Abstract
Background and objective
Incidence and predictors of endobronchial tuberculosis (EBTB) remain unknown because of the lack of prospective studies. Our objective was to assess the incidence and predictors of concomitant EBTB in patients with active pulmonary tuberculosis (PTB).
Methods
We prospectively performed routine bronchoscopic examination in all patients with PTB to detect EBTB. Clinical and bronchoscopic findings were analyzed to elucidate predictors of EBTB.
Results
Bronchoscopies of 429 PTB patients were performed at a tertiary referral hospital in Korea. Among those, 233 patients (54.3%) had EBTB. Female gender (odds ratio (OR) 4.35, 95% confidence interval (CI) 1.78–10.63), longer symptom duration (>4 weeks; OR 1.86, 95% CI 1.05–5.46), and no previous history of tuberculosis (OR 4.16, 95% CI 1.22–14.18) were found to be the independent predictors of concomitant EBTB in patients with active PTB. Most of the EBTB/PTB patients had mild stenosis, and more than 20% of them had severe stenosis at the time of diagnosis. Patients with EBTB had follow-up bronchoscopy to evaluate persistent airway stenosis. Persistent bronchostenosis with the lumen narrowed by more than one third occurred in 20.7% (30/145) of patients. The involvement length and decreased forced expiratory volume in 1 s were the risk factors for persistent bronchostenosis.
Conclusions
In patients with active PTB, 50% or more have EBTB. Female gender and longer duration of symptoms are the main predictors of concomitant EBTB. Immediate diagnostic bronchoscopy in patients with active PTB should be considered in selected patients for detection of brocnhostenosis.
Summary at a Glance
We performed a prospective study to elucidate incidence and clinical predictors of EBTB. More than 50% of patients with PTB have EBTB. Female gender and longer duration of symptoms are the main predictors of concomitant EBTB.
Keywords: bronchoscopy, bronchostenosis, endobronchial tuberculosis, incidence, predictor
Introduction
Endobronchial tuberculosis (EBTB) is defined as a tuberculous infection of the tracheobronchial tree and is the main complication of pulmonary tuberculosis (PTB). EBTB pathogenesis is not yet clear, but may involve direct seeding from parenchymal lesions or bronchial invasion of mediastinal tuberculous lymphadenitis.1,2
The reported incidence of EBTB in PTB patients varies greatly (from 6% to 50%).3–8 Most studies of concomitant EBTB in PTB patients were retrospective, and most results from these studies were obtained for small numbers of patients. The exact incidence of EBTB has not been reported.
To diagnose EBTB, bronchoscopy is the most useful method. Generally, bronchoscopy is selectively performed when EBTB is suspected in patients with severe cough, haemoptysis, wheezing or a positive acid-fast bacilli (AFB) smear with normal chest X-ray. Because EBTB is highly infectious, bronchoscopy may be beneficial, as it allows early detection of EBTB in all patients with active PTB. Even though all patients with PTB may need bronchoscopic examination, bronchoscopy is a somewhat invasive method. There are no exact guidelines or indications to conduct bronchoscopy for detecting EBTB in patients with PTB. Clinicians need to know the exact incidence of EBTB and patient selection criteria for bronchoscopy among patients with PTB. Identification of EBTB predictors is therefore needed.
We performed this prospective observational study (i) to assess the incidence of EBTB in patients with active PTB and (ii) to identify predictors of EBTB that would be useful for guiding the selection of patients who need bronchoscopy for EBTB detection.
Methods
Study population
From 2003 to 2005, we prospectively examined by bronchoscopy all patients with newly diagnosed active PTB in a tertiary referral hospital in Daejeon, Korea. We also included patients with relapsed PTB who had been successfully treated more than 1 year ago. Active PTB was defined by a positive AFB smear or positive culture of Mycobacterium tuberculosis. The Ziehl-Neelsen stain for sputum smear and both solid and liquid medium for mycobacterial culture were used. Mycobacterial nuclear amplification test (polymerase chain reaction, PCR) was done on some patients if tuberculosis was suspected clinically. Patients with both smear- and culture-negative tuberculosis, drug-resistant PTB, younger than 18 years old, with a positive human immunodeficiency virus test, with malignancy and predicted survival of less than 6 months, with laryngeal tuberculosis or with contraindications to bronchoscopy (such as severe hypoxemia, respiratory failure, recent cardiovascular event or uncontrolled arrhythmia) were excluded.
