Abstract
Purpose
Medeiros et al. developed a combined structure-function index for glaucoma by combining two ganglion cell models developed by Harwerth et al. The current study assessed assumptions of the Medeiros combined structure-function index by evaluating whether the two Harwerth models gave similar distributions of ganglion cells in an independent dataset.
Methods
The Harwerth models were applied to our previously published data for retinal nerve fibre layer (RNFL) thickness (Stratus OCT 3.4) and visual field sensitivities (24-2 SITA Standard) from one eye each of 51 patients with glaucoma and 62 age-similar control subjects free of eye disease. RNFL thicknesses and perimetric sensitivities were converted to ganglion cell numbers using the Harwerth model for perimetry and the Harwerth model for RNFL. These two estimates of ganglion cell number were compared for the inferior temporal (IT) and superior temporal (ST) sectors of the optic disc and the corresponding visual field locations. Comparisons were made with 14 visual field locations per sector (including a point in the macula for the inferior temporal sector) and with 13 locations (no point in the macula). Data for controls and patients were analysed separately, comparing mean values for RNFL perimetry models. Bonferroni correction was applied to control for repeated tests of significance. The difference between mean values for the RNFL and perimetry models was quantified by equating the means for controls through reduction of the assumed axon diameter used by the RNFL model.
Results
For the control group, the Harwerth RNFL model yielded smaller mean number of retinal ganglion cells than the Harwerth perimetry model, 23–47% lower (t > 13, p < 0.0001). This corresponded to mean axon diameters from 0.48 to 0.69 μm, with the smallest axons when the 14th location in the macula was included. With these new axon diameters, estimates of ganglion cell numbers for patients were still lower than for the RNFL model, by 19–28% (t > 6.5, p < 0.0001).
Conclusions
The Harwerth RNFL model consistently gave lower ganglion cell numbers than the Harwerth perimetry model, and this discordance persisted in patients even after reducing assumed axon diameter for controls. This finding contradicts the assumptions of the Medeiros structure-function index.
Keywords: axons, glaucoma, perimetry, retinal ganglion cells, retinal nerve fibre layer
Introduction
Assessment of glaucomatous damage often includes measurement of peripapillary retinal nerve fibre layer (RNFL) thickness, and several quantitative models have been developed that show good agreement on average between perimetric sensitivity and RNFL thickness, within the limits produced by between-subject variability in healthy eyes.1,2 The variation in the number of retinal ganglion cells3–5 and RNFL thicknesses6–8 found in healthy people is so large that a person who began at the high end of the normal range would need to lose half of their ganglion cells or RNFL thickness before they fell outside normal limits, while someone who began at the low end of the normal range would require relatively little loss to fall outside normal limits. This variability can account for the apparent discordance between RNFL and perimetric measurements.9 It also opens the possibility that quantitative methods for combining the two measures could improve ability to assess glaucomatous damage.
A recent approach to combine structure and function has been to convert both perimetric and RNFL data into estimates of numbers of ganglion cells in individual eyes, and combine those two estimates to get a single index.10 Medeiros developed a combined structure-function index and found that it detected more eyes as progressing than either RNFL or perimetry alone.10 In subsequent studies his research group has used this index to make inferences about ganglion cell numbers related to localised RNFL defects,11 early visual field defects,12 predicting progression,13 afferent pupillary defect,14 visual field index,15 optic disc hemorraghes,16 cup-to-disc ratio,17 mean deviation,18 and design of clinical trials.19
The structure-function index proposed by Medeiros combines two models developed by Harwerth to estimate the number of retinal ganglion cells in an eye. The Harwerth perimetry model20 converts perimetric sensitivity to number of ganglion cell bodies, based on histological studies on glaucomatous monkeys.21 The Harwerth RNFL model converts RNFL thickness to number of ganglion cell axons, by assuming an average axon diameter and adjusting the ratio of axons to glial cells using a model of gliosis that incorporates visual field information.22 Medeiros’ structure-function index assumes that on average both models give equal numbers of ganglion cells, so that the two can be combined in a weighted sum that emphasises RNFL estimates in early disease and visual field estimates in advanced disease. Medeiros argued that Harwerth had validated the structure-function model in monkeys and successfully extended it to two independent human cohorts. However, examination of the patient data presented by Medeiros in the initial paper reveals that for their cohort of patients the number of ganglion cells derived from RNFL was on average lower than the number derived from perimetry, at all stages of disease. This is inconsistent with the findings by Harwerth23,24 that mean numbers were on average similar until late in the disease, where RNFL yielded higher numbers than visual field. In other words, the data used by Medeiros in developing the structure-function index are not consistent with data in the Harwerth studies relied on for validation. Given the wide range of applications for the combined structure-function index, we undertook further assessment of its assumptions on a fourth cohort.
