Abstract
Immune reconstitution inflammatory syndrome (IRIS) is an “unmasking” or paradoxical worsening of a pre-existing infection after commencement of highly active antiretroviral therapy (HAART) in human immunodeficiency virus (HIV) - infected patients. The use of HAART in the management of HIV patients restores immune responses against pathogens however in few patients, the reconstituted immune system leads to IRIS. As the treatment protocols are not standardized for IRIS, this leads to short-term morbidity or in some cases also mortality. Therefore, treatment in these patients is a huge challenge and further more research regarding the immunopathogenesis, diagnosis and management of IRIS should be well thought-out. To understand the immunopathogenesis of IRIS it will be difficult to elucidate the intrinsic dynamics of immune cells after initiation of HAART but, there are few biomarkers which help to predict or diagnose IRIS and develop specific treatment, following initiation of HIV therapy. This review is an attempt to put light on those patients with IRIS with treatment guidelines for the management of the progression of it.
Keywords: CD4 cell count, highly active antiretroviral therapy, human immunodeficiency virus, immune reconstitution inflammatory syndrome, management
Introduction
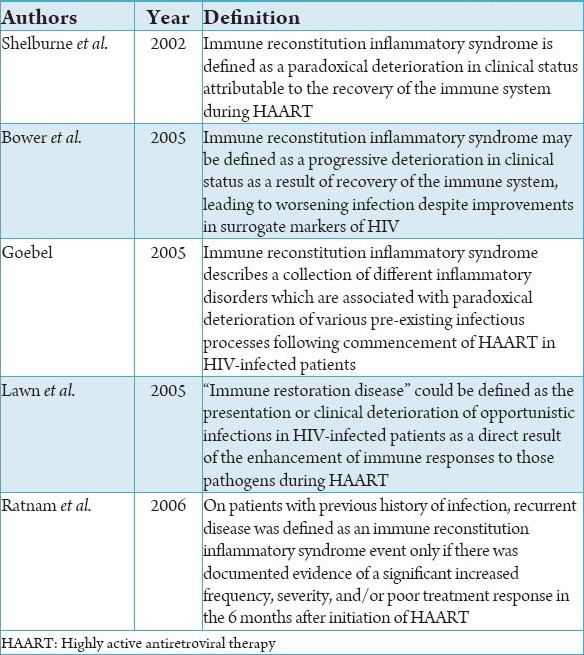
Human immunodeficiency virus (HIV) infection is a global pandemic with cases reported virtually from every country. World Health Organization (WHO) has announced it as a global emergency. HIV infection is characterized by a gradual reduction in the counts of CD4+ lymphocytes, to the point of complete depletion. This reduction in turn leads to opportunistic infections (OI) and specific neoplastic processes.1 The consequence of highly active antiretroviral therapy (HAART) initiation on HIV-infected patients is the decrease in viral load, improvement in CD4+ T cell counts and in the immune system, which reduces the OI and prolonged survival. However, after antiretroviral therapy (ART) few patients experience a clinical deterioration due to dysbalanced restoration of the CD4 T cell of the immune system. This was first noted in early 1990s following the introduction of zidovudine monotherapy, when Mycobacterium avium-intracellulare infection were observed in association with the recovery, rather than failure of immune responses. “Immune reconstitution” or immune reconstitution inflammatory syndrome (IRIS) or “restoration disease immune reconstitution syndrome” is manifested as a sequel to clinical deterioration in patients on ART due pre-existing subclinical infections.2 Definition according to various authors are listed in Table 1.3 As the mechanism and management of IRIS is not clearly understood, currently numerous research projects have been taken up but are hampered by lack of a consistent definition of this syndrome. Diagnosis is difficult because of a diverse range of clinical presentations. Despite the presence of elements warranting the inclusion of oral lesions as part of IRIS, and the presence of concrete pathogens prior to immune reconstitution, the behavior of opportunistic oral infections in subjects with immune reconstitution has not been investigated to date. Oral manifestations of IRIS remain elusive. Before the start of HAART, the patients should be screened for OI.4 Risk factors associated with patients developing IRIS are: (1) Initiation of ART after an OI, (2) decreased baseline CD4+ cell count, (3) viral load response to ART, (4) increased antigen burden of an OI.5 By knowing the pathogenesis of IRIS researcher can develop biomarkers which predict IRIS in HIV patients on HAART.6 Biomarkers of inflammation (C-reactive protein, interleukin-6), coagulation (D-dimer), and tissue fibrosis (hyaluronic acid) can be used as markers of IRIS. Early screening of patients is needed to rule out any OI before the start of HAART.4 IRIS treatment in IRIS patients is a huge challenge and further more research are required for diagnosis and management of it.