Patient history, physical examinations, blood tests, chest posterioranterior (PA) X-ray, and sputum examinations were performed at the time of diagnosis and every month during anti-tuberculosis treatment. Simple spirometry was performed at baseline and repeated after completion of the treatment. PTB severity was classified into minimal, moderately advanced and far advanced according to the chest X-ray findings.6
Treatment of EBTB was not different with PTB. Anti-tuberculosis treatment was given as a standard 6-month isoniazid, rifampicin, ethambutol and pyrazinamide or 9-month isoniazid, rifampicin, and ethambutol regimen. No additional drugs (such as steroids) for EBTB treatment were given.
The study was approved by the hospital institutional review board. Informed consent was obtained from all patients.
Bronchoscopic examination
Bronchoscopy was performed in all patients with active PTB within 1 week after the initiation of anti-tuberculosis treatment. Bronchial washing or brushing was performed on all visible endobronchial lesions. Bronchoscopic biopsy was performed in the case of differential diagnosis other than tuberculosis. The results of each bronchoscopic examination were reviewed and classified by two pulmonary specialists.
EBTB was defined by the presence of a visible endobronchial lesion proximal to a segmental bronchus, with a histological proof from bronchial biopsy or positive smear of the bronchial washing or brushing fluid. EBTB was classified into seven subtypes by using the Chung's method: actively caseating, fibrostenotic, oedematous-hyperaemic, tumorous, ulcerative, granular, and the non-specific bronchitic type.3
If a patient had two or more subtypes of EBTB simultaneously, EBTB was classified by the dominant form. We also classified EBTB by the number of involved levels. Single-level EBTB was defined when only one site of trachea, main bronchus or lobar bronchus was involved. EBTB that involved two or more bronchial levels was defined as multiple-level EBTB. For example, EBTB in trachea only was classified as single-level EBTB. EBTB that involved one main bronchus and one lobar bronchus was classified as multiple-level EBTB. EBTB that occurred proximal to the lobar bronchi was defined as central EBTB. This type has a potential to develop symptomatic stenosis. We also measured the length of the endobronchial involvement of tuberculosis by guidance of forceps.
The endobronchial narrowing was measured by comparing the area and graded as follows:
Grade 1, luminal narrowing <one third;
Grade 2, luminal narrowing ≥one third but <two thirds;
Grade 3, luminal narrowing ≥two thirds.
In case of multiple-level EBTB, the length was shown as the sum of each involvement, and the worst narrowing level among the central involvements was graded as luminal narrowing.
Follow-up bronchoscopy was planned in patients with central EBTB after at least 4 months of anti-tuberculosis chemotherapy to evaluate bronchostenosis as a late complication of EBTB and to determine whether the initial routine bronchoscopy was needed.
Statistical analysis
Statistical analysis was carried out with SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). To search for associations between clinical characteristics and concomitant EBTB, we used logistic regression analysis. A χ2 test was used to compare the differences between categorical patient characteristics. P values < 0.05 were considered significant.
Results
Incidence of EBTB and bronchoscopic findings
In total, 528 patients with newly diagnosed active PTB were observed during the study period. Among them, 99 patients refused bronchoscopy or bronchoscopy was not suitable because of hypoxemia or respiratory failure. Routine bronchoscopy was performed for evaluation of concomitant EBTB in 429 patients (247 males, 182 females) within 3 days after initiation of anti-tuberculosis treatment, and EBTB was diagnosed in 233 (54.3%). A total of 122 (28.4%) patients were biopsy-proven EBTB cases; others were diagnosed by positive bronchial washing. Among these, more clinically significant central EBTB was diagnosed in 199 (46.4%) patients. Highly infectious actively caseating EBTB was diagnosed in 114 (26.6%) patients. The incidence of EBTB was 43.3% in males and 69.2% in females. The flow chart of the study is shown in Figure 1; the results on the incidence of EBTB are summarized in Table 1.
Figure 1.
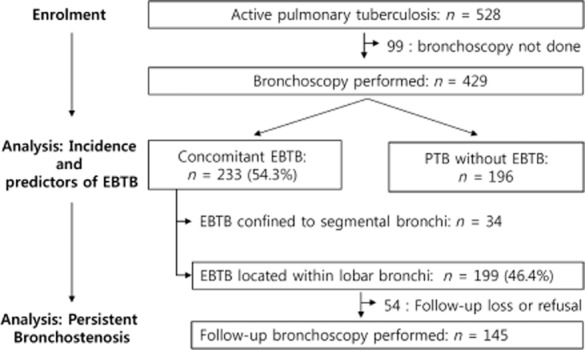
Flow chart of the routine observational bronchoscopy in patients with pulmonary tuberculosis: a total of 528 patients were diagnosed as active pulmonary tuberculosis. Among them, 429 patients were enrolled, and bronchoscopy was performed. EBTB, endobronchial tuberculosis.