The fact that RNFL yielded fewer ganglion cells than perimetry in the Medeiros cohort is consistent with a weakness in key assumption of the Harwerth RNFL model: that mean axon diameter for human retinal ganglion cells is 0.9 μm. Histological studies have found mean ganglion cell axon diameters of 0.5–0.7 μm in humans, with ganglion cells in the macula having the smallest axons.25 The assumption about axon diameter is important because the Harwerth RNFL model estimates axon density by computing how many axonal cross-sections would fill the neural component of measured RNFL thickness for a given optic disc sector. If the assumed diameter of 0.9 μm is too large, then the Medeiros structure-function index may have an artefact that leads to underestimating the number of ganglion cells in early stages of loss.
The current study assessed the assumptions of the Medeiros index about agreement between the Harwerth models for RNFL thickness and perimetry by comparing mean values of the models in a cohort of patients and controls.2 Reduction in assumed axon diameter was employed to characterise discrepancies between the RNFL and perimetry models.
Methods
Data analysed were from a recently published paper,2 which provides greater detail on inclusion and exclusion criteria, testing, and reliability criteria. Subjects were participating in a multicentre longitudinal study at three different university clinics, in Manhattan (SUNY), Indianapolis (IU) and Bloomington (IU). The research for this study adhered to the tenets of the Declaration of Helsinki. Institutional Review Board (IRB) approval was obtained from the institutional review boards at SUNY College of Optometry and at Indiana University. Informed consent was obtained from each participant after explanation of the procedures and goals of the study, before testing began. This research was HIPPA-compliant.
Inclusion and exclusion criteria
Common inclusion criteria for both groups were: best corrected visual acuity of 0.0 logMAR (6/6 or 20/20) or better (0.1 logMAR or 6/7.5 or 20/25 over age 70), spherical equivalent within −6.00 D to +2.00 D (so that lenses for perimetry at 33 cm would range from −3.00 D to +5.00 D), cylinder correction within 3.00 D, clear ocular media, absence of known eye disease during a comprehensive eye examination within 2 years (except for glaucoma in the patient group).
Common exclusion criteria for both groups were: ocular or systemic disease known to affect the visual field (e.g. diabetic retinopathy, prior vein occlusion, macular degeneration, degenerative myopia, migraines), except glaucoma in the patient group; history of intraocular surgery (except uncomplicated cataract surgery more than 1 year before enrolment, or glaucoma surgery in the patient group); usage of medications known to affect vision; inability to yield OCT images free of segmentation errors or low signal strength, inability to produce reliable perimetric data.
Additional exclusion criteria for controls were a self-reported first-degree relative with glaucoma, and IOP > 21 mmHg for two or more clinic visits. An additional exclusion criterion for patients was IOP > 30 mmHg at a clinic visit during the longitudinal study.
Study definition of glaucoma
Diagnosis of glaucoma was made by the treating physician, based on a complete ophthalmic examination including medical history, refraction, best-corrected visual acuity, slit lamp biomicroscopy (including gonioscopy), applanation tonometry, dilated fundoscopy, stereoscopic ophthalmoscopy of the optic disc, stereo photos of the optic nerve, perimetry, and optic nerve imaging. All patients had prior experience with perimetry, and had a history of reliable visual fields. An Executive Committee of five clinicians reviewed the diagnoses.
