Abstract
This paper reviews general approaches for applying activated carbon (AC) amendments as an in situ sediment treatment remedy. In situ sediment treatment involves targeted placement of amendments using installation options that fall into two general approaches: 1) directly applying a thin layer of amendments (which potentially incorporates weighting or binding materials) to surface sediment, with or without initial mixing; and 2) incorporating amendments into a premixed, blended cover material of clean sand or sediment, which is also applied to the sediment surface. Over the past decade, pilot- or full-scale field sediment treatment projects using AC—globally recognized as one of the most effective sorbents for organic contaminants—were completed or were underway at more than 25 field sites in the United States, Norway, and the Netherlands. Collectively, these field projects (along with numerous laboratory experiments) have demonstrated the efficacy of AC for in situ treatment in a range of contaminated sediment conditions. Results from experimental studies and field applications indicate that in situ sequestration and immobilization treatment of hydrophobic organic compounds using either installation approach can reduce porewater concentrations and biouptake significantly, often becoming more effective over time due to progressive mass transfer. Certain conditions, such as use in unstable sediment environments, should be taken into account to maximize AC effectiveness over long time periods. In situ treatment is generally less disruptive and less expensive than traditional sediment cleanup technologies such as dredging or isolation capping. Proper site-specific balancing of the potential benefits, risks, ecological effects, and costs of in situ treatment technologies (in this case, AC) relative to other sediment cleanup technologies is important to successful full-scale field application. Extensive experimental studies and field trials have shown that when applied correctly, in situ treatment via contaminant sequestration and immobilization using a sorbent material such as AC has progressed from an innovative sediment remediation approach to a proven, reliable technology. Integr Environ Assess Manag 2015; 11:195–207. © 2014 The Authors. Published 2014 SETAC.
Keywords: Activated carbon, Sediment, In situ treatment, Bioavailability, Remediation
Introduction
Sediments accumulated on the bottom of a waterbody are recognized as sinks for toxic substances and bioaccumulative chemicals and can be long-term reservoirs for chemicals that can be transferred via the food chain to invertebrates and fish (USEPA 2005). Establishing effective methods to reduce the ecological and human health risks contaminated sediment poses has been a regulatory priority in North America, Europe, and elsewhere since the 1970 s. Indeed, demonstrating risk reduction that is convincing to all stakeholders using traditional dredging and isolation capping approaches has been challenging (NRC 2007; Bridges et al. 2010). Although traditional approaches will continue to be an integral part of sediment cleanup remedies (e.g., when contaminated sediments are present in unstable environments), new remediation approaches are needed to either supplement or provide alternatives to existing methods.
In situ sediment treatment via contaminant sequestration and immobilization generally involves applying treatment amendments onto or into surface sediments (Luthy and Ghosh 2006; Supplemental Data Figure S1). This paper reviews the considerable advances in engineering approaches used to apply activated C (AC)-based treatment amendments in situ, summarizes field-scale demonstration pilots and full-scale applications performed through 2013, and describes lessons learned on the most promising application options. This paper also discusses the need for a balanced consideration of the potential benefits, ecological effects, and costs of in situ treatment using AC relative to other sediment cleanup technologies. The results of this work aim to identify a common set of features from engineering, chemistry, and ecology that could help guide and advance the use of in AC-based in situ sediment treatment in future sediment remediation projects.
Treatment Amendments and Mechanisms
Beginning in the early 2000s, encouraging results from laboratory tests and carefully controlled, small-scale field studies generated considerable interest in remediating, or managing, contaminated sediments in situ. Mechanisms to do so mainly suggested sorptive treatment amendments such as AC, organoclay, apatite, biochar, coke, zeolites, and zero valent iron (USEPA 2013a). Three of these amendments—AC, organoclay, and apatite—have been identified as particularly promising sorptive amendments for in situ sediment remediation (USEPA 2013b). Of these, AC has been used more widely in laboratory experiments and field-scale applications to control dissolved hydrophobic organic compounds (HOCs). This is largely because AC has been used successfully for decades as a stable treatment medium for water, wastewater, and air, and because early testing of sediment treatment with AC showed positive results.
Laboratory testing and field-scale applications of AC have demonstrated its effectiveness in reducing HOC bioavailability. Both natural and anthropogenic black carbonaceous particles in sediments, including soot, coal, and charcoal strongly bind HOCs, and the presence of these particles in sediments has been demonstrated to reduce biouptake and exposure substantially (Gustafsson et al. 1997; Cornelissen et al. 2005). Using engineered black carbons such as AC augments the native sequestration capacity of sediments, resulting in reduced in situ bioavailability of HOCs. When AC is applied at optimal, site-specific doses (often similar to the native organic C content of sediment), the porewater concentrations and bioavailability of HOCs can be reduced between 70% and 99%. Furthermore, AC-moderated HOC sequestration often becomes more effective over time due to progressive mass transfer (Millward et al. 2005; Zimmerman et al. 2005; Werner et al. 2006; Sun et al. 2009; Ghosh et al. 2011; Cho et al. 2012).
Given these promising results, in situ sediment treatment involving the use of AC amendments is receiving increased attention among scientists, engineers, and regulatory agencies seeking to expand the list of remedial technologies and address documented or perceived limitations associated with traditional sediment remediation technologies. Based on the authors' review, AC is now the most widely used in situ sediment sequestration and immobilization amendment worldwide.
A previous review of the in situ AC remediation approach (Ghosh et al. 2011) reported the results of laboratory studies and early pilot-scale trials, summarized treatment mechanisms, highlighted promising opportunities to use in situ amendments to reduce contaminant exposure risks, and identified potential barriers for using this innovative technology. Another critical review by Janssen and Beckingham (2013) summarized the dependence of HOC bioaccumulation on AC dose and particle size, as well as the potential impacts of AC amendments on benthic communities (e.g., higher AC dose and smaller AC particle size further reduce bioaccumulation of HOCs but may induce stress in some organisms). This paper builds on these earlier reviews, focusing on design and implementation approaches involving the use of AC for in situ sediment treatment and summarizing key lessons learned.
Demonstrating Efficacy in the Field
Until recently, a primary challenge for full-scale in situ treatment remedies has been that most experience has emerged from laboratory and limited field pilot studies. Through 2013, however, more than 25 field-scale demonstrations or full-scale projects spanning a range of environmental conditions were completed or underway in the United States, Norway, and the Netherlands (Table1 and Figure 1).
Table 1.
