Abstract
Background
This study examined outcomes of a technique for performing thoracoscopic left upper lobectomy (LUL) in patients with a previous left internal mammary artery (LIMA) coronary artery bypass graft, where a small wedge of lung parenchyma adjacent to the graft is left to avoid injury.
Methods
All patients undergoing thoracoscopic LUL from 1999-2010 at a single institution were reviewed. Perioperative morbidity, cancer recurrence, and long-term survival were compared between patients who had (LIMA group) or did not have (Control group) a previous LIMA graft.
Results
During the study period, 290 patients underwent thoracoscopic LUL; 14 (5%) had previous LIMA grafts. There was no perioperative mortality in the LIMA group versus 4 (1%) in the Control group (p=0.65). One patient (7%) in the LIMA group required conversion to thoracotomy, which was similar to the control group (n=16, 6%; p=0.83). Overall perioperative morbidity was also not different between the groups (LIMA 36% [5/14] versus Control 29% [81/276], p=0.61). No patient in the LIMA group had perioperative cardiac ischemia. For patients with lung cancer, 5-year survival (LIMA 50% versus Control 63%, p=0.23) and cancer recurrence rates (LIMA 27% (3/14) versus Control 15% (36/276), p=0.27) were not different between the groups. Only 1 LIMA recurrence was local, and it was not related to the parenchyma left on the LIMA graft.
Conclusions
Thoracoscopic LUL can be performed safely in patients with LIMA bypass grafts. Leaving lung parenchyma on the graft may prevent injury and does not compromise oncologic outcomes in appropriately selected patients.
Keywords: Lung Cancer Surgery, Outcomes, Lobectomy, Coronary Artery Bypass Grafts, CABG
Introduction
Anatomic pulmonary resection with lobectomy is the preferred surgical treatment for most patients with resectable early stage non-small cell lung cancer (NSCLC) [1]. Video-assisted thoracoscopic lobectomy is increasingly used to treat these patients, as it is associated with less morbidity compared to thoracotomy [2-5]. The safety of thoracoscopic lobectomy has led to its application to more complex patient populations, such as the elderly [6-8] and those undergoing sleeve resections [9].
One clinical situation that can increase the complexity of a thoracoscopic lobectomy is when patients who require a left upper lobectomy (LUL) have also had previous coronary artery bypass grafting (CABG) using the left internal mammary artery (LIMA). These patients typically have adhesions between the lung and the LIMA graft, chest wall, and mediastinum. Surgical dissection in this area must be done with extreme care to avoid LIMA manipulation or injury that could lead to disastrous myocardial ischemic events. Unfortunately, this scenario is not that uncommon, considering that both single-institution and multi-institution studies have reported the incidence of coronary artery disease (CAD) in the population of patients who undergo major pulmonary resection to be 16 to 27.5% [7, 10-12]. However, few studies have examined outcomes after thoracoscopic left upper lobectomy in this situation [13].
When performing a thoracoscopic left upper lobectomy in patients who have had a previous CABG with a LIMA graft, we utilize a strategy in which the left upper lobe is fully mobilized from the chest wall and mediastinum in all areas except in the location of the LIMA graft. To avoid inadvertent LIMA manipulation or injury, the part of the lung that is adherent to the mediastinum in this area is separated from the rest of the lung by using mechanical staplers to leave a small wedge of lung parenchyma on the LIMA graft. The purpose of this study was to examine outcomes after use of this strategy to ensure that leaving devascularized lung tissue in this fashion did not increase the chances of having a postoperative complication or a local recurrence of cancer. We specifically tested the hypothesis that utilizing this technique results in similar morbidity, mortality, and cancer recurrence as compared to standard thoracoscopic LUL in patients without previous LIMA grafts.
Patients and Methods
After local Institutional Review Board approval was granted, including waiver of the need for patient consent, the Duke University Medical Center Data Center was queried to identify patients who underwent thoracoscopic LUL from 1999-2010. Data of patients undergoing lung resection at our institution are prospectively collected as part of a quality control process, with complications recorded by clinical providers based on the definitions of postoperative events according to the Society of Thoracic Surgeons General Thoracic Surgery Database. Retrospective review of these patients documented demographics, preoperative characteristics and comorbidities, the histology and stage of disease, intraoperative details, and postoperative course. All operative and pathology reports were reviewed in detail to confirm stage (7th edition of the AJCC Cancer Staging Manual) and operative resection. Additional review of medical records was utilized as necessary to ensure complete data collection and that all postoperative events were captured.