One eye each of 51 patients with glaucoma and 62 age-similar control subjects had assessments of RNFL thicknesses and perimetric sensitivities. For patients, age ranged from 45 to 84 years, mean (SD) = 64 (9) years, for control subjects the range was 46–84 years, mean 62 (9) years. Mean Deviation (MD) ranged from −23 to +1.2 dB (mean of −5.4 dB) for patients and −2.9 to +1.5 dB (mean of −0.3 dB) for controls.
Perimetric testing was performed using the Humphrey Visual Field Analyzer II (Carl Zeiss Meditec, http://www.zeiss.com/meditec/en_de/home.html), using 24-2 SITA Standard. RNFL testing was performed with optical coherence tomography (Stratus OCT 3, Model 3000, Carl Zeiss Meditec) to obtain measurements of thickness of the peripapillary RNFL using the RNFL 3.4 Fast Scan protocol. All results were reviewed individually for artefacts.2
For each dataset from an individual patient or control, arithmetic means were computed for superior temporal (ST) and inferior temporal (IT) optic disc sectors, either for corresponding perimetric sensitivities or corresponding RNFL thicknesses. The sectors were defined by the 2002 Garway-Heath map,26 where ST is from 45 to 90° and IT is from 270 to 315°, mapped to test locations as shown in Figure 1. Thirteen locations for IT have a corresponding location for ST at the same horizontal eccentricity and opposite vertical eccentricity, while the 14th location is at an eccentricity of 4° for IT and an eccentricity of 21° for ST. When 14 vs 13 locations are used the effect on ganglion cell number and axon diameter is expected to be greater for IT than for ST, because ganglion cell density is much higher in the macula than the rest of the retina, and mean axon diameter is smallest in the macula. Therefore all calculations were performed separately with 14 vs 13 locations per sector.
Figure 1.
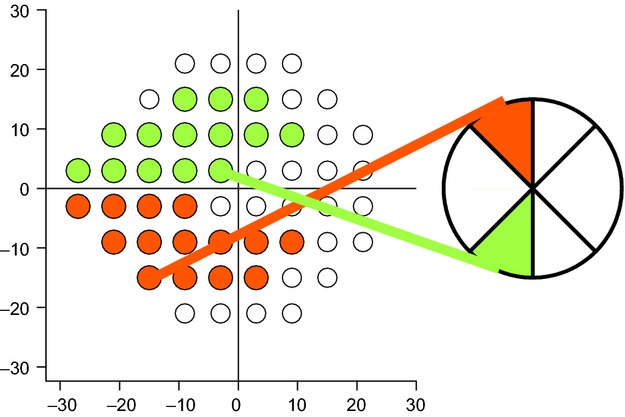
shows superior temporal (ST) and inferior temporal (IT) visual field locations mapped to optic disc sectors. Coloured regions indicate IT (green) and ST (red), lines connect the disc sector to the 14th location, which is at an eccentricity of 4° for IT and 21° for ST.
The estimates of number of ganglion cell bodies from perimetric data were derived with the 2004 Harwerth model.20 The estimates of number of ganglion cell axons from RNFL data were derived with the 2007 Harwerth model.22
Statistical approach
Student's T-test was used to compare the mean numbers of ganglion cells derived from RNFL thicknesses and perimetric sensitivities, because the Medeiros index uses mean numbers of ganglion cells. Comparisons were performed separately for the control group and the patient group. With two groups, two sectors, and two numbers of locations, there were a total of eight t-tests. Bonferonni correction was used to set a value of p < 0.00625 (0.05/8) for statistical significance.
Any differences between mean values for controls with the RNFL and perimetry models were quantified by equating the means through reduction of the assumed axon diameter used by the RNFL model. That is, if the mean number of ganglion cells was 20% lower for RNFL than for perimetry, then the new axon diameter was set giving 0.72 μm, which is 80% of Harwerth's assumed diameter of 0.90 μm, causing the new mean number of ganglion cells for RNFL to be equal to the mean number for perimetry.