In situ sediment treatment using carbon-based sorbents (mainly AC): Summary of field-scale pilot demonstrations or full-scale projects
| Site number (see Figure 1) | Year(s) | Location | Contaminant(s) | Application area (hectares) | Carbon-based amendment(s) | Delivery method(s) | Average water depth during delivery (m) | Enhancement(s) | Application equipment | Primary reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2004 | Anacostia River, Washington, DC | PAHs | 0.2 | Coke Breeze | Geotextile mat | 8 | Armored cap | Crane | McDonough et al. (2007) |
| 2 | 2004, 2006 | Hunters Point, San Francisco, CA | PCBs, PAHs | 0.01 | AC (slurry) | Direct placement | <1 | Mechanical mixing (some areas) | Aquamog, slurry injection | Cho et al. (2009 and 2012) |
| 3 | 2006 | Grasse River, Massena, NY | PCBs | 0.2 | AC (slurry) | Direct placement | 5 | Mechanical mixing (some areas) | Tine sled injection, tiller (with and without mixing) | Beckingham et al. (2007); Alcoa (2007) |
| 4 | 2006, 2008 | Trondheim Harbor, Norway | PAHs, PCBs | 0.1 | AC (slurry) | Blended cover, direct placement | 5 | Armored cap (some areas) | Tremie, agricultural spreader | Cornelissen et al. (2011) |
| 5 | 2006 | Spokane River, Spokane, WA | PCBs | 1 | Bituminous Coal Fines (slurry) | Direct placement | 5 | Armored cap | Mechanical bucket | Anchor QEA (2007 and 2009) |
| 6 | 2009 | De Veenkampen, Netherlands | Clean Sediment | <0.01 | AC (slurry) | Direct placement | 1 | None | Laboratory rollerbank | Kupryianchyk et al. (2012) |
| 7 | 2009 | Greenlandsfjords, Norway | Dioxins/Furans | 5 | AC (slurry) | Blended cover | 30/100 | None | Tremie from hopper dredge | Cornelissen et al. (2012) |
| 8 | 2009 | Bailey Creek, Fort Eustis, VA | PCBs | 0.03 | AC (SediMite®) | Direct placement | 1 | None | Pneumatic spreader | Ghosh and Menzie (2012) |
| 9 | 2010 | Fiskerstrand Wharf, Ålesund, Norway | TBT | 0.2 | AC (slurry) | Blended cover | 40 | None | Tremie with biokalk | Eek and Schaanning (2012) |
| 10 | 2010 | Tittabawassee River, Midland, MI | Dioxins/Furans | 0.1 | AC (AquaGatetm), Biochar | Blended cover | <1 | None | Agricultural disc | Chai et al. (2013) |
| 11 | 2011 | Upper Canal Creek, Aberdeen, MD | PCBs, Mercury | 1 | AC (SediMite®, AquaGatetm, slurry) | Direct placement | <1 | None | Pneumatic spreader, bark blower, hydroseeder | Bleiler et al. (2013); Menzie et al. (2014) |
| 12 | 2011 | Lower Canal Creek, Aberdeen, MD | Mercury, PCBs | 0.04 | AC (SediMite®) | Direct placement | 1 | None | Agricultural spreader | Menzie et al. (2014) |
| 13 | 2011 to 2016 | Onondaga Lake, Syracuse, NY | Various Organic Chemicals | 110 | AC (slurry) | Blended cover | 5 | Armored cap | Hydraulic spreader | Parsons and Anchor QEA (2012) |
| 14 | 2011 | South River, Waynesboro, VA | Mercury | 0.02 | Biochar (Cowboy Charcoal®) | Direct placement | <1 | None | Pneumatic spreader | DuPont (2013) |
| 15 | 2011 | Sandefjord Harbor, Norway | PCBs, TBT, PAHs | 0.02 | AC (BioBloka) | Direct placement | 30 | None | Mechanical bucket | Lundh et al. (2013) |
| 16 | 2011 | Kirkebukten, Bergen Harbor, Norway | PCBs, TBT | 0.7 | AC (BioBloka) | Direct placement | 30 | Armored cap (some areas) | Mechanical bucket | Hjartland et al. (2013) |
| 17 | 2012 | Leirvik Sveis Shipyard, Sandefjord, Norway | PCBs, TBT, Various Metals | 0.9 | AC (BioBloka) | Direct placement | 30 | Armored cap (some areas) | Hydraulic spreader (up to 30-degree slopes) | Lundh et al. (2013) |
| 18 | 2012 | Naudodden, Farsund, Norway | PCBs, PAHs, TBT, Various Metals | 0.4 | AC (BioBloka) | Direct placement | 30 | Armored cap, habitat layer | Mechanical bucket | Lundh et al. (2013) |
| 19 | 2012 | Berry's Creek, East Rutherford, NJ | Mercury, PCBs | 0.01 | AC (SediMite®, granular) | Blended cover, direct placement | <1 | None | Pneumatic spreader | USEPA (2013c) |
| 20 | 2012 | Puget Sound Shipyard, Bremerton, WA | PCBs, Mercury | 0.2 | AC (AquaGatetm) | Direct placement | 15 | Armored cap | Telebelt® (under-pier) | Johnston et al. (2013) |
| 21 | 2012 | Custom Plywood, Anacortes, WA | Dioxins/Furans | 0.02 | AC (SediMite®) | Blended cover, direct placement | 8 | None | Agricultural spreader | WDOE (2012) |
| 22 | 2012 | Duwamish Slip 4, Seattle, WA | PCBs | 1 | AC (slurry) | Blended cover | 4 | Armored cap | Mechanical bucket | City of Seattle (2012) |
| 23 | 2013 | Mirror Lake, Dover, DE | PCBs, Mercury | 2 | AC (SediMite®) | Direct placement | 1 | None | Telebelt® and air horn | DNREC (2013) |
| 24 | 2013 | Passaic River Mile 10.9, Newark, NJ | Dioxin/Furans, PCBs | 2 | AC (AquaGatetm) | Blended cover | 1 | Armored cap | Telebelt® | In preparation |
| 25 | 2013 | Little Creek, Norfolk, VA | PCBs, various metals | 1 | AC (AquaGatetm) | Direct placement | 1 | None | Pneumatic spreader (under-pier) | In preparation |
AC, activated carbon; PAH, polynuclear aromatic hydrocarbon; PCB, polychlorinated biphenyl; TBT, tributyltin.
BioBlok is licensed by AquaBlok®.
Figure 1.
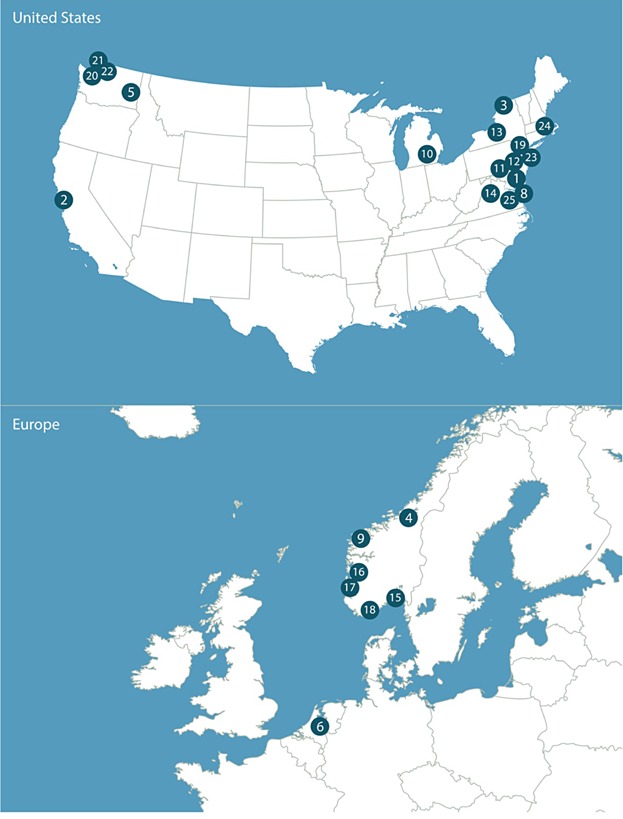
In situ sediment treatment field application sites (numbers refer to sites listed in Table1).
Among the more than 25 projects, field demonstrations in the lower Grasse River (Massena, NY, USA) and upper Canal Creek (Aberdeen, MD, USA) included the most comprehensive assessments and available documentation of the longer-term efficacy of the in situ AC remediation approach, although similar results have been reported for many of the other field projects. For this reason, the lower Grasse River and upper Canal Creek field demonstrations receive the greatest attention here, as summarized below.
Demonstration in lower Grasse River, Massena, New York
An AC pilot demonstration was conducted in the lower Grasse River as part of a program designed to evaluate available sediment cleanup options for the site. The demonstration study evaluated the effectiveness of AC as a means to sequester sediment polychlorinated biphenyls (PCBs) and reduce flux from sediments and uptake by biota.
The project began with laboratory studies and land-based equipment testing, and continued with field-scale testing of alternative placement methods. It culminated in a 2006 field demonstration of the most promising AC application and mixing methods to a 0.2-hectare pilot area of silt and fine sand sediments at average water depths of approximately 5 meters (Alcoa 2007; Beckingham and Ghosh 2011).