Table 1.
Definitions of immune reconstitution inflammatory syndrome.3
Prevention of Complications of IRIS7,8
Recommendations
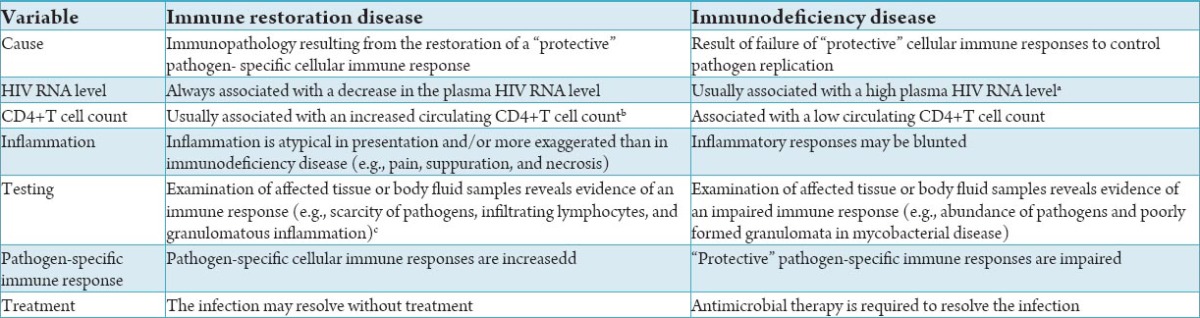
Favorable viral suppression and immune reconstitution have been observed in patients IRIS after 24 months of HAART.9 Difference between IRIS and immune deficiency disease listed in Table 2.9 The long-term outcome of these patients appears to be comparatively better than that of patients without syndrome. The main role of the clinician is thus to provide reassurance to patients. Nevertheless, patients who develop IRIS often require significant interventions to minimize short-term morbidity. There are to date no controlled prospective studies from which to formulate treatment guidelines for IRIS. The prevention of IRIS depends largely on optimal screening for OIs before commencing HAART in order to prevent unmasking and to optimally time HAART in patients who are receiving treatment for an OI. It is particularly important for clinicians to include IRIS in the differential diagnosis of a patient who presents with an inflammatory process after initiation of HAART.
Table 2.
Differing characteristics of immune restoration disease and immunodeficiency disease in patients with HIV infection.9
There are different ways to prevent the development of IRIS. Since a low CD4+ T cell count is a risk factor for the development, some authors have suggested that Starting HAART early before the CD4+ cell count drops to below 100 cells/μL.10 Others suggested that delaying HAART for 4-8 weeks until the co-existing infection resolves.11 Given that IRIS occurs more frequently if HAART is initiated early when antigens are abundant, or if there is more advanced immunosuppression delaying HAART until the antigen load is reduced by antimicrobials will, in theory, reduce the risk of IRIS. Nevertheless, patients will be at risk of other AIDS events if HAART is delayed.12 Starting HAART immediately and using prophylaxis against any suspected asymptomatic infection could be another option.13 When IRIS is diagnosed, treatment options depend on the potential hazards and the extent of discomfort for the patient. If IRIS may cause severe irreversible damage, such as liver failure or cytomegalovirus (CMV) retinitis, HAART may have to be stopped until the IRIS infection is checked. In many cases, IRIS is self-limiting and only symptomatic treatment is needed, besides treating the IRIS infection. HAART can be continued if the scenario is not life-threatening.