Table 1.
Incidence of EBTB in patients with PTB
| % | |
|---|---|
| Overall incidence | 54.3 (233/429) |
| Incidence by gender | |
| Males (n = 247) | 43.3 (107/247) |
| Females (n = 182) | 69.2 (126/182) |
| Incidence by location | |
| Located within lobar bronchi | 46.4 (199/429) |
| Confined to segmental bronchi | 7.9 (34/429) |
| Incidence by subtypes | |
| Actively caseating | 26.6 (114/429) |
| Others | 27.7 (119/429) |
(Associate Editor: Chi Chiu Leung).
EBTB, endobronchial tuberculosis; PTB, pulmonary tuberculosis.
Table 2 shows the overall bronchoscopic findings. Actively caseating EBTB was found to be the main type (48.9%, 114/233). Lobar bronchi were the most common site of endobronchial involvement (44.2%, 103/233). The mean length of involvement exceeded 20 mm. In most patients, the level of EBTB was single, but 35% of patients had multiple-level EBTB. Among patients with central EBTB, 14.5% had grade 3 bronchostenosis at the time of diagnosis.
Table 2.
Characteristics of EBTB according to bronchoscopic features
| Bronchoscopic features | n (%) |
|---|---|
| Type of EBTB | |
| Actively caseating | 114 (48.9) |
| Oedema or hyperaemia | 87 (37.3) |
| Other types† | 32 (12.8) |
| Length of involvement (mm) (±SD) | 24.31 (±21.8) |
| Site involved | |
| Central airways | 199 (85.4) |
| Trachea | 38 (16.3) |
| Main bronchi | 58 (24.9) |
| Lobar bronchi | 103 (44.2) |
| Segmental bronchi | 34 (14.6) |
| Levels involved | |
| Single | 152 (65.2) |
| Multiple | 81 (34.8) |
| Stenosis grade (central EBTB; n = 199) | |
| Grade 1 | 140 (70.4) |
| Grade 2 or 3 | 59 (29.6) |
| Grade 2 | 30 (15.1) |
| Grade 3 | 29 (14.5) |
Including 11 patients with fibrostenotic, 2 tumorous, 3 ulcerative, 4 granular, and 12 non-specific bronchitic type.
EBTB, endobronchial tuberculosis; SD, standard deviation.
Characteristics of the patients
The baseline clinical characteristics of the 429 patients are summarized in Table 3. Most of patients were smear and culture positive. A total of 27 patients with only smear positive were diagnosed by PCR positive. The median age of patients was 48 years (range 18–97 years). Approximately 20% (88/429) of patients had an underlying disease associated with the increased risk of tuberculosis. Diabetes was most common underlying disease, followed by alcoholism and chronic liver disease.
Table 3.
Clinical characteristics of 429 patients with PTB examined by bronchoscopy
| Characteristics | Overall (n = 429) | EBTB(+) (n = 233) | EBTB(−) (n = 196) | P-value |
|---|---|---|---|---|
| Age (years), Median (range) | 48 (18–97) | 47 (18–97) | 48 (18–84) | 0.930 |
| Sputum AFB | ||||
| Smear positive | 366 | 232 | 134 | <0.001 |
| Culture positive | 402 | 227 | 175 | <0.001 |
| Both smear and culture positive | 339 | 226 | 113 | |
| Gender | ||||
| Male (%) | 247 (57.6) | 107 (45.9) | 140 (71.4) | <0.001 |
| Female (%) | 182 (42.4) | 126 (54.1) | 56 (28.6) | |
| Main symptoms | <0.001 | |||
| Cough (%) | 195 (53.7) | 124 (62.3) | 71 (43.3) | |
| Non-cough (%) | 142 (39.1) | 62 (31.2) | 80 (48.8) | |
| Asymptomatic (%) | 26 (7.2) | 13 (6.5) | 13 (7.9) | |
| Symptom duration | ||||
| Mean (range, weeks) | 7.5 (0–52) | 8.25 (0–52) | 6.19 (0–48) | 0.083 |
| Duration | ||||
| <4 weeks (%) | 192 (44.7) | 67 (30.9) | 94 (51.9) | <0.001 |
| ≥4 weeks (%) | 237 (55.3) | 150 (69.1) | 87 (48.1) | |
| Previous history of PTB | 0.002 | |||
| Yes (%) | 77 (17.9) | 29 (12.7) | 47 (24.1) | |
| No (%) | 352 (82.1) | 199 (87.3) | 148 (75.9) | |
| Co-morbid disease | 0.003 | |||
| Absent (%) | 341 (79.5) | 198 (85.0) | 143 (73.0) | |
| Present (%) | 88 (20.5) | 35 (15.0) | 53 (27.0) | |
| Severity by CXR | ||||
| Minimal (%) | 152 (47.8) | 84 (47.7) | 68 (47.9) | 0.995 |
| Moderate advanced (%) | 116 (36.5) | 64 (36.4) | 52 (36.6) | |
| Far advanced (%) | 50 (15.7) | 28 (15.9) | 22 (15.5) | |
| CXR finding | ||||
| No cavitary disease (%) | 253 (79.6) | 138 (78.5) | 115 (81.0) | 0.614 |
| Cavitary disease (%) | 65 (20.4) | 38 (21.5) | 27 (19.0) | |
| No atelectasis (%) | 192 (74.7) | 98 (70.0) | 94 (80.3) | 0.164 |
| Atelectasis (%) | 65 (25.3) | 42 (30.0) | 23 (19.7) | |
| Initial FEV1 (% predicted) | 89.2 ± 22.3 | 88.3 ± 22.4 | 91.1 ± 21.8 | 0.226 |
PTB, pulmonary tuberculosis; EBTB, endobronchial tuberculosis; CXR, chest X-ray; FEV1, forced expiratory volume in 1 s.
