 days.
Post immersion testing
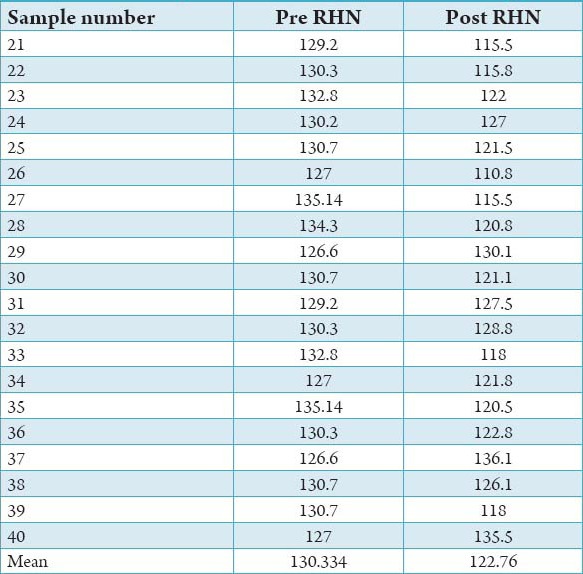
All the samples from Group A and B were evaluated for surface hardness after the completion of 30 soak cycles using rock well hardness tester (modle RASN) values. All the tests were carried out at room temperature and under standardized machine conditions. The pre and post hardness valve of Group A and Group B are shown in Tables 1 and 2.
Table 1.
Pre and post Rockwell hardness number for Group A.
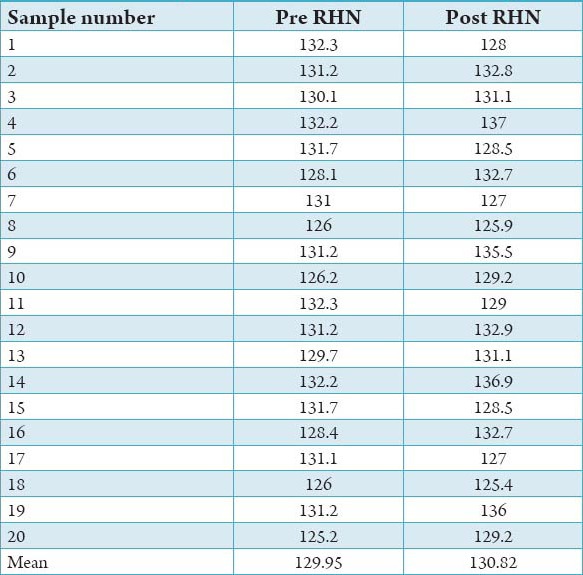
Table 2.
Pre and post Rockwell hardness number for Group B.
Color alteration
Five samples from the Experimental Group A (immersed in warm water) and five samples from the Experimental Group B (immersed in hot water) were selected randomly and were put side by side and photographed using a camera:(Nikon D 40 digital SLR Camera with attached close-up lenses 1, 2, 3. ASA 400, film sharpness 3008*2000 fine, Auto mode). The photographs were examined visually for the color changes.
Results
Percentage change in mean hardness was calculated using following formula.
% Change in Mean Hardness = Mean (Post RHN Data)− Mean (Pre RHN Data)× 100/Mean Value (Pre RHN Data)
It is observed that the mean reduction in hardness of the acrylic specimens – Samples 21-40 following treatment with Hot water (100°C) was 5.8%. The treatment of samples in Warm water (40°C), samples 1-20, on the other hand, showed a slight increase in hardness, which was 0.67%.
A paired t-test was applied for intra-group statistical analysis. Digital-software - SPSS 20.0 is used for the analysis of the data. The comparison of groups and their statistical significance is shown in Table 3.
Table 3.
Paired t-test.
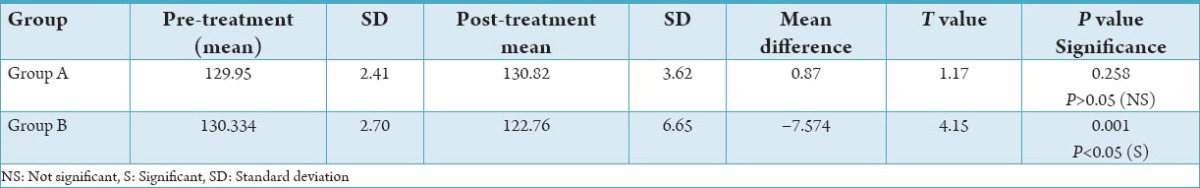
For the experimental Group A, the difference in mean is −7.57 with a t-value of 1.17. However, since the probability is 0.258 (>0.05), the results are not statistically significant.
