Abstract
Down syndrome is a common disorder associated with intellectual disability in humans. Among a variety of severe health problems, patients with Down syndrome exhibit disrupted sleep and abnormal 24-h rest/activity patterns. The transchromosomic mouse model of Down syndrome, Tc1, is a trans-species mouse model for Down syndrome, carrying most of human chromosome 21 in addition to the normal complement of mouse chromosomes and expresses many of the phenotypes characteristic of Down syndrome. To date, however, sleep and circadian rhythms have not been characterized in Tc1 mice. Using both circadian wheel-running analysis and video-based sleep scoring, we showed that these mice exhibited fragmented patterns of sleep-like behaviour during the light phase of a 12:12-h light/dark (LD) cycle with an extended period of continuous wakefulness at the beginning of the dark phase. Moreover, an acute light pulse during night-time was less effective in inducing sleep-like behaviour in Tc1 animals than in wild-type controls. In wheel-running analysis, free running in constant light (LL) or constant darkness (DD) showed no changes in the circadian period of Tc1 animals although they did express subtle behavioural differences including a reduction in total distance travelled on the wheel and differences in the acrophase of activity in LD and in DD. Our data confirm that Tc1 mice express sleep-related phenotypes that are comparable with those seen in Down syndrome patients with moderate disruptions in rest/activity patterns and hyperactive episodes, while circadian period under constant lighting conditions is essentially unaffected.
Keywords: Circadian wheel-running, Down syndrome, sleep, Tc1, trans-species aneuploid mouse line
Down syndrome is, with an incidence of about 1 in 700 live births, the most common autosomal aneuploidy in humans. The partial or whole triplication of the human chromosome 21 (Hsa21) (Lejeune et al. 1959) is associated with numerous features including congenital heart defects, gastrointestinal anomalies, craniofacial alterations, early-onset dementia and mental disturbances (reviewed by Malt et al. 2013). Among the brain deficits experienced by Down syndrome patients, increased sleep fragmentation is a consistent feature (Churchill et al. 2012; Diomedi et al. 1999; Fernandez & Edgin 2013; Levanon et al. 1999). Currently, there are numerous mouse models available partially reflecting the human syndrome. All are based on the conserved synteny between Hsa21 and mouse chromosomes 16 (Mmu16), Mmu17 and Mmu10 (Cole et al. 1998; Pletcher et al. 2001; Yu et al. 2010). Based on different regions of synteny, these mice reflect different aspects of the human condition and provide valuable models for further study. In assessing alterations in rhythmic activity and sleep–wake in mouse models, the majority of studies have focused on the Ts65Dn line containing approximately 60% of the mouse orthologues of human genes on Hsa21. Confoundingly, however, this mouse line is also trisomic for regions unrelated to Hsa21, including about 60 genes from centromeric Mmu17 (Duchon et al. 2011; Reinholdt et al. 2011). The behavioural outcomes of Ts65Dn studies are variable (Escorihuela et al. 1995; Martinez-Cue et al. 2002; Martinez-Cue et al. 2013; Reeves et al. 1995; Stewart et al. 2007).
The most novel and complete mouse model for Down syndrome is the Tc1 (transchromosomic, Tc(Hsa21)1TybEmcf) line (O'Doherty et al. 2005). This is a trans-species aneuploid line expressing a large portion of Hsa21 (83%, 269 genes) as a third copy. Several Down syndrome-related phenotypes have been detected in the Tc1 line including learning and memory deficits (Morice et al. 2008; O'Doherty et al. 2005), increased stereotypic grooming and activity in the open field (Galante et al. 2009), impaired balance and coordination on a static rod and impairments in motor skill learning on an accelerating Rotarod (Galante et al. 2009).
Given that Tc1 mice express many of the characteristic features of Down syndrome, we were prompted to determine their circadian activity and sleep-related parameters. Our approach has been to use two independent non-invasive methodologies. First, we used conventional wheel-running screens to monitor circadian locomotor activity under several lighting conditions. Second, a non-invasive video monitoring approach, estimating sleep based on pre-defined periods of immobility, was utilized (Fisher et al. 2012; Pack et al. 2007). This dual methodological approach allows one to assess multiple specific components of behaviour. These include estimates of circadian period, tau (τ), under constant conditions and wheel-running performance using the conventional wheel-running approach. Furthermore, the video-tracking method provides information about the timing, duration and consolidation of immobility-based sleep assessment and activity. By assessing animals in both tests, we have been able to detect significant latencies in Tc1 animals for light-induced activity suppression while rest–wake patterns in these animals are significantly disrupted reflecting the sleep-related findings in Down syndrome patients (Gigli et al. 1987; Levanon et al. 1999).