Treatment should aim at targeting the infectious agents and monitoring for complications secondary to the inflammatory process. Empirical treatment of symptomatic IRIS using non-steroidal anti-inflammatory drugs has been reported in a number of case reports.14 If a corticosteroid is used, the risk-benefit should be carefully assessed, particularly for patients with mycobacterial disease. Affected patients are capable of generating an inflammatory response, so many of them ultimately discontinue secondary prophylaxis against the offending pathogen.15
Complications of IRIS can be prevented by careful monitoring the patients with low CD4+ cell count and a thorough history of co-infections. Clinicians should be aware of the fact that in spite of the initial restoration of CD4+ counts the patient has high chances to develop IRIS in a course of time. Patients with low CD4+ counts at the start of therapy have suspicion for ophthalmologic manifestations of IRIS, which could further result in CMV retinitis after initiation of ART. Thereby the patient is advised to receive a dilated ophthalmologic examination every 3 months.16 Transaminase levels should be monitored prior to initiation and monthly monitoring for first 3 months after ART is mandatory for hepatitis B or C patients. Any altered bilirubin levels, loss of synthetic functions or elevated transaminase levels in these jaundice patients (e.g. - Elevated prothrombin time/international normalized ratio or decreased albumin) should be evaluated the help of hepatologist.
Management and Treatment Recommendations
Symptomatic treatment and supportive care should be considered for patients with IRIS by the practitioners. Prednisone 1-2 mg/kg or equivalent for 1-2 weeks should be considered in severe cases by the clinicians. Patients on corticosteroids should be carefully monitored by the clinicians. There are chances of developing OI including CMV, retinitis and tuberculosis (TB) diseases. IRIS reduces over time in most of the patients and if not severe, symptomatic treatment and supportive care is required.
-
Management and Treatment of Mild IRIS17
Clinicians and patients should be aware that mild IRIS presentations are an initiation of immune reconstitution and not the progression of HIV disease. These mild cases can be treated with the standard protocols. In addition to standard therapy for the offending OI, the following treatments may increase inflammation in patients with mild IRIS
-
Management and Treatment of Severe IRIS
In severe IRIS patients a threat to patient’s functional status or permanent disability is examples of this are a vision loss from CMV or decrease in pulmonary capacity from TB or M. avium complex (MAC), neurologic complications from corticosteroid therapy which is the most commonly used line of treatment in severe IRIS patients. A study done on patients with severe TB-IRIS treated with 10-80 mg of prednisone daily showed improved signs in all patients within 3 days.18 Another study was performed on patients with MAC - IRIS, few patients responded to prednisone.19 Although no trials on the base dose of recommended corticosteroids levels have been conducted, but according to some experts, recommended 1-2 mg/kg prednisone, or the equivalent, for 1-2 weeks and then taper the dose. Risks of corticosteroid therapy should be adjusted against the severity of the IRIS manifestations and the potential benefits, particularly in HIV infected patients with the high prevalence of hypertension, diabetes mellitus, and mental health disorders. Risks of corticosteroid therapy are associated with following factors:
Hypertension
Hyperglycemia
Pre worsening of an existing infection
Disposition to a new infection
Mental status changes.
In severe cases, ART should not be interrupted in IRIS patients. Despite the risks that develop on stoppage of combination ART, reports suggest that there is recurrence of IRIS on restarting the therapy. Treatment of CMV vitritis with intraocular steroids has been suggested but has not been useful in uveitis.
Prognosis
Majority of patients with IRIS is a self-limiting disease. Mortality associated with IRIS is relatively uncommon; however, associated high morbidity plays considerable burden on the healthcare system.9 Morbidity and mortality rates vary according to the pathogen and organs involved. IRIS in the setting of OI involving the central nervous system (CNS) has a high mortality rates. The heightened immune response in a relatively closed space leads to raised intracranial pressures, with potentially irreversible damage leading to increased morbidity and mortality.
High mortality rates are reported for cryptococcal meningitis.20 Overall mortality rate of TB-IRIS is low; however, significant morbidity and mortality may be seen with ARDS and CNS involvement in TB-IRIS.