The main symptom was cough (53.7%). In asymptomatic patients (26 patients or 7.2%), active PTB was diagnosed due to an abnormality detected by regular chest X-ray examination. The average time from PTB symptoms to diagnosis was 7.5 weeks (range, 0–52 weeks). Symptom duration was <4 weeks in 44.7% (192/429) and >4 weeks in 55.3% (237/429).
Some patients (17.9%) had a history of PTB diagnosed by history of complete anti-tuberculosis treatment more than 1 year before the study, but most of patients (82.1%) had no previous history of PTB. PTB severity revealed by chest X-ray was as follows: minimal disease was found in 47.8% of the patients, moderately advanced in 36.5% and far advanced in 15.7%. According to chest X-ray, only 20.4% patients had cavities, and 25.3% had atelectasis. Simple spirometry was almost normal in all patients: mean forced expiratory volume in 1 s (FEV1) was 2.56 L (89% of the predicted normal value).
To elucidate the predictors of EBTB, we compared the clinical parameters (sex, underlying diseases, symptoms, symptom duration, pulmonary function test and chest X-ray findings) between EBTB-positive and EBTB-negative PTB patients. Age was not significantly different between the two groups. EBTB was more frequent in females than in males (P < 0.001). Patients with cough as the main complaint had higher incidence of EBTB than patients with non-cough symptoms (P = 0.001). Patients without past history of tuberculosis also had a significantly higher incidence of concomitant EBTB than patients with a previous history of tuberculosis (P = 0.002). Other parameters, such as simple spirometry test, severity of PTB by chest X-ray, and the presence or absence of cavity or atelectasis were similar in patients with and without EBTB. EBTB was more frequent in patients with longer symptom duration (>4 weeks; P < 0.001) (Table 3).
Predictors of EBTB
Univariate logistic regression analysis revealed that female gender, symptoms with cough, no previous history of PTB, co-morbid disease and symptom duration of more than 4 weeks were significant predictors of concomitant EBTB. However, multivariate regression analysis confirmed only female gender (odds ratio (OR) = 4.35, 95% confidence interval (CI): 1.78–10.63), symptom duration of more than 4 weeks (OR = 1.86, 95% CI: 1.05–5.46), and no previous history of PTB (OR = 4.16, 95% CI: 1.22–14.18) as the significant predictors of concomitant EBTB (Table 4).
Table 4.
Predictors of concomitant EBTB
| Variables | Univariate | Multivariate† | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | P-value | Odds ratio | 95% Confidence interval | P-value | |
| Age, ≥50 years old | 1.00 | 0.99–1.01 | 0.954 | 1.24 | 0.52–2.93 | 0.632 |
| Sex, female | 3.02 | 2.02–4.52 | 0.000 | 4.35 | 1.78–10.63 | 0.001 |
| Cough, present | 2.17 | 1.42–3.30 | 0.000 | 1.50 | 0.34–2.00 | 0.660 |
| Symptom duration, ≥4 weeks | 2.42 | 1.61–3.64 | 0.000 | 1.86 | 1.05–5.46 | 0.049 |
| Previous history, none | 2.18 | 1.31–3.63 | 0.003 | 4.16 | 1.22–14.18 | 0.023 |
| Co-morbid disease, absent | 2.10 | 1.30–3.38 | 0.002 | 2.15 | 0.73–6.36 | 0.168 |
| Atelectasis on CXR, present | 1.79 | 1.00–3.20 | 0.048 | 1.24 | 0.14–1.17 | 0.093 |
| Cavity on CXR, present | 1.17 | 0.68–2.04 | 0.571 | 1.26 | 0.39–4.06 | 0.695 |
| Severity by CXR, moderate or far advanced | 1.01 | 0.65–1.57 | 0.977 | 1.15 | 0.54–2.42 | 0.718 |
| Initial FEV1, <80% of the predicted value | 1.46 | 0.73–2.95 | 0.289 | 1.731 | 0.65–4.59 | 0.269 |
Adjusted by all other variables in the table.