Materials and methods
Animals
Tc1 mice and wild-type littermate controls were bred at the Mary Lyon Centre, Harwell, and tested between 8 and 12 weeks of age; a total number of 31 mice were used in this study. The colony was maintained as an F1 (C57BL/6Jx129S8) colony, with a stable transmission frequency of more than 40% of progeny inheriting Hsa21 from their mothers. Owing to the loss of transmission of Hsa21, this mouse line cannot be kept on a pure genetic background (O'Doherty et al. 2005). DNA for genotyping was extracted from ear biopsies using 100 µl of 50 mm NaOH at 95°C for 90 min and buffered with 10 µl of 1 m Tris pH 7.5. Hsa21 present in Tc1 mice was identified by PCR using primers D21S55F (5′-GGT TTG AGG GAA CAC AAA GCT TAA CTC CCA-3′) and D21S55R (5′-ACA GAG CTA CAG CCT CTG ACA CTA TGA ACT-3′) specific to Hsa21 and control primers for myosin (MyoF: 5′-TTA CGT CCA TCG TGG ACA GCA T-3′ and MyoR: 5′-TGG GCT GGG TGT TAG TCT TAT-3′) resulting in PCR products of 208 and 245 bp, respectively.
All animal experiments were carried out under the guidance issued by the Medical Research Council in ‘Responsibility in the Use of Animals for Medical Research’ (July 1993) and Home Office Project Licence (No. 30/2686) and in accordance with the Animal (Scientific Procedures) Act 1986, UK. All experiments conformed to international guidelines on the ethical use of animals.
Circadian wheel-running
Ten adult Tc1 male mice and ten littermate control males were singly housed in cages equipped with running wheels with food and water available ad libitum in light tight chambers with ambient temperature kept at 21 ± 2°C and 45–65% humidity (Banks & Nolan 2011). Mice were entrained under a standard 12:12-h LD cycle with the onset of light at zeitgeber time (ZT) 0 and dark onset at ZT12. After 8 days under light/dark (LD) conditions, animals were transferred to free-running conditions for 12 days in constant darkness (DD) followed by 14 days in constant light (LL). Wheel-running data were recorded and analysed using ClockLab (Actimetrics, Wilmette, IL, USA) using default settings to calculate all parameters measured. anova tests (SPSS, IBM, Armonk, NY, USA) were performed to identify differences between experimental groups.
Video-tracking
Five adult Tc1 male mice and six littermate control males were singly housed in video-monitored standard home cages placed in light tight chambers with food and water available ad libitum. Video-tracking and sleep estimation were performed as described previously (Fisher et al. 2012) using a validated methodology which showed a correlation coefficient of more than 94% when compared with electroencephalography (EEG) recordings. This correlation has been shown not only for baseline conditions but also been confirmed following administration of sedatives (Zolpidem) or stimulants (caffeine) in a dose-dependent manner (Fisher et al. 2012). Mice were first kept under a standard 12:12-h LD cycle with at least a 72-h habituation period to the home cage prior to any recordings. After baseline data collection for a single 24-h LD cycle, a 3-h acute light pulse (LP) was presented during the dark period at ZT16 and data recorded for the duration of the LP and for 2-h segments immediately before and after the LP. Finally, animals were transferred to DD and data recorded over a full circadian cycle.
Videos were recorded at 12.5 frames per second (FPS) and saved in AVI format. Stored videos were analysed using ANYmaze software (Stoelting, Wood Dale, IL, USA) by tracking the centre of the animal with an immobility detection rate of 95%, a validated setting (Fisher et al. 2012) to prevent detection of movement caused by breathing during sleep. According to this validation, animals were recorded as asleep when immobile for more than 40 seconds. Data for LD or constant conditions were analysed in hourly bins, whereas data for the LP study (including pre- and post-LP) were analysed in 10 min bins to detect rapid changes in sleep–wake behaviour following acute changes in lighting conditions. Time spent immobile (asleep) is displayed as a percentage of the total time in a particular bin (1 h or 10 min). For example, if an animal is immobile for an entire 1-h bin, then immobility is scored as 100%. Immobility in this context is subsequently referred to as asleep. Furthermore, in order to facilitate comparison with wheel-running data, the y-axes for immobility were inverted so that higher percentages of immobility, representing estimated periods of sleep, correspond to lower y-axis values. Lower percentages of immobility, representing active periods, are displayed as higher y-axis values. Statistical analysis was performed using two-way anovas in SPSS (IBM).