Between-subject variability in the control group was characterised using bivariate Gaussian ellipses.27 Each ellipse provides an assessment of when two measured values are below normal at p < 0.05, based on the variability of the two measures and the strength of their correlation. The aspect ratio of a Gaussian ellipse reflects the ratio of the variances of the two measures, and its orientation reflects the strength of the correlation. The ellipses were computed to include 90% of the control data, so that a datapoint falling outside the bottom half of the ellipse would have p < 5%.
Tangents to these ellipses were used as limits of agreement for patients, based on between-subject variability in controls. These tangents were parallel to the line connecting the centroid of the ellipse with the origin (0,0). This reflects the assumption of the Medeiros index that both measures proceed from mean normal to zero as disease progresses, and also incorporates normal between-subject variability.27
Results
Figures 2 and 3 show the number of ganglion cell bodies from the Harwerth model for perimetry plotted as a function of the number of ganglion cell axons from Harwerth model for RNFL thickness, with horizontal and vertical lines showing the means of the control group, and a diagonal line representing the line of equality (equal numbers from the two models). For each graph, a diagonal line passes through the origin and the means for the controls, an ellipse outlines 90% of the normal range, and tangents to the ellipse represent the prediction interval for the patients based on between-subject variability of the controls. Figure 2 shows results with 14 locations per sector (including the macula for IT), and Figure 3 shows results with 13 locations per sector (excluding the macula). The left panels show results for Harwerth's assumption that mean axon diameter is 0.90 μm, and the right panels show results when axon diameter was reduced to equate the means for the controls. In all eight panels, almost all patient datapoints (except those inside the ellipse or near the origin) were above the line of equality, having more ganglion cell bodies from perimetry than ganglion cell axons from RNFL thickness.
Figure 2.
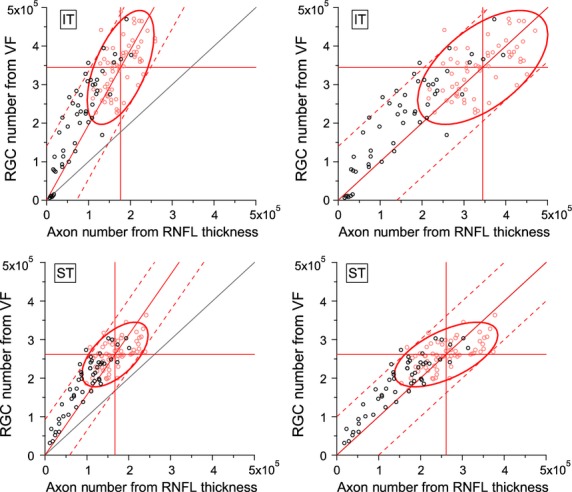
Comparison of results for Harwerth models for numbers of ganglion cell bodies from perimetry (y-axis) and numbers of ganglion cell axons from retinal nerve fibre layer thickness (x-axis). The left panels show results for the Harwerth models as published, and the right panels show results with axon diameter reduced to equate means for controls. Black circles show patient data, red circles show control data. Horizontal and vertical lines show means for the controls, ellipses show Gaussian limits for the controls at p = 0.90. Black diagonal line shows equality, red solid diagonal line passes through zero and mean normal, dashed red lines represent limits of agreement for patients based on between-subject variability of the controls.
Figure 3.