EBTB, endobronchial tuberculosis; CXR, chest X-ray; FEV1, forced expiratory volume in 1 s.
We analyzed clinical and bronchoscopic characteristics of all 233 patients with EBTB by gender. Clinical characteristics such as median age, symptoms and their duration, and a previous history of PTB were not different by gender. Fewer female than male patients with EBTB had underlying diseases or risks for PTB (P = 0.001) (data not shown).
The differences in bronchoscopic findings by gender are shown in Table 5. Female patients had a higher incidence of actively caseating EBTB (P = 0.003). Bronchostenosis of grade 2 or 3 was more frequent in females (37.3%) than in males (20.7%; P = 0.009). The frequencies of central EBTB and multiple levels of involvement were higher in female patients, but the difference did not reach statistical significance.
Table 5.
Comparison of EBTB by sex according to bronchoscopic features
| Bronchoscopic features | Overall (n = 233) | Males (n = 107) | Females (n = 126) | P-value |
|---|---|---|---|---|
| Type of EBTB | ||||
| Actively caseating (%) | 114 (48.9) | 37 (35.2) | 77 (62.6) | 0.003 |
| Other types (%) | 119 (51.1) | 68 (64.8) | 46 (37.4) | |
| Length of involvement (mm) (±SD) | 24.31 (±21.8) | 19.05 (±16.5) | 29.19 (±25.0) | 0.485 |
| Central airway involvement | 199 (85.4) | 89 (83.2) | 110 (87.3) | 0.360 |
| Trachea (%) | 38 (16.3) | 16 (15.0) | 22 (17.5) | |
| Main bronchi (%) | 58 (24.9) | 21 (19.6) | 37 (29.4) | |
| Lobar bronchi (%) | 103 (44.2) | 52 (48.6) | 51 (40.4) | |
| Level of involvement | ||||
| Single (%) | 151 (65.0) | 75 (70.7) | 76 (60.3) | 0.097 |
| Multiple (%) | 81 (35.0) | 31 (29.3) | 50 (39.7) | |
| Stenosis grade in central EBTB | ||||
| Grade 1 (%) | 138 (70.1) | 69 (79.3) | 69 (62.7) | 0.009 |
| Grade 2 or 3 (%) | 59 (29.9) | 18 (20.7) | 41 (37.3) |
EBTB, endobronchial tuberculosis; SD, standard deviation.
Endobronchial stenosis after treatment
Table 6 shows follow-up bronchoscopic results on late bronchostenosis. Most EBTB patients improved following standard anti-tuberculosis therapy without steroids. However, grade 2 or 3 bronchostenosis developed in some patients (20.7%, 30/145). We analyzed the risk factors for persistent grade 2 or 3 bronchostenosis. In univariate analysis, female gender, decreased FEV1 and the involvement length of >20 mm were the risk factors for persistent stenosis. Yet, multivariate analysis confirmed only the involvement length, and decreased FEV1 as the risk factors for persistent stenosis (Table 7).
Table 6.
Bronchostenosis in patients with central EBTB after standard anti-tuberculosis therapy
| Persistent bronchostenosis after treatment | P-value | |||
|---|---|---|---|---|
| Grade 1 (%) | Grade 2 or 3 (%) | |||
| Initial luminal narrowing in central EBTB | Grade 1 (n = 106) | 86 (81.1) | 20 (18.9) | 0.450 |
| Grade 2 (n = 19) | 13 (68.4) | 6 (31.6) | ||
| Grade 3 (n = 20) | 16 (80.0) | 4 (20.0) | ||
| Total | 145 | 115 (79.3) | 30 (20.7) | |
EBTB, endobronchial tuberculosis.
Table 7.