For the experimental Group B, the difference in mean was 9.045 with a t value of 4.15. The results are extremely statistically significant since the P = 0.001.
Discussion
During clinical use, denture base resin materials in the oral cavity are exposed to thermal changes and saliva, therefore during denture storage they are soaked in water or an aqueous cleansing solution. As a result of this immersion, water or saliva may be absorbed by the denture base resins.14
Acrylic denture bases absorb relatively small amounts of water when placed in an aqueous environment. This water exerts a significant effect on the mechanical and dimensional properties of the polymer. Although the absorption is facilitated by the polarity of polymethyl methacrylate molecules, a diffusion mechanism is primarily responsible for the ingress of water. The introduction of water molecules within the polymerized mass produces two important effects. First, it causes a slight expansion of the polymerized mass. Second, water molecules interfere with the entanglement of polymer chains and thereby act as plasticizers.15,16
This water absorption along with the change in temperature of the environment in which the denture base is may cause degradation of the denture polymers.17
Mechanical properties of the acrylic resin are affected by the heat and water sorption due to the changes in polymer chains which in turn soften the denture.1,8 on the other hand some studies showed a gradual increase in surface hardness of some denture base resins after water immersion.14
This improvement in the hardness property has been attributed in part to leaching of the residual monomer from the resin as the residual monomer may adversely affect the mechanical properties of denture base resins by having a plasticizing effect.17 This rate of diffusion of monomer out of the resin into water decreases progressively.14
A routine denture cleaning regimen which includes the mechanical and chemical cleansing is also a very important part of the denture use and maintenance to remove and prevent reaccumulation of microbial plaque and also to remove mucin, food debris, calculus, and exogenous discoloration.
Alkaline peroxides cleansers which are used in this study when dissolved in water converts into hydrogen peroxide reduces the surface tension, and effervescent oxygen helps in mechanical cleaning which are more effective over a long period of immersion.
According to some studies, prolonged immersion of heat or chemically cured acrylic resin in peroxide cleansers did not affect the surface of the acrylic resins.
According to some studies, prolonged immersion of heat or chemically cured acrylic resin in peroxide cleansers did not affect the surface of the acrylic resins. However, when these alkaline are regularly used may change the color of the denture due their bleaching action.12,18 Conversely there are some other studies which state that the major factor responsible for whitening is a high temperature of water in which the dentures are soaked, irrespective of the presence of the denture cleaning agent.17,19
Previous studies have measured hardness using a variety of shaped indentors (e.g., Rockwell and Vickers hardness measurement systems) and loading conditions subjecting the acrylic samples to thermal cycling at different temperatures.11,14,20 There have also been a lot of studies regarding the effect of different denture cleansers on the mechanical properties of the acrylic surfaces.3,6,12,21-23 In this study it has been observed that when the acrylic samples were repeatedly immersed in hot water at 100°C along with alkaline peroxide denture cleanser tablet for a period of 30 days there was a significant reduction of 5.8% in the surface hardness of acrylic and a slight increase of 0.67% in the surface hardness when immersed in warm water at 40°C. These results are in correlation with the results of similar studies conducted earlier by Devlin and Kaushik11 and Goiato et al.24 Color changes are measured objectively using a spectrophotometer where the standardization cannot be achieved. Visual changes in the color are more easily appreciated than a measurable value.
This study concludes that the hot alkaline peroxide solution caused a water supersaturation of the acrylic surface, resulted in a clinically insignificant surface whitening of acrylic. Peracini et al.19 concluded with similar findings in his study. Study done by Unlü et al.12 found that clinically significant whitening effect of regular use of denture cleansers is seen on auto polymerized acrylic resin and not on the heat polymerized resin denture bases. But studies conducted by Goiato et al.24 and Polyzois et al.25 have found clinically significant changes in the gloss and color of denture base acrylic after long-term immersion in disinfectant.