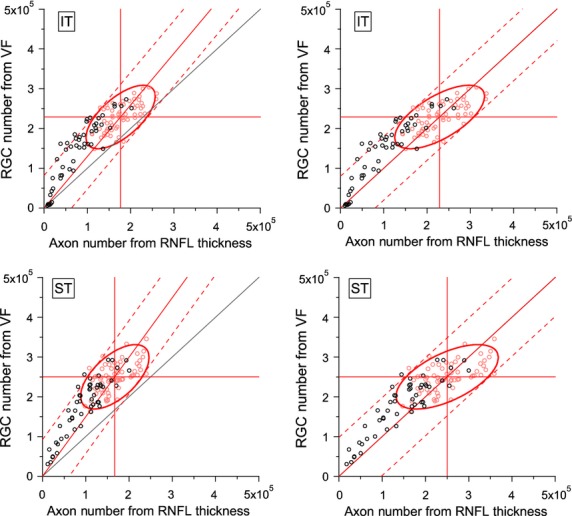
Comparison of results for Harwerth models for numbers of ganglion cells from perimetry and axon numbers from retinal nerve fibre layer thickness, as shown in Figure 2 except that the 14th locations were removed before computing number of ganglion cell bodies from perimetric sensitivities mapped to ST and IT (removed locations are outlined in Figure 1).
The mean numbers of ganglion cell bodies from the Harwerth model for perimetric sensitivities were substantially higher than the mean numbers of ganglion cell axons from the Harwerth model for RNFL, as shown in the left panels of Figures 2 and 3. For the control group, with 14 locations IT averaged 157 608 more cells from perimetry than RNFL, and 52 236 more cells with 13 locations. This corresponds to RNFL having 47% fewer cells with 14 locations and 23% fewer cells with 13 locations (t61 > 13, p < 0.0001). To equate these means, assumed axon diameter was reduced to 0.48 μm with 14 locations and 0.69 μm with 13 locations. For ST, the control group averaged 94 100 more cells with 14 locations and 84 311 more cells with 13 locations. This corresponds to RNFL having 36% fewer cells with 14 locations and 34% fewer cells with 13 locations (t61 > 19, p < 0.0001). To equate these means, assumed axon diameter was reduced to 0.58 μm with 14 locations and 0.60 μm with 13 locations.
For the patient group, even with these new axon diameters the RNFL model yielded fewer ganglion cells than perimetry, as illustrated in the right panels of Figures 2 and 3. The mean differences for IT were 41 431 cells with 13 locations and 49 553 cells with 14 locations, corresponding to 25% and 28% fewer cells (t50 > 6.5, p < 0.0001). The mean differences for ST were 33 047 cells with 13 locations and 34 497 cells with 14 locations, corresponding to 19% fewer cells (t50 > 7, p < 0.0001).
Discussion
This study arose from the observation that the cohort that Medeiros10 analysed for developing a combined structure-function index had lower ganglion cell numbers for the Harwerth RNFL model than for the Harwerth perimetry model. This means that data from the Medeiros cohort are inconsistent with data from the two cohorts used by Harwerth to extend the models from monkeys to humans. A close analysis of Figure1 of the Medeiros paper10 reveals that the Harwerth RNFL model systematically produced lower numbers of ganglion cells than the Harwerth perimetry model. In this study we replicated this aspect of the Meideros cohort, using a separate cohort, and confirmed that the Harwerth RNFL model systematically produced lower numbers of ganglion cells than the Harwerth perimetry model. The fact that the same discrepancy is seen in the Medeiros cohort and our cohort contradicts the assumptions of the structure-function index.
Harwerth compared his RNFL and perimetry models in terms of clustering around the line of equality and the 95% limits of agreement, which is a traditional method for assessing agreement. Harwerth found that data for both Houston and Miami cohorts clustered around the line of equality, except in severely damaged eyes where the RNFL model tended to give a larger number of ganglion cells than the perimetry model. Medieros et al. did not draw a line of equality or limits of agreement, but observation of his Figure1 shows that most datapoints fell above the line of equality.
We demonstrated in the control group that the discrepancy was consistent with the Harwerth RNFL model over-estimating mean axon diameter. We reduced the value used for axon diameter in order to make the mean number of ganglion cells equal for the RNFL and perimetry models. This yielded results consistent with the literature25 on human ganglion cell axon diameter: means from 0.48 to 0.69 μm, with the smallest diameter found when the macula was included. These values are consistent with histological findings that human retinal ganglion cell axons have mean diameters from 0.46 to 0.73 μm at different locations across the retina, with cell bodies near the fovea having the smallest axon diameters.25 However, for patients this adjustment was not sufficient to equate the means for the two models.