Risk factors for grade 2 or 3 stenosis after treatment
| Variables | Univariate | Multivariate† | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | P-value | Odds ratio | 95% Confidence interval | P-value | |
| Age, ≥50 years old | 0.99 | 0.96–1.02 | 0.570 | 0.79 | 0.28–22.68 | 0.891 |
| Sex, female | 2.34 | 1.01–5.40 | 0.047 | 2.66 | 0.72–98.41 | 0.595 |
| Cough, present | 1.50 | 0.63–3.59 | 0.364 | 1.82 | 0.03–105.83 | 0.774 |
| Symptom duration, ≥4 weeks | 0.73 | 0.34–1.87 | 0.601 | 2.63 | 0.17–40.88 | 0.490 |
| Previous history of PTB, none | 1.57 | 0.44–5.67 | 0.491 | 10.89 | 0.01–951.32 | 0.490 |
| Atelectasis on CXR, present | 2.65 | 0.86–8.21 | 0.091 | 5.57 | 0.02–189.73 | 0.564 |
| Cavity on CXR, present | 0.80 | 0.27–2.84 | 0.687 | 3.51 | 0.05–264.10 | 0.569 |
| Severity by CXR, moderate or far advanced | 1.17 | 0.47–9.28 | 0.742 | 1.05 | 0.02–63.90 | 0.359 |
| Initial FEV1, <80% of the predicted value | 6.98 | 1.74–28.07 | 0.006 | 4.97 | 1.01–23.06 | 0.041 |
| Length of involvement, ≥20 mm | 5.68 | 2.22–14.83 | 0.000 | 16.33 | 1.90–141.10 | 0.011 |
| Type of EBTB, actively caseating | 1.94 | 0.87–4.33 | 0.107 | 4.32 | 0.10–179.51 | 0.442 |
| Level of involvement, multiple | 2.15 | 0.99–4.64 | 0.052 | 20.60 | 0.24–181.30 | 0.185 |
| Initial stenosis, grade 2 or 3 | 1.33 | 0.56–3.14 | 0.518 | 0.14 | 0.00–66.40 | 0.243 |
Adjusted by all other variables in the table.
EBTB, endobronchial tuberculosis; PTB, pulmonary tuberculosis; CXR, chest X-ray; FEV1, forced expiratory volume in 1 s.
As a result, three patients underwent bronchoscopic interventions (one balloon dilatation only, two stent insertion after balloon dilatation). All these three patients were women and had stenosis in the left main bronchus.
Discussion
To the best of our knowledge, this is the first report of a prospective study of the incidence and predictors of EBTB, whereas the data available so far were provided only by retrospective studies or pre-fibre-optic bronchoscopic reports. By using prospective routine bronchoscopy in 429 patients with PTB, we found that the incidence of EBTB was 54.3%. The data from autopsy of patients with active PTB showed that the frequency of EBTB was 30–50%.9–11 Despite the higher incidence of EBTB suggested by these autopsy data, the frequency of EBTB was generally known to be about 10%.12 Our estimate is consistent with the previous autopsy studies9–11 and is higher than the estimates from other retrospective studies.9–12
High incidence of EBTB raises questions related to clinical applications. One question is whether bronchoscopic examination should be performed in every patient with active PTB. The other question is what the risk factors are for EBTB in patients with PTB. Our results show that female patients with longer symptom duration (>4 weeks) or no previous history of PTB need bronchoscopic examination for detection of concomitant EBTB.
Our findings are in line with some retrospective studies that also showed that female gender was associated with concomitant EBTB.12–16 But there have been no prospective studies demonstrating the female gender as a risk factor for EBTB in patients with PTB. Our study is the first to show that female gender is the main risk factor for concomitant EBTB.
Why is concomitant EBTB more likely in female patients? Some possible causes have been proposed. One may be a longer exposure to tubercle bacilli, because female patients have less expectorated sputum containing bacilli due to sociocultural and aesthetic factors.6 Rikimaru et al. postulated that a structural difference may play a role. Female bronchi are narrower than those of males, which may make females more susceptible to EBTB.17 Smoking may also be related to concomitant EBTB. Frequent smokers are less likely to develop EBTB, so in females, who are more likely to smoke less, EBTB arises more frequently.17 Our study was not designed in a way to allow us to elucidate the reasons for higher frequency of EBTB in females. Female gender was also a risk factor for a higher grade of stenosis, and females had a more active type of EBTB and a tendency to have multiple-level EBTB. Further observational studies are needed to define the differences between females and males in this respect.
Symptom duration is also presumed to be a risk factor of concomitant EBTB. A previous retrospective study showed that longer symptom duration was associated with a higher frequency of EBTB.18 In the present study, longer symptom duration (>4 weeks) was an independent risk factor for EBTB in patients with PTB. We believe that longer symptom duration may be related to a longer exposure to tubercle bacilli and thus contributes to EBTB development. We expected that longer symptom duration would also be related to the levels of involvement and to the grade of stenosis, but our study did not confirm this relation for symptom duration. More data about the grade of stenosis are required to perform this analysis properly.