The following application techniques were implemented in the Grasse River (Supplemental Figure S2):
Applying (spraying) an AC slurry onto the submerged sediment surface and then mixing the material into near-surface sediments using a rototiller-type mechanical mixing unit (tiller)
Injecting an AC slurry directly into near-surface sediments using a tine sled device (tine sled)
Applying (spraying) an AC slurry onto the sediment surface within a temporary shroud enclosure, with no sediment mixing
All three application techniques successfully delivered the AC slurry onto or into surface sediments, and no detectable losses of AC to the water column or water quality impacts (e.g., turbidity monitored using instrumentation) were observed during placement (Alcoa 2007). A chemical oxidation method developed by Grossman and Ghosh (2009) was used to quantitatively confirm AC doses delivered onto or into sediment. This particular analytical method was used because typical total organic C and thermal (375 °C) oxidation methods were found to be imprecise and inaccurate, respectively, for AC analysis in sediment. Spraying the slurry onto the sediment successfully delivered AC to the sediment surface, and both the tiller with mixing and the tine sled applied all of the delivered AC into the 0- to 15-cm sediment layer. The tine sled application achieved more spatially (laterally) uniform doses, with an average AC concentration delivered to the 0- to 15-cm sediment layer of approximately 6.1 ± 0.8% AC (dry wt; ± 1 SE around the mean based on core and surface grab sample data). This target (and applied) dose was approximately 1.5× the native organic C content of the lower Grasse River. Cost comparisons of the different placement techniques indicate the tine sled unit would be a more cost-effective delivery method under full-scale deployment.
Detailed post-construction monitoring of the AC pilot area was performed in 2007, 2008, and 2009 (Beckingham and Ghosh 2011). Key findings are summarized below:
AC addition decreased sediment porewater PCB concentrations, and reductions improved during the 3-year, post-placement monitoring period. Greater than 99% reductions in PCB aqueous equilibrium concentrations were observed during the third year of postplacement monitoring in plots where the AC dose in the 0- to 15-cm layer was 4% or greater (Figure 2), effectively demonstrating that PCB flux from sediments to surface water was almost completely contained.
AC addition decreased PCB bioavailability as measured by in situ and ex situ bioaccumulation testing (using Lumbriculus variegatus). The overall decrease improved during the 3-year, post-placement monitoring period, with greater than 90% reductions observed during the third year of post-placement monitoring in plots where the AC dose in the 0- to 15-cm layer was greater than 4% (Figure 2).
Benthic recolonization occurred rapidly after application and no changes to the benthic community structure or number of individuals were observed in AC amendment plots relative to background (Beckingham et al. 2013).
In laboratory studies using site sediment, aquatic plants grew at a moderately reduced rate (approximately 25% less than controls) in sediment amended with a dose of greater than 5% AC. The reduced growth rate was likely attributable to nutrient dilution of the sediment (Beckingham et al. 2013).
Although other project data (not shown) indicated the AC amendment slightly increased the erosion potential of sediments (although within the range of historical data for native sediments), all of the delivered AC remained in the sediments throughout the 3-year, post-placement monitoring period.
Up to several centimeters of relatively clean, newly deposited sediment accumulated on the sediment surface in the pilot area over the 3-year, postplacement monitoring period. Passive sampling measurements revealed a downward flux of freely dissolved PCBs from the overlying water column into the AC amended sediments throughout the postconstruction monitoring period. This suggested that the placed AC will continue to reduce PCB flux from sediments in the long term.
Figure 2.
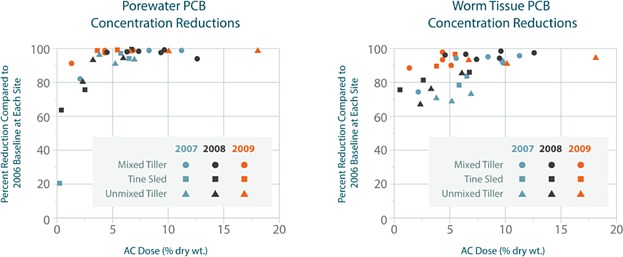
Reductions in porewater and worm tissue PCB concentrations at lower Grasse River, NY.
Demonstrations in upper Canal Creek, Aberdeen Proving Ground, Maryland
Two interrelated, pilot-scale, field demonstration projects were performed in 2011 to evaluate AC amendment additions to hydric soils at a tidal estuarine wetland in upper Canal Creek, at the Aberdeen Proving Ground, Maryland. (A third, separate treatment study was also carried out in the channelized portion of lower Canal Creek, but those results are only described minimally here.)
The first demonstration pilot (Menzie et al. 2014) evaluated in situ treatment with SediMite® pellets, a proprietary system for delivering powdered AC treatment materials with a weighting agent and an inert binder (Ghosh and Menzie 2012). The second demonstration pilot (Bleiler et al. 2013) evaluated two different powdered AC-bearing treatment materials: AquaGate + PACtm (AquaGate) and a slurry containing AC. The proprietary AquaGate product typically includes a dense aggregate core, along with clay-sized materials, polymers, and powdered AC additives. For both field demonstrations and all AC-bearing materials, the objective was to reduce PCB exposure to invertebrates living on or within surface sediments of the wetland area and thus reduce exposure to wildlife that might feed on these invertebrates.
All three AC-containing treatment materials for these pilot projects were applied onto the surface of the wetland and creek sediments during seasonal and tidal conditions with little or no overlying water. A total of 20 plots (each 8 × 78 meters) were used for the demonstration projects; sampling was conducted prior to application and at 6 and 10 months following application. Performance measurements used in one or both of the pilot projects included porewater and macroinvertebrate tissue PCB concentrations; phytotoxicity bioassays; ecological community abundance, diversity, and growth surveys; and nutrient uptake studies. Treatment efficacy was evaluated by comparing pre- versus post-treatment metrics and by evaluating treated plots relative to control (no action) and conventional sand cap plots.
The three treatment materials—SediMite®, AquaGate, and AC in a slurry—were applied using a pneumatic spreader, a bark blower, and a hydroseeder, respectively (Supplemental Figure S3). Figure S3 also shows a barge-mounted agricultural spreader that was used to demonstrate delivery of SediMite® to a portion of lower Canal Creek.
For both field demonstrations and all AC-bearing materials, the treatment goal was to achieve a 3% to 7% (dry wt) AC concentration in wetland surface sediment, which was operationally defined as the upper 10 cm (SediMite® studies) and 15 cm (AquaGate and slurry studies). Because the materials contained different amounts of AC, the applications differed in target thickness on the wetland surface. SediMite® contains approximately 50% AC by dry weight, so the target dose of 5% in the top 10 cm of sediment resulted in a target amendment layer thickness of roughly 0.7 cm. In contrast, AquaGate contained a coating of 5% powdered AC and was thus applied as a thicker 3-cm to 5-cm target layer over the sediment. The slurry system delivered roughly 0.2 cm to 0.5 cm of concentrated AC on the surface of the marsh. All of the treatments relied on natural processes (bioturbation, sediment deposition, and other physical processes) to mix AC placed onto the sediment surface into the wetland and creek sediment over time (see post-construction monitoring discussion below).
The AC amendments were applied effectively onto wetland and creek sediments in all of the applications. Measurements made over time indicated that close to 100% of the AC was retained within the plots, but vertical mixing into native wetland sediments via natural processes was slower than originally anticipated. As a result of low bioturbation rates, AC applied in more concentrated forms (i.e., as SediMite® and as AC in a slurry) remained at concentrations greater than the target dose of 5% in the upper 2 cm of the wetland sediment layer 10 months following application (Supplemental Data Figure S4). During the 10-month, post-application monitoring period, AC was incorporated into the biologically active zone largely from localized root elongation processes (Bleiler et al. 2013). Based on the two post-application monitoring rounds, approximately 60% of the recovered AC was found in the top 2 cm of sediment, whereas the remaining 40% penetrated mostly in the 2- to 5-cm depth interval. It is expected that further incorporation of the AC into the deeper layers of sediment will occur slowly over time via natural mixing processes and deposition of new sediment and organic matter.
The effectiveness of the AC amendments applied to the upper Canal Creek wetlands was assessed by measuring reductions in PCB concentrations in porewater (in situ measurements) and macroinvertebrate tissue (ex situ bioaccumulation testing). PCB concentrations exhibited a large spatial variability (1 order of magnitude) and vertical variability (up to 2 orders of magnitude within a sediment depth of 20 cm) in sediments across the plots, which was a site condition before the AC was applied. This finding posed some challenges in interpreting data and was therefore taken into account when evaluating other metrics. The findings of the upper Canal Creek demonstration pilot are reported in detail in Menzie et al. (2014) and Bleiler et al. (2013).