Operative deaths were defined as deaths that occurred within 30 days after operation or those that occurred later but during the same hospitalization. Deaths were captured both by chart review and use of the Social Security Death Index Database. Overall morbidity was defined as the occurrence of at least one postoperative event.
Postoperative surveillance was surgeon-dependent but typically involved a computed tomography (CT) scan of the chest every six to twelve months for at least five years following resection. Patterns of failure were assessed by means of follow-up imaging studies and information obtained from procedures such as CT–guided transthoracic biopsies, bronchoscopy, and endobronchial ultrasound. Locoregional recurrence was defined as recurrence of disease at the surgical resection margin or in lymph nodes in the ipsilateral hilum or in the mediastinum. All other sites of failure, including the supraclavicular fossa and contralateral hilum, were considered distant recurrences.
Patient selection and operative technique for thoracoscopic lobectomy have been previously described [14]. When performing a thoracoscopic left upper lobectomy in patients who have had a previous CABG with a LIMA grant, the left upper lobe was fully mobilized from the chest wall and mediastinum in all areas except in the location of the LIMA graft using dissection done with electrocautery and scissors. To avoid inadvertent LIMA manipulation or injury, the part of the lung adherent to the mediastinum in this area was separated from the rest of the lung by using mechanical staplers (typically a Covidien [Dublin, Ireland] EndoGIA purple load) to leave a small wedge of lung parenchyma (1-2cm) on the LIMA graft. The stapler is fired from the 5th intercostal space anterior access incision, while the camera remains in the 8th intercostal space port. Topic thrombotic agents are very infrequently used after pulmonary resection at our institution. Due to an at least theoretical possibility of harvesting malignant cells that could be subsequently recirculated, cell saver is never used in cancer operations at our institution. This technique of LUL resection was only utilized if the operating surgeon judged preoperatively based on the tumor location in relation to the LIMA graft that residual disease would not be left behind in the wedge. Postoperative care, including 24 hours of perioperative prophylactic antibiotics, is the same as for all thoracoscopic lobectomy patients [7].
Patients who had undergone thoracoscopic LUL were divided into two groups: those with no history of CABG using the LIMA (Control Group) and those who had previously undergone CABG using the LIMA (LIMA Group). Comparisons of patient characteristics between the two groups were performed using a chi-square test for categorical (frequency, percentages) and two-sample, unpaired t-test for continuous variables (mean, standard deviations). Overall morbidity and cardiac specific morbidity were compared between LIMA and Control groups using chi-squared tests. Overall survival analyses were performed according to the Kaplan-Meier method, and included all deaths from any cause in the follow-up period, with patients still alive censored at the last available follow-up. Median follow-up was calculated from the date of lobectomy to death or most recent follow-up at Duke University Medical Center. Survival of patients who had resection for lung cancer was compared between the two groups using the log-rank test.
Patient and tumor characteristics are presented as raw number (percentage) for categorical data, mean ± standard deviation for continuous data that are normally distributed, and median (interquartile range) for non-normally distributed variables. Statistical analyses were performed using STATA/SE version 11.2 (Stata Corporation, College Station, TX) and JMP Version Pro 9.0.0 (SAS Institute Inc., Cary, NC). A two-sided probability value of ≤0.05 was used for all comparisons.
Results
There were 290 patients who underwent thoracoscopic LUL during the study interval, 14 (5%) of whom had previous LIMA grafts. Demographic, baseline characteristics, and comorbid conditions of the patients who had and who did not have LIMA grafts are shown in Table 1. Patients in the LIMA and Control group were mostly similar in regards to age, gender, tobacco use history, diabetes, induction chemotherapy, induction radiation therapy, pulmonary function, and tumor pathology. Considering that by definition all patients in the LIMA group had a cardiac history, the LIMA group expectedly had more congestive heart failure (36% vs. 3%, p<0.001) and coronary artery disease (100% vs. 16%, p<0.001). Preoperative cardiac testing in LIMA patients was at the discretion of the surgeon and included cardiac catheterization (n=3), transthoracic echocardiography (n=3), treadmill stress test (n=2), and stress echocardiography (n=2). One LIMA patient had an occlusion of the LIMA graft at its insertion in the left anterior descending coronary artery noted preoperatively.
Table 1.