Sakin et al. found that EBTB was more common in patients with advanced cavitary tuberculosis.19 In cavitary tuberculosis, more tubercle bacilli are usually dispersed, and longer exposure to bacilli on the airway increases the chance of bronchial spread. Thus, patients with cavitary tuberculosis may be more frequently affected by EBTB. However, our study did not show the relationship between cavitary tuberculosis or advanced tuberculosis and concomitant EBTB. A possible explanation of this result could be that although patients with cavitary tuberculosis had numerous bacilli, the release of these bacilli was less pronounced than expected.
In the present study, the incidence of EBTB differed according to the previous history of tuberculosis: EBTB was more frequent in the initial treatment group than in the retreatment group. We do not know why patients without history of treatment were more at risk of having EBTB. In univariate analysis, atelectasis was related to concomitant EBTB, but in multivariate analysis, it was not a risk factor. Atelectasis was only found in 15% of EBTB cases. Some studies claimed that young age was a risk factor for EBTB.20 However, our study did not find any differences in concomitant EBTB frequencies by age group. In summary, we found female gender, longer symptom duration (>4 weeks) and the initial treatment to be the independent clinical predictors for concomitant EBTB in patients with PTB.
High incidence of EBTB may be relevant for clinical practice. Routine bronchoscopy may be needed for female patients or patients with longer symptom duration. However, follow-up bronchoscopy did not show the effectiveness of early diagnostic bronchoscopy in terms of active identification and prevention of persistent bronchostenosis. Most patients with EBTB improved after standard anti-tuberculosis therapy. Female gender was not a risk factor for persistent bronchostenosis. Thus, the risk factors for EBTB and persistent bronchostenosis are different. Bronchoscopic finding (the involvement length of >20 mm) was the only risk factor for grade 2 or 3 persistent bronchostenosis. In a previous retrospective study based on only follow-up bronchoscopy performed only in patients with severe EBTB, fibrostenotic type is identified as one of the predictor of persistent bronchostenosis.4 But in our study based on all patients with EBTB, type of EBTB was not the predictor of bronchostenosis.
The present study has several limitations. First, some patients enrolled in this study could have had non-tuberculous mycobacterial (NTM) infection as we did not perform nuclear amplification test (PCR) in all patients. Up to 10% of patients with positive AFB stain may actually be suffering from NTM pulmonary disease in Korea.21 But endobronchial lesions as a result of NTM are extremely rare. Just a few cases were reported.22 So high incidence of EBTB would not have been affected by the eventual presence of NTM cases. Second, we did not consider the sputum AFB grade as a variable for risk factor analysis because the method of sputum collection, such as timing, was not controlled. Other possible risk factors such as smoking or alcohol drinking history were not analyzed. Third, about half of the patients with EBTB were diagnosed by bronchial biopsy, and other patients diagnosed by bronchial washing only. Some patients might thus have showed false positive EBTB results, especially in non-caseating types.
The importance of this study is that it has prospectively revealed the incidence and the predictors of EBTB in patients with active PTB, and also the risk factors for persistent bronchostenosis after treatment. The incidence of EBTB in patients with active PTB was at least 50% and was strikingly higher than that reported by previous retrospective studies. Female gender and longer symptom duration were the main independent predictors of concomitant EBTB. Bronchoscopy revealed length of involvement as an independent risk factor for persistent bronchostenosis. Thus, our findings suggest that females and patients with longer symptom duration should be considered for bronchoscopy to detect endobronchial involvement. But in terms of bronchostenosis after treatment, recommendation for immediate diagnostic bronchoscopy in patients with active PTB should be considered in selected patients.
Acknowledgments
We would like to thanks all technicians in bronchoscopy unit for assisting bronchoscopic procedure. This study was supported by the Chungnam National University Hospital, Daejeon, Korea.
Glossary
- AFB
acid-fast bacilli
- CI
confidence interval
- EBTB
endobronchial tuberculosis
- FEV1
forced expiratory volume in 1 s
- NTM
non-tuberculous mycobacterial
- OR
odds ratio
- PCR
polymerase chain reaction
- PTB
pulmonary tuberculosis
References
- Smart J. Endobronchial tuberculosis. Br. J. Dis. Chest. 1951;45:61–68. [Google Scholar]
- Shim YS. Endobronchial tuberculosis. Respirology. 1996;1:95–106. doi: 10.1111/j.1440-1843.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest. 2000;117:385–392. doi: 10.1378/chest.117.2.385. [DOI] [PubMed] [Google Scholar]
- Um SW, Yoon YS, Lee SM, Yim JJ, Yoo CG, Chung HS, Kim YW, Han SK, Shim YS, Kim DK. Predictors of persistent airway stenosis in patients with endobronchial tuberculosis. Int. J. Tuberc. Lung Dis. 2007;11:57–62. [PubMed] [Google Scholar]
- Ozkaya S, Bilgin S, Findik S, Kök HC, Yuksel C, Atıcı AG. Endobronchial tuberculosis: histopathological subsets and microbiological results. Multidiscip. Respir. Med. 2012;7:34–39. doi: 10.1186/2049-6958-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Tuberculosis Association of USA. 1961. Diagnostic Standards and Classification of Tuberculosis New York NTA.