The resemblance between the whitening of acrylic due to long-term water immersion and crazing is, at present, poorly understood. Solvents such as chloroform readily cause crazing of acrylic but not whitening or opacity of the surface. High residual monomer content does affect the mechanical properties of acrylic by acting as a plasticizer, but there is no evidence that it contributes to whitening of the acrylic surface.11,26,27
Hence, the possible effect of thermal cycling and denture cleansers on the acrylic resins may influence the performance of the acrylic prostheses.1
Conclusion
The study concludes:
Acrylic samples immersed in hot water showed a small but statistically significant decrease of 5.8% in surface hardness.
Acrylic samples immersed in warm water showed a slight but statistically insignificant increase of 0.67% in surface hardness.
Samples immersed in hot water showed clinically insignificant color change when compared to samples immersed in warm water on examination of the photographs.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.de Silva CS, Machado AL, de Chaves CA, Pavarina AC, Vergani CE. Effect of thermal cycling on denture base and autopolymerizing reline resins. J Appl Oral Sci. 2013;21(3):219–24. doi: 10.1590/1679-775720130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagger D, Harrison A, Jagger R, Milward P. The effect of the addition of poly (methyl methacrylate) fibres on some properties of high strength heat-cured acrylic resin denture base material. J Oral Rehabil. 2003;30(3):231–5. doi: 10.1046/j.1365-2842.2003.01011.x. [DOI] [PubMed] [Google Scholar]
- 3.Ural C, Şanal FA. Effect of different denture cleansers on surface roughness of denture base materials. J Clin Dent Res. 2011;35(2):14–20. [Google Scholar]
- 4.Brosky ME, Pesun IJ, Morrison B, Hodges JS, Lai JH, Liljemark W. Clinical evaluation of resilient denture liners. Part 2: Candida count and speciation. J Prosthodont. 2003;12(3):162–7. doi: 10.1016/S1059-941X(03)00041-X. [DOI] [PubMed] [Google Scholar]
- 5.Gornitsky M, Paradis II, Landaverde G, Malo AM, Velly AM. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J Can Dent Assoc. 2002;68(1):39–45. [PubMed] [Google Scholar]
- 6.Felipucci DN, Davi LR, Paranhos HF, Bezzon OL, Silva RF, Pagnano VO. Effect of different cleansers on the surface of removable partial denture. Braz Dent J. 2011;22(5):392–7. doi: 10.1590/s0103-64402011000500008. [DOI] [PubMed] [Google Scholar]
- 7.Cheng YY, Cheung WL, Chow TW. Strain analysis of maxillary complete denture with three-dimensional finite element method. J Prosthet Dent. 2010;103(5):309–18. doi: 10.1016/S0022-3913(10)60064-9. [DOI] [PubMed] [Google Scholar]
- 8.Durkan RK, Özdemir T, Pamir AD, Usanmaz A. Water absorption of two different denture base resins reinforced with dental fiber systems. J Appl Polym Sci. 2010;117(3):1750–3. [Google Scholar]
- 9.Ernst CP, Canbek K, Euler T, Willershausen B. In vivo validation of the historical in vitro thermocycling temperature range for dental materials testing. Clin Oral Investig. 2004;8(3):130–8. doi: 10.1007/s00784-004-0267-2. [DOI] [PubMed] [Google Scholar]
- 10.Barclay CW, Spence D, Laird WR. Intra-oral temperatures during function. J Oral Rehabil. 2005;32(12):886–94. doi: 10.1111/j.1365-2842.2005.01509.x. [DOI] [PubMed] [Google Scholar]
- 11.Devlin H, Kaushik P. The effect of water absorption on acrylic surface properties. J Prosthodont. 2005;14(4):233–8. doi: 10.1111/j.1532-849X.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 12.Jin C, Nikawa H, Makihira S, Hamada T, Furukawa M, Murata H. Changes in surface roughness and colour stability of soft denture lining materials caused by denture cleansers. J Oral Rehabil. 2003;30(2):125–30. doi: 10.1046/j.1365-2842.2003.01014.x. [DOI] [PubMed] [Google Scholar]