The two cohorts that Harwerth evaluated had data that clustered around the line of equality, rather than above it as in the Medeiros cohort and our cohort. One difference is that Harwerth converted his ganglion cell numbers to log units before performing statistical analysis, while the Medeiros index does not. The log transform decreases variability when ganglion cell number is high and increases variability when ganglion cell number is low. We applied the log transform to our data for IT with 13 locations, and found that on average RNFL gave smaller numbers of cells, by −0.12 (0.07) log unit for controls (t > 12, p < 0.0001), which is equivalent to the 23% reduction found in our linear analysis. For patients the difference was −0.25 (0.18) log unit, or −0.13 log unit more, which converts to 26% reduction as compared to 25% reduction for our linear analysis. We conclude that the use of the log transform cannot account for the difference.
Measurements of RNFL thickness and perimetric sensitivities are affected by a range of factors that may differ systematically across cohorts due to inclusion and exclusion criteria. We had a more strict acuity criterion than the two cohorts used by Harwerth, so it possible that our cohort on average had less forward light scatter than his cohorts, which could have decreased perimetric sensitivity in his cohorts. However, Medeiros used a more lax acuity criterion, so difference in acuity criterion cannot explain his cohort. Measurement of RNFL thickness are affected by axial length, because the scan circle is physically larger in longer eyes and measures locations where RNFL is thinner than for the smaller scan circles in shorter eyes.28 None of the cohorts used axial length as an exclusion criterion, and it is possible that the Harwerth cohorts had larger axial length on average than the Medeiros cohort or our cohort. The acuity criteria will often exclude eyes with long axial lengths, but pseudophakic eyes may be emmetropic despite long axial length. These factors illustrate why the structure-function index would be complicated to use in clinical practice, because the clustering around the line of equality found in the Harwerth cohorts was not present in the Medeiros cohort or our cohort.
The Harwerth model for estimating ganglion cell numbers from RNFL thickness was developed using a 10-sector map of visual field to the optic disc,22 while the current study used a six-sector map.26 The difference in field-to-disc maps cannot explain the lower mean number of retinal ganglion cells for RNFL, because the difference is also seen when the global RNFL numbers are compared to global perimetric numbers, as shown in Figure 4. The left panel shows that mean number of cells from RNFL for controls was 15% lower than from perimetry (t61 > 7, p < 0.0001). The right panel shows that almost all patients still had ganglion cell numbers from RNFL that were lower than numbers from perimetry, even after equating the means for controls.
Figure 4.
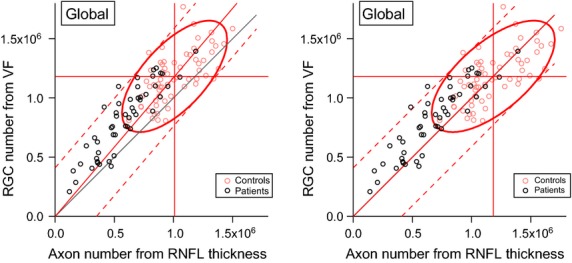
Computed numbers of ganglion cell bodies from perimetry vs numbers of ganglion cell axons from RNFL thickness, for global RNFL and perimetric values. Left shows results for the Harwerth model of RNFL, right shows results when axon diameter is reduced to equate the means for controls.
Medeiros10 adapted Harwerth's models in an effort to assess rate of ganglion cell loss using an index that relied primarily on RNFL in early disease and increasingly on perimetry in advanced disease. Our evaluation is that the Harwerth RNFL model under-estimated ganglion cell number relative to Harwerth perimetry model, due to an assumption about axon diameters. This makes it likely that the Medeiros index underestimates ganglion cell number in early disease relative to ganglion cell number in late disease, a potential artefact when assessing progression of ganglion cell loss.