Regardless of the above challenges, all AC-treated wetland plots showed reduced PCB bioavailability as measured by reductions in both benthic organism tissue and porewater concentrations during the post-application monitoring period. In addition, no significant phytotoxicity or changes in species abundance, richness or diversity, vegetative cover, or shoot weight or length were observed between the AC treatment and control plots. Furthermore, plant nutrient uptake in the AC treatment plots was not significantly lower than control plots. Although the overall findings of these pilot projects suggest that adding AC can sequester PCBs in wetland sediments, more monitoring will take place given the slow mixing of the placed AC into the underlying wetland and creek sediments.
The lower Grasse River and upper Canal Creek projects, along with the other field-scale projects summarized in Table1, collectively demonstrate the efficacy of full-scale in situ sediment sequestration and immobilization treatment technologies. Such efforts reduce the bioavailability and mobility of several HOC and other contaminants, including PCBs, polynuclear aromatic hydrocarbons, dioxins and furans, tributyltin, methylmercury, and similar chemicals. Results from these field applications indicate that in situ treatment of contaminants can reduce risks rapidly by addressing key exposures (e.g., bioaccumulation in invertebrates), often becoming more effective over time due to progressive mass transfer.
Application Methods and Examples
The AC application projects summarized in Table1 involved placing amendments using several options that fall into two broad categories (Figure 3):
Figure 3.
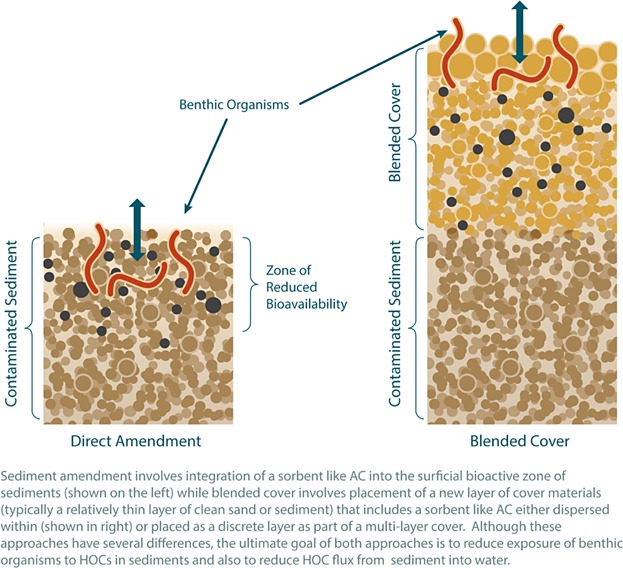
Direct amendment versus blended cover application methods for in situ sorbent application.
Direct application of a thin layer of sorptive, carbon-based amendments (which potentially incorporates weighting or binding materials) onto the surface sediment, with or without initial mixing
Incorporating amendments into a pre-mixed, blended cover material of clean sand or sediment, which is also applied onto the sediment surface
Although these approaches have several differences, the ultimate goal of both is to reduce exposure of benthic organisms to HOCs in sediment and reduce HOC flux from sediment into water (Figure 3). Under either approach, the applied AC may mix eventually throughout the biologically active layer via bioturbation. Application methods are described further in the next sections.
Direct application method
Using this approach, the bioavailability of HOCs in surface sediments is reduced by directly applying a strong carbon-based sorbent such as AC. At the lower Grasse River, upper Canal Creek, and many other field demonstration or full-scale projects (Table1), AC amendment was applied successfully using several methods with or without mixing, weighting agents, inert binders, or other proprietary systems. The specific application method was optimized to site-specific conditions. Adding weighting agents or inert binders can often improve the placement accuracy of finer-grained AC materials.
When the amendment introduced consists primarily of the sorbent, the direct application approach introduces minimal new material (an advantage), with little or no change in bathymetry or ecological habitat including the sediment's physical and mineralogical characteristics. Applying amendment to sediment surfaces also allows for some capacity to treat new contaminated sediments that may be deposited after constructing the remedy. This approach may have particular advantages at ecologically sensitive sites, where maintaining water depth is critical, and also where the potential for erosion is low.
The Delaware Department of Natural Resources and Environmental Control conceived and funded the first full-scale example of direct placement of AC in the United States, which was implemented in Mirror Lake, a reservoir on the St. Jones River in Dover, Delaware (Table1; Site 23). The sediment cleanup remedy at this site aimed to enhance the sorption capacity of native sediments in the lake, such that PCB bioavailability to the food chain is reduced without greatly altering the existing sediment bed. The remedy included placing SediMite® over an approximate 2-hectare area in the lake and river, along with integrated habitat restoration (DNREC 2013).
Placing AC at Mirror Lake was performed in the fall of 2013 using two application methods (Supplemental Figure S5): a Telebelt® application for the most accessible parts of the lake and an air horn device to pneumatically deliver SediMite® from a boat and along nearshore areas. Heavy equipment could not be deployed in the lake due to shallow water depth (averaging roughly 1 meter), as well as soft bottom sediments. The SediMite® application was completed safely in approximately 2 weeks. The target (and measured) thickness of the applied SediMite® material was approximately 0.7 cm, with the material expected to integrate naturally into the surficial sediment over time. Grab samples (13 stations) were collected from the top 10 cm of sediment in the lake 2 weeks after application to measure AC based on a method described in Grossman and Ghosh (2009). Applying SediMite® achieved an average AC dose of 4.3 ± 1.6% (Supplemental Data Figure S6).
Blended cover application method
The blended cover application method is a variation of the enhanced natural recovery remedy described by the US Environmental Protection Agency (USEPA 2005). In this approach, the carbon-based sorbent material is premixed with relatively inert materials such as clean sand or sediment and placed onto the contaminated sediment surface. Although this approach involves introducing materials in addition to the sorbent, it may have advantages at sites where a more spatially (vertically and laterally) uniform application of AC to the sediment surface is desired (because the AC can be mixed more thoroughly with the sand or sediment) or where more rapid control of HOC flux is desired.
Laboratory experiments and modeling studies (Murphy et al. 2006; Eek et al. 2008; Gidley et al. 2012), as well as field demonstrations (McDonough et al. 2007; Cornelissen et al. 2011, 2012) have confirmed the effectiveness of the blended cover application approach in reducing flux of mobile HOCs. At sites where additional isolation or erosion protection of underlying contaminated sediments may be needed, a related but separate option is to apply the sorbent as a layer within a conventional armored isolation cap. This paper, however, does not review either conventional or reactive isolation caps as defined by the USEPA (2005).
A full-scale example of blended AC application began in 2012 at Onondaga Lake, located in Syracuse, New York. The sediment cleanup remedy included placing bulk granular AC (GAC) blended with clean sand over approximately 110 hectares of lake sediments, along with related armored capping, dredging, and habitat restoration actions (NYSDEC and USEPA 2005; Parsons and Anchor QEA 2012). Full-scale implementation began following a successful field demonstration in fall 2011 and is currently scheduled to be completed in 2016.
Placing the blended GAC material in Onondaga Lake is being accomplished using a hydraulic spreading unit with advanced monitoring and control systems capable of placing approximately 100 cubic meters per hour of material in 6-meter-wide lanes (Figure 4). Granular AC amendment is mixed with sand and hydraulically transported and spread over sediment (average water depth of approximately 5 meters) through a diffuser barge. The GAC is presoaked for at least 8 hr prior to hydraulic mixing with the sand, to improve the settlement of the GAC through the water column. The spreader barge is equipped with an energy diffuser to distribute the blended materials evenly. The spreader barge incorporates electronic position tracking equipment and software so that the location of material placement can be tracked in real time. The spreader barge is also equipped with instruments for measuring the density of the slurry and the flow rates, which together provide the instantaneous production rate of the blended material being placed. Granular AC application rates are also tightly controlled and monitored using peristaltic metering pumps and a slurry density flow meter. The land-based slurry feed system is metered to the desired GAC dose.
Figure 4.

Hydraulic spreading application unit at Onondaga Lake, Syracuse, NY.