Patient Characteristics
| Variable | Control Group (N=276) |
LIMA Group (N=14) |
P |
|---|---|---|---|
| Patient age (years) | 65.3 ± 10.5 | 68.1 ± 7.0 | 0.33 |
| Gender (female) | 134 (48.6) | 4 (28.6) | 0.14 |
| History of tobacco use | 168 (60.9) | 13 (92.9) | 0.50 |
| Diabetes | 44 (15.9) | 4 (28.6) | 0.23 |
| Congestive heart failure | 8 (2.9) | 5 (35.7) | <0.001 |
| Coronary artery disease | 43 (15.6) | 14 (100.0) | <0.001 |
| Neoadjuvant chemotherapy | 18 (6.5) | 1 (7.1) | 0.95 |
| Neoadjuvant radiation therapy | 11 (4.0) | 0 | 0.44 |
| DLCO (% predicted) | 77.9 ± 20.4 | 73.5 ± 18.5 | 0.52 |
| FEV1 (% predicted) | 73.7 ± 20.9 | 71.3 ± 16.1 | 0.52 |
Values are presented as raw number [percent] or mean ± standard deviation. DLCO-diffusion capacity of the lung for carbon monoxide; FEV1-forced expiratory volume in 1 second; NSCLC-non-small cell lung cancer.
The pathologic details of the lesions resected via LUL are shown in Table 2. The two groups had a similar distribution of NSCLC by stage, as well as benign and malignant lesions. However, the LIMA group had smaller tumors (1.9 ± 1.3cm vs. 3.0 ± 2.0cm, p=0.02).
Table 2.
Pathologic Details
| Variable | Control Group (N=276) |
LIMA Group (N=14) |
P |
|---|---|---|---|
| Lung cancer size (cm) | 3.0 ± 2.0 | 1.9 ± 1.3 | 0.02 |
|
| |||
| Pathology | |||
| Stage 1 NSCLC | 160 (58.0) | 10 (71.4) | 0.46 |
| Stage 2 NSCLC | 60 (21.7) | 1 (7.1) | |
| Stage 3 NSCLC | 20 (7.3) | 0 | |
| Stage 4 NSCLC | 2 (0.7) | 0 | |
| Benign | 19 (6.9) | 1 (7.1) | |
| Metastasis | 15 (5.4) | 2 (14.3) | |
Values are presented as raw number [percent] or mean ± standard deviation.
Perioperative and complication details are listed in Table 3. There was no perioperative mortality in the LIMA group versus 4 (1%) in the control group; this difference was not statistically significant (p=0.65). There was also no statistically significant difference in the postoperative length of stay or the rate of conversion to thoracotomy between the two groups (1 conversion of 14 patients in the LIMA group [7%] vs. 16 conversions in 276 patients in the Control group [6%], p=0.83). The rate of complications overall was similar between the two groups: 5 in the LIMA group (36%) vs. 81 in the Control group (29%; p=0.61; Table 3). Cardiac complications occurred in 5 (36%) in the LIMA group versus 47 (17%) in the control group, although this difference did not reach statistical significance (p=0.08). No patient in the LIMA group had any measured evidence of perioperative cardiac ischemia. The rate of takeback for bleeding and the need for blood transfusion were higher in the LIMA group (Table 3).
Table 3.
Postoperative Complications
| Variable | Control Group (N=276) |
LIMA Group (N=14) |
P |
|---|---|---|---|
| Perioperative mortality | 4 (1.5) | 0 | 0.65 |
| Length of hospital stay (median, IQR) | 4 (3,5) | 4 (4,5) | 0.29 |
| Conversion from thoracoscopy to thoracotomy |
16 (5.8) | 1 (7.1) | 0.83 |
| Overall morbidity | 81 (29.4) | 5 (35.7) | 0.61 |
| Cardiac complications overall | 47 (17.0) | 5 (35.7) | 0.08 |
| Myocardial infarction | 2 (0.7) | 0 | 0.75 |
| Atrial fibrillation | 45 (16.3) | 5 (35.7) | 0.06 |
| Air leak > 5 days | 29 (10.5) | 2 (14.3) | 0.66 |
| Need for post-op bronchoscopy | 13 (4.7) | 0 | 0.41 |
| Need for transfusion | 9 (3.3) | 2 (14.3) | 0.04 |
| Pneumonia | 6 (2.2) | 0 | 0.58 |
| Empyema | 2 (0.7) | 2 (14.3) | <0.001 |
| Takeback for bleeding | 1 (0.4) | 1 (7.1) | 0.003 |
Values are presented as raw number [percent]. IQR-interquartile range
The rate of empyema was higher in the LIMA group (2 [14%] vs. 2 [0.7%], p<0.001). The first LIMA empyema patient was an 80-year-old female with hemoptysis. Her FEV1 was 98% predicted and her DLCO was 70% predicted. She was a former smoker with a history of diabetes, renal insufficiency, and congestive heart failure. Her left upper lobectomy required a complete pneumolysis, as she had fusion of all pleural surfaces. Operative time was 3 hours and 20 minutes. Postoperatively, she required a blood transfusion and also developed atrial fibrillation. She developed a methicillin resistant staphylococcus aureus (MRSA) empyema and required thoracoscopic decortication 17 days after lobectomy. The second LIMA empyema patient was a 69-year-old male with a T2aN0 squamous cell lung cancer. His preoperative FEV1 was 54% predicted and his DLCO was 38% predicted. He was a previous smoker with congestive heart failure. He had complete fusion of his pleural surfaces and a thick cortex around his left lung requiring extensive pneumolysis. Operative time was 8 hours and 51 minutes. Postoperative complications included a prolonged air leak, need for blood transfusion, and atrial fibrillation. He required a decortication 1 month after lobectomy for a MRSA empyema.