The Harwerth model for perimetry is only one of several models relating perimetry to ganglion cell number.9,29–31 If other models had been used, different estimates for axon diameter would have been obtained. The purpose of reducing axon diameter was to assess the choice of axon diameter as an explanation for why the Harwerth models for perimetry and RNFL do not agree. Reducing axon diameter to equate means for controls did not equate means for patients, so further work is needed to determine how these results change when other perimetry models are used.
The clinical ‘ISNT’ rule for optic nerve rim area suggests the inferior rim has the most nerve fibres, but histological data show greater retinal ganglion cell density in superior retina over inferior retina.3 Our results with 13 vs 14 locations show that the difference between histology and clinical observation can be accounted for by the finding that macular visual field projections include the inferior temporal (IT) sector but not the superior temporal (ST) optic disc sectors.32 This result shows that inclusion or exclusion of a single perimetric location can dramatically affect the estimated number of ganglion cells, and underscores the role of mapping the macula to the optic disc in structure-function analyses.
The purpose of this study was scientific, varying assumed axon diameter to characterise systematic discrepancies between ganglion cell estimates from perimetry and RNFL thickness. Axon diameter was a fixed parameter that has been utilised in the structure-function index developed by Medeiros, and our finding is that this was an overestimate of mean human axon diameter that causes the Harwerth model for RNFL thickness22 to give lower retinal ganglion cell estimates than the Harwerth model for perimetry20. This means that the Medeiros index10 may systematically overestimate the amount of neural loss compared to perimetry, and the extent of this bias may vary with disc sector. Further research is needed before structure and function can be converted into reliable estimates of ganglion cell number. This study did not directly address the clinical value of the Medeiros index or its ability to evaluate progression of glaucoma.
Acknowledgments
Funding for this research was provided by the National Institutes of Health, Bethesda, Maryland; Grant numbers R01EY007716 and R01EY024542.
Disclosure
Dr. Swanson is a consultant for Heidelberg Engineering and Carl Zeiss Meditec. Dr. Horner reports no conflicts of interest. The authors have no proprietary interest in any of the materials mentioned in this article.
References
- 1.Hood DC, Anderson SC, Wall M, et al. A test of a linear model of glaucomatous structure-function loss reveals sources of variability in retinal nerve fiber and visual field measurements. Invest Ophthalmol Vis Sci. 2009;50:4254–4266. doi: 10.1167/iovs.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson WH, Malinovsky VE, Dul MW, et al. Contrast sensitivity perimetry and clinical measures of glaucomatous damage. Optom Vis Sci. 2014;91:1302–1311. doi: 10.1097/OPX.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curcio CA. Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 4.Jonas JB, Schmidt AM, Muller-Bergh JA, et al. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–2018. [PubMed] [Google Scholar]
- 5.Blanks JC, Torigoe Y, Hinton DR. Blanks RH. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–384. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 6.Hood DC, Fortune B, Arthur SN, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. 2008;17:519–528. doi: 10.1097/IJG.0b013e3181629a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong SW, Ahn MD, Kang SH. Im SK. Analysis of peripapillary retinal nerve fiber distribution in normal young adults. Invest Ophthalmol Vis Sci. 2010;51:3515–3523. doi: 10.1167/iovs.09-4888. [DOI] [PubMed] [Google Scholar]