20 patients with long-term control of HIV replication may rarely have persistent immune defects that are complicated by OI
The circulating CD4+ T cell count is not increased in some patients with immune restoration disease (e.g. in 10% of patients with MAC, immune restoration disease do not have an increased CD4+ T cell count)
Viable pathogens may be isolated from tissue exudate, or body fluid samples in “unmasking” immune restoration disease
This has been demonstrated directly for only a small number of pathogens but has been demonstrated indirectly by clinicopathological and serological studies for many others.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Gaitan Cepeda LA, Ceballos Salobreña A, López Ortega K, Arzate Mora N, Jiménez Soriano Y. Oral lesions and immune reconstitution syndrome in HIV+/AIDS patients receiving highly active antiretroviral therapy. Epidemiological evidence. Med Oral Patol Oral Cir Bucal. 2008;13(2):E85–93. [PubMed] [Google Scholar]
- 2.Surendra K, Soneja S, Soneja M. HIV & immune reconstitution inflammatory syndrome (IRIS) Indian J Med Res. 2011;134:866–77. doi: 10.4103/0971-5916.92632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang CS, Samaranayake LP. Immune reconstitution inflammatory syndrome after highly active antiretroviral therapy: a review. Oral Dis. 2010;16(3):248–56. doi: 10.1111/j.1601-0825.2009.01628.x. [DOI] [PubMed] [Google Scholar]
- 4.Dagnachew M, et al. Immunopathogenesis, treatment and prevention of immune reconstitution inflammatory syndrome (IRIS) Int J Med Med Sci. 2012;2(11):211–7. [Google Scholar]
- 5.Ortega KL, Ceballos-Salobreña A, Gaitán-Cepeda LA, Magalhães MG. Oral manifestations after immune reconstitution in HIV patients on HAART. Int J STD AIDS. 2008;19(5):305–8. doi: 10.1258/ijsa.2007.007261. [DOI] [PubMed] [Google Scholar]
- 6.Bonham S, Meya DB, Bohjanen PR, Boulware DR. Biomarkers of HIV Immune Reconstitution Inflammatory Syndrome. Biomark Med. 2008;2(4):349–61. doi: 10.2217/17520363.2.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng CF, Ma LL, Jones GJ, Gill MJ, Krensky AM, Kubes P, et al. Cytotoxic CD4 T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109(5):2049–57. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 8.Acosta RD, Mays BC, Wong RK. Electronic clinical challenges and images in GI. CMV colitis with immune reconstitution syndrome. Gastroenterology. 2008;134(2):e1–2. doi: 10.1053/j.gastro.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 9.French MA, Lenzo N, John M, Mallal SA, McKinnon EJ, James IR, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1(2):107–15. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 10.Jevtovic DJ, Salemovic D, Ranin J, Pesic I, Zerjav S, Djurkovic-Djakovic O. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2005;6(2):140–3. doi: 10.1111/j.1468-1293.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 11.Lortholary O, Fontanet A, Mémain N, Martin A, Sitbon K, Dromer F, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19(10):1043–9. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 12.Puthanakit T, Aurpibul L, Oberdorfer P, Akarathum N, Kanjananit S, Wannarit P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44(4):599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French AM. Immune reconstitution inflammatory syndrome: A reappraisal clinical. Infect Dis. 2009;48:101–7. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SK, Dhooria S, Barwad P, Kadhiravan T, Ranjan S, Miglani S, et al. A study of TB-associated immune reconstitution inflammatory syndrome using the consensus case-definition. Indian J Med Res. 2010;131:804–8. [PubMed] [Google Scholar]
- 15.King MD, Reznik DA, O’Daniels CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34(5):641–8. doi: 10.1086/338637. [DOI] [PubMed] [Google Scholar]
- 16.Shankar EM, Vignesh R, Velu V, Murugavel KG, Sekar R, Balakrishnan P, et al. Does CD4+CD25+foxp3+cell (Treg) and IL-10 profile determine susceptibility to immune reconstitution inflammatory syndrome (IRIS) in HIV disease? J Inflamm (Lond) 2008;5:2. doi: 10.1186/1476-9255-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips P, Bonner S, Gataric N, Bai T, Wilcox P, Hogg R, et al. Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin Infect Dis. 2005;41(10):1483–97. doi: 10.1086/497269. [DOI] [PubMed] [Google Scholar]
- 18.Bell C, Nelson M, Kaye S. A case of immune reconstitution rheumatoid arthritis. Int J STD AIDS. 2002;13(8):580–1. doi: 10.1258/095646202760159747. [DOI] [PubMed] [Google Scholar]
- 19.Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, et al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103(10):1391–8. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158(1):157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]