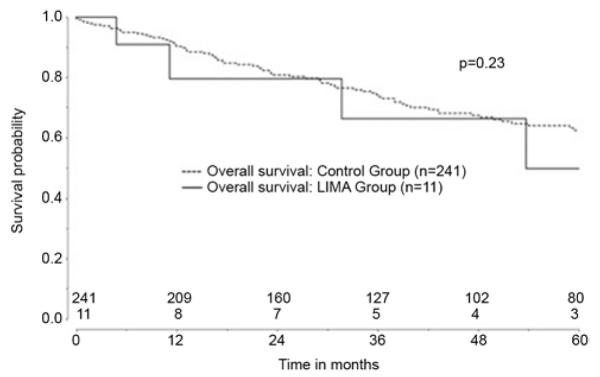
Median follow-up was 38 months (IQR 17-68). For patients with lung cancer, overall actuarial five-year survival was not different: 50% in the LIMA group vs. 63% in the Control group (p=0.23; Figure 1). In regards to overall lung cancer recurrence in the two groups, there was also no difference: three in the LIMA group (27%) vs. 36 among Controls (15%; p=0.27). In the subset of patients with stage I lung cancer, the rate of cancer recurrence was LIMA 3/10 (30%) vs. Control 16/160 (10%), p=0.051. However, only 1 of the recurrences in the LIMA group was local. This LIMA patient had undergone a lingular-sparing left upper lobectomy for a 1cm pathologic stage IA moderately differentiated adenocarcinoma. He had annual surveillance with CT and developed a left hilar mass 6.5 years after his initial resection. The patient also had bilateral mediastinal lymphadenopathy and bilateral pulmonary nodules. Biopsy of the hilar mass and a paratracheal lymph node demonstrated poorly differentiated adenocarcinoma. Retrospective imaging review showed that the new disease was not adjacent to the LIMA graft and was likely not related to any parenchyma left along the LIMA graft. The patient was treated with chemotherapy and died approximately eight years after his initial resection.
Figure 1.
Overall survival in Control (dashed line) versus LIMA group (solid line).
Of the other two recurrences in the LIMA group, one was locoregional (level 5 mediastinal lymph node) and one was distant (right leg). Of the recurrences in the control group, 16 were locoregional, 18 were distant, 1 was both, and 1 was unknown.
Comment
This study shows that thoracoscopic LUL can be performed safely in patients after CABG with LIMA bypass grafts. Leaving lung parenchyma on the graft helps to prevent LIMA injury and cardiac ischemic events. This technique results in similar rates of cancer recurrence and long-term survival compared to standard thoracoscopic lobectomy.
The management of patients requiring LUL who have histories of LIMA grafts after CABG is not well-described in the literature. Singhatanadgige and colleagues published a case report describing the procedure in 2006 [15]. Funaki and colleagues published a report of 2 patients with histories of internal mammary bypass grafts undergoing thoracoscopic lobectomy [13]. One patient had a previous LIMA-to-left anterior descending coronary artery graft and underwent LUL. The other had a previous right internal thoracic artery-left anterior descending coronary artery graft and underwent a right upper lobectomy. A rim of parenchyma was left around the graft in both cases. There were no postoperative complications, and at 5-year follow-up neither patient had local recurrence.