Through the first 2 years of the 5-year construction project, the blended GAC material was placed in Onondaga Lake without any detectable losses to the water column. Verifying GAC placement was performed using both in situ catch pans located on the sediment surface prior to placement, as well as cores collected after placement. Results of these verifications demonstrated that the GAC was placed uniformly both horizontally and vertically within the sand layer applied to the lake (Supplemental Data Figure S7).
Site Evaluation and Design Considerations
The more than 25 field-scale demonstrations or full-scale projects performed through 2013 span a range of application methods and environmental conditions (including marine, brackish, and freshwater sites; tidal wetlands and mudflats; deep depths; steep slopes; under piers; and moving water [Table1]). Collectively, these projects demonstrate the efficacy of in situ sediment treatment using sorptive, carbon-based amendments, particularly AC. As a result, in situ sediment treatment using AC is ready for full-scale application at a range of sites, subject to careful site-specific design analyses, generally as outlined in the next paragraphs.
To determine if site conditions are favorable for AC amendment, relatively simple bench testing of AC amendments can be performed by mechanically mixing AC into the sediments and performing straightforward porewater or bioaccumulation testing (e.g., Sun and Ghosh 2007). Short-term bench testing performed in this manner can rapidly identify sediment sites that are amenable to sediment treatment with AC and can be coupled with focused modeling or column studies to evaluate HOC behavior associated with groundwater flux. Bench testing can also be used to optimize AC materials (e.g., grain size or porosity) and dosing based on site-specific conditions. (Note that at most of the sites listed in Table1, optimal AC doses were similar to the native organic C content of sediment.)
Although much has been learned to date, additional focused field-scale demonstrations may be particularly helpful to evaluate certain site-specific HOCs such as dioxins, furans, and methylmercury for which treatment effectiveness has been either variable or slow to develop (i.e., after the AC is mixed in) and in environments where sorptive carbon-based amendments have not yet been piloted (e.g., high-energy, erosion-prone locations). It is also important to note that at some sites, AC application may not provide additional protection compared to traditional sediment cleanup technologies. For example, mixing AC into a blended cover at Grenlandsfjords, Norway resulted in only marginal additional dioxin and furan flux reductions at 9 and 20 months compared with unamended clean sand or sediment cover materials, attributable in part to relatively slow sediment-to-AC transfer rates for large molecular volume dioxins and furans (Cornelissen et al. 2012; Eek and Schaanning 2012).
Based on a critical review of the results of the field-scale projects listed in Table1, specific-site and sediment characteristics can reduce the effectiveness of AC application compared to other potential sediment cleanup technologies. These characteristics include (but are not likely limited to) relatively high native concentrations of black carbonaceous particles and slow sediment-to-AC transfer rates for relatively large molecular volume HOCs (Choi et al. 2014). Properly accounting for these and factors such as erosional forces and mixing or bioturbation in site-specific AC application design is necessary to ensure the effectiveness of the in situ remedial approach.
Experimental, modeling, and long-term monitoring lines of evidence from the case studies summarized in Table1 have all confirmed that the effectiveness of AC applications increases over time at sites where there is not a significant flux from the underlying sediment to the surface. In many settings, full treatment effectiveness of AC amendments is achieved years after installation (e.g., Werner et al. 2006; Cho et al. 2012). The delay can be caused by (among other factors) the heterogeneity of AC distribution (even on a small scale), particularly at sites with relatively low bioturbation rates, as well as progressive mass transfer (Figure 5).
Figure 5.
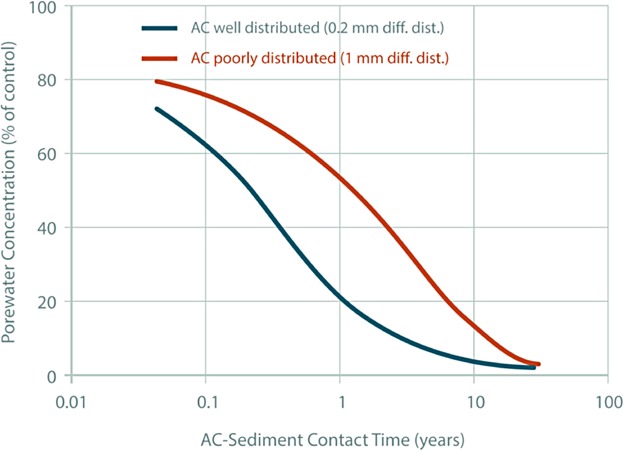
Model simulations of porewater PCB concentration reductions with different mixing scenarios (adapted from Cho et al. 2012).
Site-specific evaluations of natural sediment deposition and bioturbation rates (as well as ongoing contaminant sources) and their effect on AC mixing and resultant restoration time frames are important design factors in developing appropriate site-specific in situ treatment strategies. Rates of natural sediment deposition and bioturbation-induced mixing of AC into the biologically active zone vary widely between sediment environments. For example, surface sediment bioturbation rates have been shown to vary more than 2 orders of magnitude between sediment environments, with relatively lower rates in wetlands and offshore sediments and relatively higher rates in productive estuaries and lakes (e.g., Officer and Lynch 1989; Wheatcroft and Martin 1996; Sandnes et al. 2000; Parsons and Anchor QEA 2012; Menzie et al. 2014). If relatively slow rates of natural deposition and mixing are anticipated, applying AC directly could be staggered over multiple applications to incorporate the amendment more evenly into the depositing sediments, albeit with potential cost implications.
As the USEPA (2005), NRC (2007), Bridges et al. (2010), ITRC (2014), and others have emphasized, the effectiveness of all sediment cleanup technologies depends significantly on sediment- and site-specific conditions. For example, resuspension and release of sediment contaminants occurs during environmental dredging, particularly at sites with debris and other difficult dredging conditions (Patmont et al. 2013). Optimizing risk management at contaminated sediment sites can often be informed by comparative evaluations of sediment cleanup technologies applied to site-specific conditions, considering quantitative estimates of risk reduction, risk of remedy, and remedy cost (e.g., Bridges et al. 2012). A hypothetical comparative risk reduction evaluation is presented in Figure 6 and highlights some of the short- and long-term tradeoffs that can occur between different sediment remediation technologies. Consistent with the example presented in Figure 6, at many sites, AC placement can achieve risk reductions similar to conventional capping but at a lower cost (see below), and may also provide better overall risk reduction than environmental dredging. Although Figure 6 presents a relatively common sediment remedial alternatives evaluation scenario in North America, it is important to note that site-specific conditions will result in varying risk reduction outcomes from alternative sediment remedies.
Figure 6.
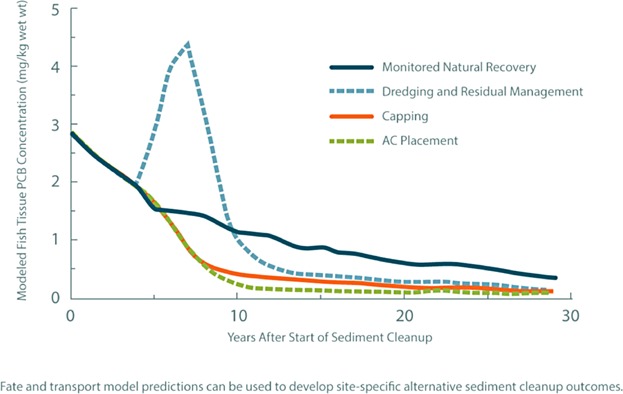
Hypothetical comparative net risk reduction of alternative sediment remedies. Example presented for illustrative purposes using the following fate and transport model input assumptions: average environmental dredge production rate of 400 m3 per day and release of 3% of the PCB mass dredged (Patmont et al. 2013); average water flow through the cleanup area of 500 m3 per second; implementation of effective upstream source controls; net sedimentation rate of 0.1 cm per year; and typical PCB mobility and bioaccumulation parameters.