Results
Using a conventional circadian wheel-running recording system, mice of both genotypes, Tc1 carrier mice and wild-type littermate controls, showed normal photoentrainment under a 12:12 LD cycle, with 90% of wheel-running activity occurring in the nocturnal phase and similar alpha (Table1, Figs. 1 and 2a). Comparison of wheel-running activity showed a trend towards a delay in the acrophase of wheel-running activity at the beginning of the dark phase for Tc1 animals, although this was not significant (Fig. 2a, wild-type acrophase at 22.00 ± 0.41 h and Tc1 at 20.96 ± 0.25 h, F1,17 = 1.553, P = 0.230). Furthermore, Tc1 animals ran significantly less during the dark phase of the LD cycle compared with their wild-type littermate controls resulting in lower average rotations per night (Table1). Remarkably, however, video-tracking of Tc1 animals showed a 6-h period sustained wakefulness (0% immobility), with the onset of the dark phase. Inspection of videos during this time confirmed that mutant animals conduct all of the behaviours expected, including locomotor activity, vertical activity, climbing, feeding and grooming, more frequently than wild-type littermate controls. Controls were asleep for approximately 20% of the time with sleep increasing at later stages in the dark phase (Fig. 2b). Tc1 animals were not only more mobile than littermate controls but more active with greater distances travelled (F1,54 = 100.91; P < 0.001; Fig. 2c). Inspection of video recordings during this period of hyperactivity showed that the number of immobile episodes was significantly lower for Tc1 animals (F1,54 = 45.88; P < 0.001), which is consistent with a constant and uninterrupted wakefulness (Fig. 2e). Average estimated sleep bout length was also significantly shorter (Fig. 2g, F1,10 = 0.842, P = 0.0218). In the light phase of the LD cycle, no significant differences in distance travelled and percentage of immobility could be observed (Fig. 2). However, the number of immobile episodes during the light phase was significantly higher for Tc1 animals compared with littermate controls (F1,108 = 5.72; P = 0.018), while average estimated sleep bout length was not significantly different (F1,10 = 7.367, P = 0.38), which is indicative of greater sleep disruption/fragmentation in triploid animals (Fig. 2f).
Table 1.
Wheel-running parameters in Tc1 and control mice under three separate lighting conditions
| Wt | Tc1 | |
|---|---|---|
| Mean revolutions LD (± SEM) | 2871 ± 789 | 2078 ± 430* |
| Nocturnal activity LD [%] (± SEM) | 94.65 ± 1.5 | 96.27 ± 1.1 |
| Amplitude LD (± SEM) | 926 ± 95 | 824 ± 43 |
| Alpha length LD (± SEM) | 8.89 ± 0.38 | 8.10 ± 0.41 |
| Mean revolutions DD (± SEM) | 2956 ± 782 | 3106 ± 491 |
| Tau DD (± SEM) | 23.74 ± 0.05 | 23.79 ± 0.09 |
| Amplitude DD (± SEM) | 1183 ± 231 | 1137 ± 124 |
| Alpha length DD (± SEM) | 11.06 ± 0.49 | 9.26 ± 0.50* |
| Mean revolutions LL (± SEM) | 1624 ± 417 | 1626 ± 744 |
| Tau LL (± SEM) | 24.83 ± 0.11 | 25.08 ± 0.10 |
| Amplitude LL (± SEM) | 869 ± 171 | 986 ± 187 |
| Alpha length LL | 9.01 ± 0.64 | 5.86 ± 0.50* |
Average wheel-running revolutions under light/dark (LD) conditions and percentage of nocturnal wheel-running activity, amplitude and alpha in Tc1 and littermate controls (Wt). Mean wheel-running revolutions, Tau, amplitude and alpha during constant darkness (DD) and constant light (LL).
Asterisk (*) indicates a significant difference between genotypes (P < 0.05).
Figure 1.

Double plotted actogram for Tc1 animals and littermate controls. Actogram showing wheel-running data in 12:12 light/dark (LD) for 8 days, constant darkness (DD) for 12 days, and constant light (LL) conditions for 14 days for two Tc1 carriers and two littermate controls. An acute LP for some of the animals was given at ZT 14 on the third night.