Catastrophic complications are rare after thoracoscopic lobectomy [16]. As more surgeons have become familiar with thoracoscopic techniques, these procedures are being used in more complex situations [17]. Given the proven decreased morbidity of thoracoscopic lobectomy, including decreased pain and shorter hospital stay [4,5,18], thoracoscopy as opposed to thoracotomy is the approach of choice at our institution if considered technically feasible. However, injury to a patent LIMA bypass graft during lobectomy could result in cardiac ischemia and significant potential morbidity, including immediate or delayed hemodynamic instability. A known major graft injury such as inadvertent transection could lead to significant hypotension or arrhythmias and would likely require immediate repair, which would certainly increase the complexity and scope of the operation. Even an injury considered minor or an injury that occurs due to graft manipulation that is not appreciated at the time of surgery could lead to immediate or delayed cardiac issues, potentially due to thrombosis at the site of injury or distal emboli. Although our sample size of 14 is small, we feel that having no major cardiac ischemic events demonstrates that this technique of minimizing LIMA graft manipulation during the performance of a thoracoscopic LUL is safe.
Given the higher-risk nature of this procedure, preoperative planning is critical to avoid complications. At our institution, cardiac and thoracic surgeons share an operating room suite, and thus cardiac surgeons and perfusionists are readily available in case of inadvertent injury to the LIMA graft. Thoracic surgeons who do not have these resources immediately available should carefully consider whether they have safe back-up options available before proceeding with LUL in this setting, whether performed thoracoscopically or via thoracotomy. Additionally, we recommend obtaining the operative report of the prior CABG procedure, as well as the actual images of the most recent cardiac catheterization. This will assist in identifying the location of the graft(s), as well assessing the patency of the LIMA. We typically do not add or request any further preoperative testing, aside from the standard testing done for a lung resection. However, a contrasted study with either cardiac MRI or CT angiography should be considered if the tumor is close to the LIMA graft or otherwise central in location to define the tumor’s relationship to both the graft as well as other hilar structures. If there are any other concerning clinical symptoms such as chest pain with exertion, we recommend cardiology evaluation and consideration of coronary angiography.
Leaving a rim of lung parenchyma near the bypass graft provides a measure of safety, in that we do not attempt to dissect the lung off of the graft and risk injury. However, this may result in an increased risk of infection due to leaving devascularized lung tissue along the mediastinum. The higher rate of empyema in the LIMA group in this series may be a result of this, although it is difficult to attribute causation given the small number of patients and high-risk characteristics (poor pulmonary function, diabetes, etc.) of the 2 LIMA patients who developed empyemas. This technique also does have a theoretical risk of worse oncologic outcome, including cancer recurrence, as it is not a complete anatomic resection. The results of this study have shown, however, that the rate of cancer recurrence was similar to patients who underwent LUL without a previous LIMA graft. Only one patient was found to develop disease in the area near the original cancer resection in the LIMA group, and this did not appear to be a result of leaving lung parenchyma along the graft in the mediastinum. It is important to recognize that we did not utilize this technique if a complete resection was not considered likely due to the tumor location in relation to the LIMA and mediastinum. However, it is also important to note that these procedures are also technically challenging even when thoracotomy is used, and enhanced visualization with thoracoscopy may allow more precise and less blunt dissection than what may be necessary in some aspects of the chest during thoracotomy.
Although there was no statistically significant difference in the rate of cardiac complications between LIMA and non-LIMA patients, the study was underpowered for this comparison. There was a trend toward increased cardiac complications in the LIMA group, mostly attributed to an increased rate of atrial fibrillation. This finding is not necessarily unexpected, given the known cardiac comorbidities of patients in the LIMA group. Additionally, the LIMA group had an increased need for blood transfusion and takeback for bleeding. This finding may be related to more blood loss from performing lobectomy in a reoperative field that requires extensive lysis of adhesions or perhaps a lower threshold for blood transfusion in patients with coronary artery disease.
The similar outcomes between the LIMA and non-LIMA patients in this study suggest this technique is safe and does not compromise oncologic efficacy. Although this is a potentially higher-risk procedure than LUL via thoracotomy, we believe providing patients with a minimally invasive approach is still beneficial. Given that numerous studies that have demonstrated advantages of thoracoscopic lobectomy over thoracotomy [2-5], we do not feel that patients should be denied these benefits based on their previous CABG, especially since this study demonstrates that a thoracoscopic LUL can be performed safely in the setting of a prior LIMA graft. Additionally, patients with histories of CABG typically are older and have other comorbidities such as diabetes and extensive smoking histories that can be associated with impaired pulmonary function. In our experience, these patients tend to derive the most benefit from thoracoscopic surgery [7,10,19].