Potential Negative Ecological Impacts
The acceptability of any sediment remediation option will depend on whether the benefits of the approach outweigh potential adverse environmental or ecological impacts, compared to other options. Because in situ treatment technologies involve adding a new material to sediments, in situ remedies have the potential to impact the native benthic community and vegetation, at least temporarily. A recent review by Janssen and Beckingham (2013) found that impacts to benthic organisms resulting from AC exposure were observed in one-fifth of 82 tests (primarily laboratory studies). Importantly, community effects have been observed more rarely in AC field pilot demonstrations compared to laboratory tests and often diminish within 1 or 2 years following placement (Cornelissen et al. 2011; Kupryianchyk et al. 2012), particularly in depositional environments where new (typically cleaner) sediment continues to deposit over time.
Although applying relatively higher AC doses or smaller AC particle sizes provide greater bioaccumulation reductions of HOCs, higher doses and smaller particle size may induce greater stress in some organisms (Beckingham et al. 2013). Negative impacts to benthic macroinvertebrates and aquatic plants resulting from adding AC, particularly at relatively high doses, may be attributable to nutrient reductions associated with AC amendment.
Although the available dose–dependent effects data for AC are not comprehensive, field trials and experimental studies suggest that potential negative ecological effects can be minimized by maintaining finer-grained AC doses below approximately 5% (dry wt basis; e.g., see discussion of the lower Grasse River AC demonstration). Similar to the net risk reduction comparisons summarized in Figure 6, the positive effects of reduced bioaccumulation of HOCs need to be balanced against potential negative short-term impacts. In addition, site-specific outcomes from in situ AC applications should be compared with outcomes resulting from other remediation approaches such as dredging and conventional capping, which are often greater than those resulting from in situ treatment.
Relative Sustainability of Different Carbon Amendments
Although amendments produced from different carbon source materials often exhibit similar effectiveness and negative ecological effects, different types of C amendments have different sustainability attributes. For example, life cycle analyses have demonstrated that AC produced from anthracite coal is less sustainable than AC produced from biomass feedstock (Sparrevik et al. 2011; e.g., agricultural residues), even though anthracite-derived AC may bind HOCs very effectively (Josefsson et al. 2012). One important positive effect of biomass AC related to sustainability is that its C is sequestered and removed from the global C cycle (Sparrevik et al. 2011). Even better sustainability outcomes can result from using non-activated pyrolyzed C, or “biochar” (Ahmad et al. 2014), because considerable amounts of energy are required for the activation process. However, the sorption capacity of biochars for many HOCs is more than an order of magnitude lower than AC (Gomez-Eyles et al. 2013).
Cost
Based on a critical review of the field-scale projects listed in Table1 for which adequate cost information was available, we summarized approximate low- and high-range unit costs for a full-scale AC application to a hypothetical 5-hectare sediment cleanup site. Cost summaries for the primary implementation components, not all of which may be needed at a particular site, are summarized in Table2. Based on this summary, AC application is often likely to be less costly than either traditional dredging or capping approaches. Again, site-specific conditions can result in varying cost outcomes from alternative sediment remedies.
Table 2.
Summary of low- and high-range unit costs of AC applicationa
| Component | Low-range Unit Cost | High-range Unit Cost |
|---|---|---|
| Activated Carbonb | $50,000/hectare | $100,000/hectare |
| Facilitating AC Placement Using Binder/Weighting Agentsc | $0/hectare | $70,000/hectare |
| Facilitating AC Placement by Blending with Sediment or Sandc | $0/hectare | $100,000/hectare |
| Field Placement | $30,000/hectare | $200,000/hectare |
| Long-term Monitoring | $20,000/hectare | $100,000/hectared |
| Total | $100,000/hectare | $500,000/hectare |
Estimated costs for a 4 percent AC dose (dry weight basis) over the top 10-cm sediment layer at a 5-hectare site.
Powdered activated carbon (PAC) and/or granular activated carbon (GAC), depending on site-specific designs.
To facilitate AC placement, binder or weighting agent amendments such as SediMite® or AquaGateTM, or clean sediment or sand (but typically not both) may be required in some applications depending on site-specific conditions and designs.
High-end monitoring cost of $100,000 per hectare reflects prior pilot projects and likely overestimates costs for full-scale remedy implementation.
Conclusion
In situ sediment treatment using AC can rapidly address key exposures (e.g., bioaccumulation in invertebrates and fish), often becoming more effective over time due to progressive mass transfer. Due to its relatively large surface area, pore volume, and absorptive capacity, AC has a decades-long track record of effective use as a stable treatment medium in water, wastewater, and air. As such, AC is well suited for in situ sequestration and immobilization of HOCs in various sediment environments.
When designed correctly to address site-specific conditions, controlled (accurate and spatially uniform) placement of AC-bearing treatment materials has been demonstrated using a range of conventional construction equipment and delivery mechanisms and in a wide range of aquatic environments (Table1), including wetlands. When contaminated sediments are present in unstable environments, traditional capping or dredging remedies might be the preferred option. Depending on sediment and site conditions, however, using AC can achieve short-term risk reduction similar to conventional capping and better overall risk reduction than environmental dredging, with lower costs and environmental impacts than traditional sediment cleanup technologies.
With a growing international emphasis on sustainability, in situ sediment treatment remedies offer an opportunity to realize significant environmental benefits, while avoiding the environmental impacts often associated with more invasive sediment cleanup technologies. Less invasive remediation strategies—such as treatment using in situ AC applications—are also typically far less disruptive to communities and stakeholders than dredging or conventional capping remedies. Important environmental, economic, and other sustainability issues can be associated with in situ sediment treatment, such as low-impact reduction of the bioavailable or mobile fractions of sediment contaminants through sequestration, improved recovery time frames, and reduced energy use and emissions (e.g., C; ITRC 2014).
Proper site-specific balancing of the potential benefits, negative ecological effects, and costs of in situ treatment relative to other sediment cleanup technologies is important to applying this approach successfully at full-scale. As discussed in USEPA (2005) and ITRC (2014), at most sites, a combination of sediment cleanup technologies applied to specific zones within the sediment cleanup site will result in a remedy that achieves long-term protection while minimizing short-term negative impacts and achieving greater cost effectiveness. It is evident from the extensive experimental studies and field-scale projects presented here that when applied correctly, in situ treatment of sediment HOCs using sorptive, AC-bearing materials has progressed from an innovative sediment remediation approach to a proven, reliable technology. Indeed, it is one that is ready for full-scale remedial application in a range of aquatic sites.
Acknowledgments
The authors gratefully acknowledge the advice and support of the Sediment Management Work Group, whose members (including Steve Nadeau, Larry McShea, Bill Hague, Will Gala, Steve Brown, and Joe Chai, among others) provided encouragement and constructive reviews of early drafts of this paper.
Disclaimer
Views or opinions expressed in this paper do not necessarily reflect the policy or guidance of the USEPA.
Supporting Information
All Supplemental Data may be found in the online version of this article.
Figure S1. Simplified food chain model of in situ treatment.
Figure S2. Pilot area and tine sled or tiller application units at lower Grasse River, NY.
Figure S3. Dry broadcasting and slurry spray applications, Canal Creek, Aberdeen Proving Ground, MD.
Figure S4. Vertical distribution of AC in wetland sediments at Canal Creek, Aberdeen Proving Ground, MD.
Figure S5. SediMite® delivery at Mirror Lake, Dover, DE.
Figure S6. Post-placement surface sediment AC concentrations at Mirror Lake, Dover, DE.
Figure S7. Applied versus measured AC dose at Onondaga Lake, Syracuse, NY.
References
- Ahmad M, Rajapaksha A, Lim J, Zhang M, Bolan N, Mohan D, Vithanage M, Lee S, Ok Y. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Alcoa. 2007. Grasse River activated carbon pilot study: Construction documentation report. November. [cited 2014 April] Available from http://www.thegrasseriver.com/activated_carbon.html.
- Anchor QEA (formerly Anchor Environmental). 2007. Cleanup action completion report, Upriver Dam PCBs sediment site. [cited 2014 April] Available from https://fortress.wa.gov/ecy/gsp/Sitepage.aspx?csid=4213.
- Anchor QEA. 2009. Year 2 Upriver Dam Cap Monitoring Results, Project 080306–01. Memorandum to Brendan Dowling, Washington State Department of Ecology. [cited 2014 April]. Available from https://fortress.wa.gov/ecy/gsp/Sitepage.aspx?csid=4213.