- 8.Tariq YM, Li H, Burlutsky G. Mitchell P. Retinal nerve fiber layer and optic disc measurements by spectral domain OCT: normative values and associations in young adults. Eye (Lond) 2012;26:1563–1570. doi: 10.1038/eye.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood DC. Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medeiros FA, Zangwill LM, Anderson DR, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012;154:814–24 e1. doi: 10.1016/j.ajo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatham AJ, Weinreb RN, Zangwill LM, et al. Estimated retinal ganglion cell counts in glaucomatous eyes with localized retinal nerve fiber layer defects. Am J Ophthalmol. 2013;156:578–87 e1. doi: 10.1016/j.ajo.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros FA, Lisboa R, Weinreb RN, et al. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology. 2013;120:736–744. doi: 10.1016/j.ophtha.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meira-Freitas D, Lisboa R, Tatham A, et al. Predicting progression in glaucoma suspects with longitudinal estimates of retinal ganglion cell counts. Invest Ophthalmol Vis Sci. 2013;54:4174–4183. doi: 10.1167/iovs.12-11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatham AJ, Meira-Freitas D, Weinreb RN, et al. Estimation of retinal ganglion cell loss in glaucomatous eyes with a relative afferent pupillary defect. Invest Ophthalmol Vis Sci. 2014;55:513–522. doi: 10.1167/iovs.13-12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marvasti AH, Tatham AJ, Zangwill LM, et al. The relationship between visual field index and estimated number of retinal ganglion cells in glaucoma. PLoS One. 2013;8:e76590. doi: 10.1371/journal.pone.0076590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gracitelli CP, Tatham AJ, Zangwill LM, et al. Estimated rates of retinal ganglion cell loss in glaucomatous eyes with and without optic disc hemorrhages. PLoS One. 2014;9:e105611. doi: 10.1371/journal.pone.0105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatham AJ, Weinreb RN, Zangwill LM, et al. The relationship between cup-to-disc ratio and estimated number of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2013;54:3205–3214. doi: 10.1167/iovs.12-11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medeiros FA, Zangwill LM, Bowd C, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53:6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisboa R, Weinreb RN. Medeiros FA. Combining structure and function to evaluate glaucomatous progression: implications for the design of clinical trials. Curr Opin Pharmacol. 2013;13:115–122. doi: 10.1016/j.coph.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harwerth RS, Carter-Dawson L, Smith EL, 3rd, et al. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–3160. doi: 10.1167/iovs.04-0227. [DOI] [PubMed] [Google Scholar]
- 21.Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–2250. [PubMed] [Google Scholar]
- 22.Harwerth RS, Vilupuru AS, Rangaswamy NV. Smith EL., 3rd The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–773. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 23.Harwerth RS, Wheat JL, Fredette MJ. Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29:249–271. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harwerth RS. Charles F. Prentice Award Lecture 2006: a neuron doctrine for glaucoma. Optom Vis Sci. 2008;85:436–444. doi: 10.1097/OPX.0b013e31817841b5. [DOI] [PubMed] [Google Scholar]
- 25.FitzGibbon T. Taylor SF. Mean retinal ganglion cell axon diameter varies with location in the human retina. Jpn J Ophthalmol. 2012;56:631–637. doi: 10.1007/s10384-012-0185-9. [DOI] [PubMed] [Google Scholar]
- 26.Garway-Heath DF, Holder GE, Fitzke FW. Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–2220. [PubMed] [Google Scholar]
- 27.Alexander KR, Derlacki DJ, Fishman GA. Szlyk JP. Grating, vernier, and letter acuity in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992;33:3400–3406. [PubMed] [Google Scholar]
- 28.Patel NB, Garcia B. Harwerth RS. Influence of anterior segment power on the scan path and RNFL thickness using SD-OCT. Invest Ophthalmol Vis Sci. 2012;53:5788–5798. doi: 10.1167/iovs.12-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner SK, Demirel S, Johnson CA. Swanson WH. Assessment of linear-scale indices for perimetry in terms of progression in early glaucoma. Vision Res. 2011;51:1801–1810. doi: 10.1016/j.visres.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garway-Heath DF, Caprioli J, Fitzke FW. Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41:1774–1782. [PubMed] [Google Scholar]
- 31.Drasdo N, Millican CL, Katholi CR. Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007;47:2901–2911. doi: 10.1016/j.visres.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hood DC, Raza AS, de Moraes CG, et al. The nature of macular damage in glaucoma as revealed by averaging optical coherence tomography data. Transl Vis Sci Technol. 2012;1:3. doi: 10.1167/tvst.1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]