However, the study group is relatively small, although we could not find a larger series in the literature that examined outcomes of thoracoscopic LUL in patients with LIMA grafts. Another limitation is that there may have been patients who had LIMA grafts and were eligible, but not offered, lobectomy. We were not able to capture these patients from our database. The patients in our study were likely carefully selected as appropriate operative candidates, and we advocate very careful pre-operative assessment when considering left upper lobectomy in patients who have had a LIMA graft. This careful assessment is necessary whether considering a minimally invasive approach or a thoracotomy.
In conclusion, thoracoscopic LUL can be performed safely in patients with LIMA bypass grafts. Leaving lung parenchyma on the graft avoids inadvertent injury and cardiac ischemic events.
Acknowledgements
Dr. Berry has received support from the National Institute of Health (NIH) funded Cardiothoracic Surgical Trials Network. We also wish to thank the Duke Tumor Registry for their support in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ettinger DS, Akerly W, Bepler G, et al. National Comprehensive Cancer Network (NCCN). Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 2.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic Lobectomy has Increasing Benefit in Patients with Poor Pulmonary Function: A Society of Thoracic Surgeons Database Analysis. Ann Surg. 2012;256:487–93. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139:976–81. doi: 10.1016/j.jtcvs.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 4.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–78. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–25. doi: 10.1016/j.jtcvs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Berry MF, Onaitis MW, Tong BC, Balderson SS, Harpole DH, D’Amico TA. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg. 2012;41:888–92. doi: 10.1093/ejcts/ezr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88:1093–9. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Port JL, Mirza FM, Lee PC, Paul S, Stiles BM, Altorki NK. Lobectomy in octogenarians with non-small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg. 2011;92:1951–7. doi: 10.1016/j.athoracsur.2011.06.082. [DOI] [PubMed] [Google Scholar]
- 9.Mahtabifard A, Fuller CB, McKenna RJ., Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg. 2008;85:S729–32. doi: 10.1016/j.athoracsur.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary Function Tests Do Not Predict Pulmonary Complications After Thoracoscopic Lobectomy. Ann Thorac Surg. 2010;89:1044–52. doi: 10.1016/j.athoracsur.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro M, Swanson SJ, Wright CD, et al. Predictors of Major Morbidity and Mortality After Pneumonectomy Utilizing The Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg. 2010;90:927–935. doi: 10.1016/j.athoracsur.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Wright CD, Gaissert HA, Grab JD, O’Brien SM, Peterson ED, Allen MS. Predictors of Prolonged Length of Stay after Lobectomy for Lung Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk-Adjustment Model. Ann Thorac Surg. 2008;85:1857–1865. doi: 10.1016/j.athoracsur.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Funaki S, Inoue M, Shigemura N, Okumura M. Thoracoscopic lobectomy for lung cancer after coronary artery bypass grafting using internal thoracic artery. Interact Cardiovasc Thorac Surg. 2012;15:928–9. doi: 10.1093/icvts/ivs299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amico TA. Operative techniques in early-stage lung cancer. J Natl Compr Canc Netw. 2010;8:807–13. doi: 10.6004/jnccn.2010.0057. [DOI] [PubMed] [Google Scholar]
- 15.Singhatanadgige S, Sindhvanada W, Kittayarak C. Left upper lobectomy after CABG with the left internal mammary artery graft. J Med Assoc Thai. 2006;89:887–9. [PubMed] [Google Scholar]
- 16.Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg. 2011;142:1412–7. doi: 10.1016/j.jtcvs.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda N, Saji H, Hagiwara M, Ohira T, Usuda J, Kajiwara N. Recent advances in video-assisted thoracoscopic surgery for lung cancer. Asian J Endosc Surg. 2013;6:9–13. doi: 10.1111/ases.12013. [DOI] [PubMed] [Google Scholar]
- 18.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg. 1999;68:194–200. doi: 10.1016/s0003-4975(99)00467-1. [DOI] [PubMed] [Google Scholar]
- 19.Berry MF, Onaitis MW, Tong BC, Harpole DH, D’Amico TA. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg. 2011;39:989–994. doi: 10.1016/j.ejcts.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]