- Beckingham B, Ghosh U. Field-scale reduction of PCB bioavailability with activated carbon amendment to river sediments. Environ Sci Technol. 2011;45:10567–10574. doi: 10.1021/es202218p. [DOI] [PubMed] [Google Scholar]
- Beckingham B, Buys D, Vandewalker H, Ghosh U. Observations of limited secondary effects to benthic invertebrates and macrophytes with activated carbon amendment in river sediments. Environ Toxicol Chem. 2013;32:1504–1515. doi: 10.1002/etc.2231. [DOI] [PubMed] [Google Scholar]
- Bleiler J, Gardner K, Greenwood S, McCarthy R, Ruiz N. 2013. Evaluation of activated-carbon treatment in a PCB-contaminated wetland, in soils/sediments. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 2013, 4–7 February, Dallas (TX) USA. Battelle. C–029.
- Bridges T, Gustavson K, Schroeder P, Ells S, Hayes D, Nadeau S, Palermo M, Patmont C. Dredging processes and remedy effectiveness: Relationship to the 4 Rs of environmental dredging. Integr Environ Assess Manag. 2010;6:619–630. doi: 10.1002/ieam.71. [DOI] [PubMed] [Google Scholar]
- Bridges T, Nadeau S, McCulloch M. Accelerating progress at contaminated sediment sites: Moving from guidance to practice. Integr Environ Assess Manag. 2012;8:331–338. doi: 10.1002/ieam.1271. [DOI] [PubMed] [Google Scholar]
- City of Seattle. 2012. Lower Duwamish Waterway Slip 4 Early Action Area: Removal Action Completion Report prepared for USEPA Region 10 by Integral Consulting and City of Seattle. July. [cited 2014 April]. Available from http://yosemite.epa.gov/r10/cleanup.nsf/ldw/slip+4+early+action+area.
- Chai Y, Davis J, Currie R, Fishman V, Martin G, Wilken M, Lucas S, Wandor D, Ghosh U. 2013. A field pilot study using activated carbon and biochar to reduce the availability of PCDD/Fs in soils/sediments. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 2013, 4–7 February, Dallas (TX) USA. Battelle. C–028.
- Cho Y, Ghosh U, Kennedy A, Grossman A, Ray G, Tomaszewski J, Smith H, Bridges T, Luthy R. Field application of activated carbon amendment for in-situ stabilization of polychlorinated biphenyls in marine sediment. Environ Sci Technol. 2009;43:3815–3823. doi: 10.1021/es802931c. [DOI] [PubMed] [Google Scholar]
- Cho Y, Werner D, Choi Y, Luthy R. Long-term monitoring and modeling of the mass transfer of polychlorinated biphenyls in sediment following pilot-scale in-situ amendment with activated carbon. J Contam Hydrol. 2012;129–130:25–37. doi: 10.1016/j.jconhyd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Choi Y, Cho Y, Werner D, Luthy R. In situ sequestration of hydrophobic organic contaminants in sediments under stagnant contact with activated carbon 2. Mass transfer modeling. Environ Sci Technol. 2014;48(3):1843–1850. doi: 10.1021/es404209v. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Gustafsson O, Bucheli T, Jonker M, Koelmans A, Van Noort P. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol. 2005;39:6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Kruså M, Breedveld G, Eek E, Oen A, Arp H, Raymond C, Samuelsson G, Hedman J, Stokland Ø, Gunnarsson J. Remediation of contaminated marine sediment using thin-layer capping with activated carbon—A field experiment in Trondheim Harbor, Norway. Environ Sci Technol. 2011;45:6110–6116. doi: 10.1021/es2011397. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Amstaetter K, Hauge A, Schaanning M, Beylich B, Gunnarsson J, Breedveld G, Oen A, Eek E. Large-scale field study on thin-layer capping of marine PCDD/F contaminated sediments in Grenlandfjords, Norway: Physicochemical effects. Environ Sci Technol. 2012;46:12030–12037. doi: 10.1021/es302431u. [DOI] [PubMed] [Google Scholar]
- [DNREC] Delaware Department of Natural Resource and Environmental Control. 2013. Mirror Lake Remediation and Restoration - 100% Plans and Specifications. DNREC Watershed Assessment Division, Dover, DE. February. [cited 2014 November] Available from http://www.dnrec.delaware.gov/swc/wa/Documents/Mirror_Lake_plan_02182013.pdf.
- DuPont. 2013. Use of a carbon amendment to reduce bio-uptake of mercury in a South River floodplain pond: Technical Briefing Paper. South River Remediation Proposal: Appendix A. Prepared by Anchor QEA, URS Corporation, and EI duPont de Nemours and Company. July. [cited 2014 April]. Available from http://southriverscienceteam.org/news/techdocs/SR_RemediationProposal_2013-10-23_Final.pdf.
- Eek E, Cornelissen G, Kibsgaard A, Breedveld G. Diffusion of PAH and PCB from contaminated sediments with and without mineral capping; measurement and modelling. Chemosphere. 2008;71:1629–1638. doi: 10.1016/j.chemosphere.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Eek E, Schaanning M. 2012. Opticap Report [cited 2014 April]. Available from http://www.ngi.no/upload/Prosjektweb/opticap/Opticap%20sluttrapport.pdf.
- Ghosh U, Menzie C. 2010. A low-impact delivery system for in-situ treatment of contaminated sediment. Washington DC: CNY US Patent Office. Patent 7,824,129.
- Ghosh U, Menzie C. 2012. Pilot-scale research of novel amendment delivery for in-situ sediment remediation. Final progress report. Grant 5R01ES16182. Submitted to NIEHS Superfund Research Program. August. [cited 2014 April]. Available from http://tools.niehs.nih.gov/srp/programs/Program_detail.cfm?Project_ID=R01ES16182.
- Ghosh U, Luthy R, Cornelissen G, Werner D, Menzie C. In situ sorbent amendments: A new direction in contaminated sediment management. Environ Sci Technol. 2011;45:1163–1168. doi: 10.1021/es102694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley P, Kwon S, Yakirevich A, Magar V, Ghosh U. Advection dominated transport of polycyclic aromatic hydrocarbons in amended sediment caps. Environ Sci Technol. 2012;46:5032–5039. doi: 10.1021/es202910c. [DOI] [PubMed] [Google Scholar]
- Gomez-Eyles J, Yupanqui C, Beckingham B, Riedel G, Gilmour C, Ghosh U. Evaluation of Biochars and Activated Carbons for In Situ Remediation of Sediments Impacted with Organics, Mercury and Methylmercury. Environ Sci Technol. 2013;47:13721–13729. doi: 10.1021/es403712q. [DOI] [PubMed] [Google Scholar]
- Grossman A, Ghosh U. Measurement of Activated Carbon and Other Black Carbons in Sediments. Chemosphere. 2009;75:469–475. doi: 10.1016/j.chemosphere.2008.12.054. [DOI] [PubMed] [Google Scholar]
- Gustafsson O, Haghseta F, Chan C, MacFarlane J, Gschwend P. Quantification of the Dilute Sedimentary Soot Phase: Implications for PAH Speciation and Bioavailability. Environ Sci Technol. 1997;31:203–209. [Google Scholar]
- Hjartland T, Jersak J, Collins J, Soldal O. 2013. Using Carbon-Enriched Materials for Capping Contaminated Sediments at the Kirkebukten Site in Bergen, Norway. In: Proceedings Seventh International Conference on Remediation of Contaminated Sediments, 2013, 4–7 February, Dallas (TX) USA. Battelle. C–060.