- Surender K, Prasanta RM, Varinder S. Endobronchial tuberculosis. Indian J. Chest Dis. Allied Sci. 2003;45:247–256. [PubMed] [Google Scholar]
- Han JK, Im JG, Park JH, Han MC, Kim YW, Shim YS. Bronchial stenosis due to endobronchial tuberculosis: successful treatment with self-expanding metallic stent. Am. J. Roentgenol. 1992;159:971–972. doi: 10.2214/ajr.159.5.1414809. [DOI] [PubMed] [Google Scholar]
- Bugher JC, Littig J, Culp J. Tuberculous tracheobronchitis: its pathogenesis. Am. J. Med. Sci. 1937;193:515–525. [Google Scholar]
- Judd AR. Tuberculous tracheobronchitis. J. Thorac. Surg. 1947;16:512–523. [PubMed] [Google Scholar]
- Auerbach O. Tuberculosis of trachea and major bronchi. Am. Rev. Tuberc. 1949;60:604–620. doi: 10.1164/art.1949.60.5.604. [DOI] [PubMed] [Google Scholar]
- Pierson DJ, Lakshminarayan S, Petty TL. Endobronchial tuberculosis. Chest. 1973;64:537–539. doi: 10.1378/chest.64.4.537. [DOI] [PubMed] [Google Scholar]
- Olsen DE, Jones FS, Murray D. Bronchial disease in lungs for pulmonary tuberculosis. Am. Rev. Tuberc. 1953;68:657–677. doi: 10.1164/art.1953.68.5.657. [DOI] [PubMed] [Google Scholar]
- Marry SM, So SY, Lam WK, Mok CK. Endobronchial tuberculosis revisited. Chest. 1987;89:727–730. doi: 10.1378/chest.89.5.727. [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Maeda M. Tracheobronchoplasty for endobronchial tuberculosis. Kekkaku. 1991;66:789–792. [PubMed] [Google Scholar]
- Kurasawa T, Kuze F, Kawai M, Amitani R, Murayama T, Tanaka E, Suzuki K, Kubo Y, Matsui Y, Sato A, et al. Diagnosis and management of endobronchial tuberculosis. Intern. Med. 1992;31:593–598. doi: 10.2169/internalmedicine.31.593. [DOI] [PubMed] [Google Scholar]
- Rikimaru T, Kinosita M, Yano H, Ichiki M, Watanabe H, Shiraisi T, Huruno H, Ookubo Y, Oizumi K, Kondo M. Diagnostic features and therapeutic outcome of erosive and ulcerous endobronchial tuberculosis. Int. J. Tuberc. Lung Dis. 1998;2:558–562. [PubMed] [Google Scholar]
- Toyota E, Kobayashi N, Takahara M, Yoshizawa A, Kawada H, Suzuki T, Kudo K, Inagaki K. Clinical investigation on endobronchial tuberculosis. Kekkaku. 1999;74:347–351. [PubMed] [Google Scholar]
- Sakin O, Cadden V, Edson RC. Natural history of tuberculous tracheobronchitis. Am. Rev. Tuberc. 1943;47:351–359. [Google Scholar]
- Lee JH, Chung HS. Bronchoscopic, radiologic and pulmonary function evaluation of endobronchial tuberculosis. Respirology. 2000;5:411–417. [PubMed] [Google Scholar]
- Jeon K, Koh WJ, Kwon OJ, Suh GY, Chung MP, Lee NY, Park YK, Bai GH. Recovery rate of NTM from AFB smear-positive sputum specimens at a medical centre in South Korea. Int. J. Tuberc. Lung Dis. 2005;9:1046–1051. [PubMed] [Google Scholar]
- Kang SH, Mun SK, Lee MJ, Kim SY, Choi HG, Byun J, Kim CH, Kim HR, Cho SY. Endobronchial mycobacterium avium infection in an immunocompetent patient. Infect. Chemother. 2013;45:99–104. doi: 10.3947/ic.2013.45.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]