- 13.Budtz-Jørgensen E. Materials and methods for cleaning dentures. J Prosthet Dent. 1979;42:619–23. doi: 10.1016/0022-3913(79)90190-2. [DOI] [PubMed] [Google Scholar]
- 14.Neppelenbroek KH, Pavarina AC, Vergani CE, Giampaolo ET. Hardness of heat-polymerized acrylic resins after disinfection and long-term water immersion. J Prosthet Dent. 2005;93(2):171–6. doi: 10.1016/j.prosdent.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Anusavice KJ. Phillips’ Science of Dental Materials. 11th ed. Philadelphia: Saunders Elsevier Science; 2003. [Google Scholar]
- 16.Unemori M, Matsuya Y, Matsuya S, Akashi A, Akamine A. Water absorption of poly (methyl methacrylate)containing 4-methacryloxyethyl trimellitic anhydride. Biomaterials. 2003;24(8):1381–7. doi: 10.1016/s0142-9612(02)00521-5. [DOI] [PubMed] [Google Scholar]
- 17.Hargreaves AS. The effects of cyclic stress on dental polymethylmethacrylate. I. thermal and environmental fluctuation. J Oral Rehabil. 1983;10(1):75–85. doi: 10.1111/j.1365-2842.1983.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 18.Arab J, Newton JP, Lloyd CH. The effect of an elevated level of residual monomer on the whitening of a denture base and its physical properties. J Dent. 1989;17(4):189–94. doi: 10.1016/0300-5712(89)90073-0. [DOI] [PubMed] [Google Scholar]
- 19.Unlü A, Altay OT, Sahmali S. The role of denture cleansers on the whitening of acrylic resins. Int J Prosthodont. 1996;9(3):266–70. [PubMed] [Google Scholar]
- 20.Peracini A, Davi LR, de Queiroz Ribeiro N, de Souza RF, Lovato da Silva CH, de Freitas Oliveira Paranhos H. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. J Prosthodont Res. 2010;54(2):78–83. doi: 10.1016/j.jpor.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Haywood J, Wood DJ, Gilchrist A, Basker RM, Watson CJ. A comparison of three hard chairside denture reline materials. Part II. Changes in colour and hardness following immersion in three commonly used denture cleansers. Eur J Prosthodont Restor Dent. 2003;11(4):165–9. [PubMed] [Google Scholar]
- 22.Yuan SP, Lin H, Pan S, Lou LL, Xu YX. Effect of polident denture cleansers on the properties of heat-polymerized denture base acrylic resin. Beijing Da Xue Xue Bao. 2012;44:946–9. [PubMed] [Google Scholar]
- 23.Huddar DA, Hombesh MN, Sandhyarani B, Chandu GS, Nanjannawar GS, Shetty R. Effect of denture cleanser on weight, surface roughness and tensile bond strength of two resilient denture liners. J Contemp Dent Pract. 2012;13(5):607–11. [PubMed] [Google Scholar]
- 24.Goiato MC, Dos Santos DM, Baptista GT, Moreno A, Andreotti AM, Bannwart LC, et al. Effect of thermal cycling and disinfection on colour stability of denture base acrylic resin. Gerodontology. 2013;30(4):276–82. doi: 10.1111/j.1741-2358.2012.00676.x. [DOI] [PubMed] [Google Scholar]
- 25.Polyzois G, Niarchou A, Ntala P, Pantopoulos A, Frangou M. The effect of immersion cleansers on gloss, colour and sorption of acetal denture base material. Gerodontology. 2013;30(2):150–6. doi: 10.1111/j.1741-2358.2012.00657.x. [DOI] [PubMed] [Google Scholar]
- 26.Pavarina AC, Vergani CE, Machado AL, Giampaolo ET, Teraoka MT. The effect of disinfectant solutions on the hardness of acrylic resin denture teeth. J Oral Rehabil. 2003;30(7):749–52. doi: 10.1046/j.1365-2842.2003.01145.x. [DOI] [PubMed] [Google Scholar]
- 27.Consani RL, Pucciarelli MG, Mesquita MF, Nogueira MC, Barão Valentim AR. Polymerization cycles on hardness and surface gloss of denture bases. Int J Contemp Dent Med Rev. 2014;2014 Doi: 10.15713/ins.ijcdmr.8. [Google Scholar]