- [ITRC] Interstate Technology and Regulatory Council. 2014. Remedy selection for contaminated sediments. [cite 2014 August]. Available from http://www.itrcweb.org/contseds_remedy-selection/
- Janssen E, Beckingham B. Biological responses to activated carbon amendments in sediment remediation. Environ Sci Technol. 2013;47:7595–7607. doi: 10.1021/es401142e. [DOI] [PubMed] [Google Scholar]
- Josefsson S, Schaanning M, Samuelsson G, Gunnarsson J, Olofsson I, Eek E, Wiberg K. Capping efficiency of various carbonaceous and mineral materials for in situ remediation of polychlorinated dibenzo-p-dioxin and dibenzofuran contaminated marine sediments: Sediment-to-water fluxes and bioaccumulation in boxcosm tests. Environ Sci Technol. 2012;46:3343–3351. doi: 10.1021/es203528v. [DOI] [PubMed] [Google Scholar]
- Johnston R, Kirtay V, Chadwick D, Rosen G, Guerrero J, Collins J, Ortega C, Webb R, May R, Germano J, Browning D, Beaver E, Wicklein M, Pittz J, Leisle D, Doyle L, Hsu L. 2013. Installing an activated carbon sediment amendment at the Puget Sound Naval Shipyard and Intermediate Maintenance Facility, Bremerton, WA. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 2013, 4–7 February, Dallas (TX) USA. Battelle. B–024.
- Kupryianchyk D, Peters E, Rakowska M, Reichman E, Grotenhuis J, Koelmans A. Long-term recovery of benthic communities in sediments amended with activated carbon. Environ Sci Technol. 2012;46:10735–10742. doi: 10.1021/es302285h. [DOI] [PubMed] [Google Scholar]
- Lundh T, Hansen T, Nordvik H. 2013. Demonstrating in situ remedies for contaminated sediment in Norway: Applicability to Sandefjord Harbor and beyond. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 2013, 4–7 February, Dallas (TX) USA. Battelle. C–031.
- Luthy R, Ghosh U. 2006. In situ stabilization of persistent hydrophobic organic contaminants in sediments using coal- and wood-derived carbon sorbents. Washington DC: CNY US Patent Office. Patent 7,101,115.
- McDonough K, Murphy P, Olsta J, Yuewei Z, Reible D, Lowry GV. Development and placement of a sorbent-amended thin layer sediment cap in the Anacostia River. Soil Sediment Contam. 2007;16(3):313–322. [Google Scholar]
- Millward R, Bridges T, Ghosh U, Zimmerman J, Luthy R. Addition of activated carbon to sediments to reduce PCB bioaccumulation by a polychaete (Neanthes arenaceodentata) and an amphipod (Leptocheirus plumulosus. Environ Sci Technol. 2005;39:2880–2887. doi: 10.1021/es048768x. [DOI] [PubMed] [Google Scholar]
- Menzie C, Amos B, Kane-Driscoll S, Ghosh U, Gilmour C. 2014. Evaluating the efficacy of a low-impact delivery system for in-situ treatment of sediments contaminated with methylmercury and other hydrophobic chemicals. ESTCP Environmental Restoration Project ER-200835. [cited 2014 November]. Available from http://www.serdp.org/Program-Areas/Environmental-Restoration/Contaminated-Sediments/ER-200835.
- Murphy P, Marquette A, Reible D, Lowry G. Predicting the performance of activated carbon-, coke-, and soil-amended thin layer sediment caps. J Environ Eng. 2006;132:787–794. [Google Scholar]
- [NRC] National Research Council. 2007. Sediment dredging at superfund megasites: Assessing the effectiveness. The National Academies Press: Washington DC. [cited 2014 April]. Available from http://www.nap.edu/catalog.php?record_id=11968.
- [NYSDEC] New York State Department of Environmental Conservation and [USEPA] US Environmental Protection Agency. 2005. Record of Decision: Onondaga Lake bottom subsite of the Onondaga Lake superfund site. NYSDEC, Albany, NY and USEPA Region II, New York, NY. July. [cited 2014 April]. Available from http://www.dec.ny.gov/chemical/34481.html.
- Officer C, Lynch D. Bioturbation, sedimentation, and sediment–Water exchanges. Estuarine, Coastal, and Shelf Science. 1989;28:1–12. [Google Scholar]
- Parsons and Anchor QEA. 2012. Onondaga Lake capping, dredging, habitat and profundal zone (sediment management unit 8) final design. Prepared for Honeywell. March. [cited 2014 April]. Available from http://www.dec.ny.gov/chemical/83049.html.
- Patmont C, Nadeau S, McCulloch M. 2013. Learning from the past to enhance remedy evaluation, selection, and implementation. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 2013, 4–7 February, Dallas (TX) USA. Battelle. B–049.
- Sandnes J, Forbes T, Hansen R, Sandnes B, Rygg B. Bioturbation and irrigation in natural sediments, described by animal-community parameters. Mar Ecol Prog Ser. 2000;197:169–179. [Google Scholar]
- Sparrevik M, Saloranta T, Cornelissen G, Eek E, Fet A, Breedveld G, Linkov I. Use of life cycle assessments to evaluate the environmental footprint of contaminated sediment remediation. Environ Sci Technol. 2011;45:4235–4241. doi: 10.1021/es103925u. [DOI] [PubMed] [Google Scholar]
- Sun X, Ghosh U. PCB bioavailability control in Lumbriculus Variegatus through different modes of activated carbon addition to sediments. Environ Sci Technol. 2007;41:4774–4780. doi: 10.1021/es062934e. [DOI] [PubMed] [Google Scholar]
- Sun X, Werner D, Ghosh U. Modeling PCB mass transfer and bioaccumulation in a freshwater oligochaete before and after amendment of sediment with activated carbon. Environ Sci Technol. 2009;43:1115–1121. doi: 10.1021/es801901q. [DOI] [PubMed] [Google Scholar]
- [USEPA] US Environmental Protection Agency. 2005. Contaminated sediment remediation guidance for hazardous waste sites. EPA-540-R-05–012. [cited 2014 April]. Available from http://www.epa.gov/superfund/health/conmedia/sediment/pdfs/cover.pdf.
- 2013a. [USEPA] US Environmental Protection Agency Use of amendments for in situ remediation at superfund sediment sites. Office of Superfund Remediation and Technology Innovation. OSWER Directive 9200.2–128FS. April. [cited 2014 April]. http://www.epa.gov/superfund/health/conmedia/sediment/pdfs/In_situ_AmendmentReportandAppendix_FinalApril2013.pdf.
- 2013b. [USEPA] US Environmental Protection Agency. Superfund remedial program review action plan. Washington DC. November. [cited 2014 April]. Available from: http://www.epa.gov/superfund/cleanup/pdfs/Final_SPR_Action_Plan-11_26_2013_(2).pdf.
- 2013c. [USEPA] US Environmental Protection Agency. Berry's Creek study area: Community update. USEPA Region 2, New York, NY. August. [cited 2014 April]. Available from: http://www.epa.gov/region2/superfund/npl/berryscreek/bcsa_comunityupdate_%20august2013.pdf.
- [WDOE] Washington State Department of Ecology. 2012. Custom Plywood Interim Action: Thin-layer capping pilot study, Anacortes, Washington. Work plan prepared by Hart Crowser, Inc. July. [cited 2014 April]. Available from https://fortress.wa.gov/ecy/gsp/CleanupSiteDocuments.aspx?csid=4533.
- Werner D, Ghosh U, Luthy R. Modeling polychlorinated biphenyl mass transfer after amendment of contaminated sediment with activated carbon. Environ Sci Technol. 2006;40:4211–4218. doi: 10.1021/es052215k. [DOI] [PubMed] [Google Scholar]
- Wheatcroft R, Martin W. Spatial variation in short-term (234Th) sediment bioturbation intensity along an organic-carbon gradient. J Mar Res. 1996;54:763–792. [Google Scholar]
- Zimmerman J, Werner D, Ghosh U, Millward R, Bridges T, Luthy R. The effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environ Toxicol Chem. 2005;24:1594–1601. doi: 10.1897/04-368r.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Simplified food chain model of in situ treatment.
Figure S2. Pilot area and tine sled or tiller application units at lower Grasse River, NY.
Figure S3. Dry broadcasting and slurry spray applications, Canal Creek, Aberdeen Proving Ground, MD.
Figure S4. Vertical distribution of AC in wetland sediments at Canal Creek, Aberdeen Proving Ground, MD.
Figure S5. SediMite® delivery at Mirror Lake, Dover, DE.
Figure S6. Post-placement surface sediment AC concentrations at Mirror Lake, Dover, DE.
Figure S7. Applied versus measured AC dose at Onondaga Lake, Syracuse, NY.