Figure 2.
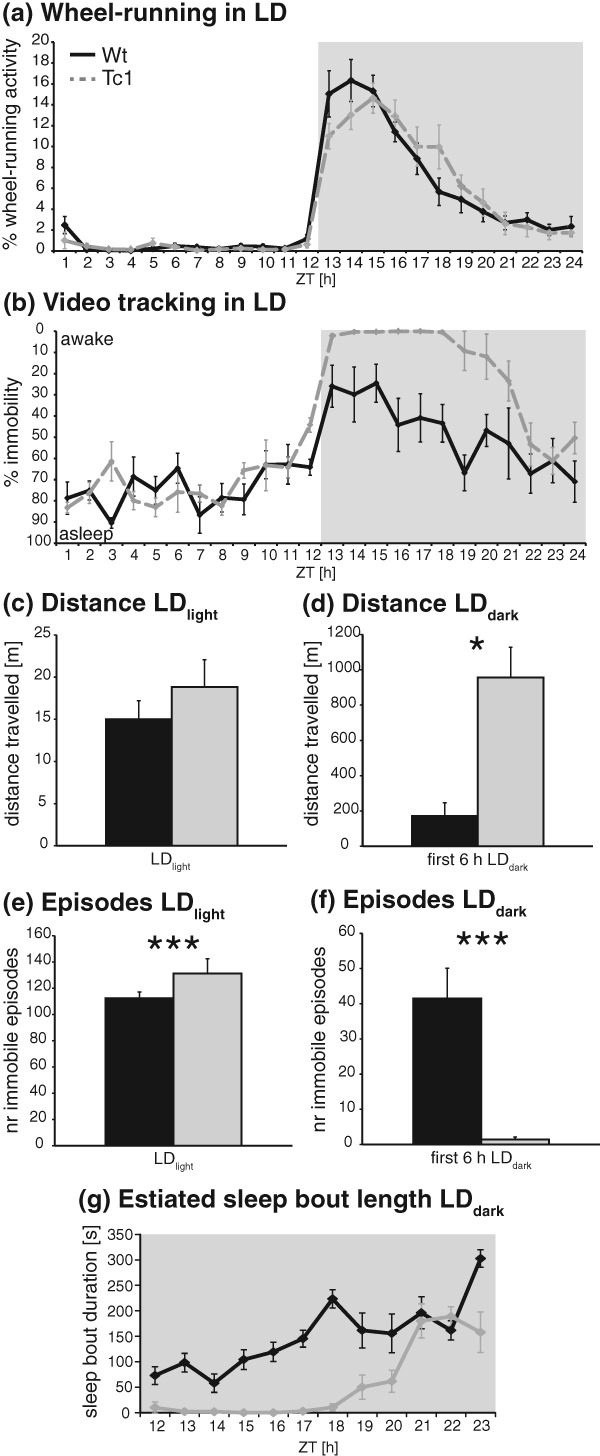
Activities of Tc1 and control mice under LD conditions. (a) Wheel-running: percentage of activity for wild-type (black) and Tc1 (grey) animals during 12:12-h light/dark (LD) conditions. (b) Video-tracking: percentage of immobility for wild-type and Tc1 animals during LD. For some data points, all Tc1 animals showed 0% immobility. (c–f) Video-tracking. (c) Total distance travelled during full 12 h of the light period. (d) Total distance travelled during first 6 h of the dark period. (e) Number of immobile episodes during full 12 h of the light period. (f) Number of immobile episodes during first 6 h of the dark period. (g) Estimated average sleep bout lengths during 12 h of the dark period. Averages were calculated per hourly bin. *P < 0.05; **P < 0.005; ***P < 0.002.
Under free-running conditions in DD, no significant differences in wheel-running amplitude or in average revolutions were identified. Neither was the circadian period significantly different between genotypes, but Tc1 animals displayed shorter alpha compared with wild-type littermates (Table1). When investigated in DD, the acrophase of wheel-running activity again showed a non-significant advanced trend for Tc1 carriers with a more defined and narrow peak of activity in comparison to wild-type littermate controls (Fig. 3a, F1,18 = 0.726, P = 0.405). In LL conditions, the internal period lengthened for all animals as expected. Average τLL, revolutions, and amplitude were not significantly different for wild-type and Tc1 animals, but alpha was shorter for Tc1 animals compared with that for littermate controls (Table1).
Figure 3.
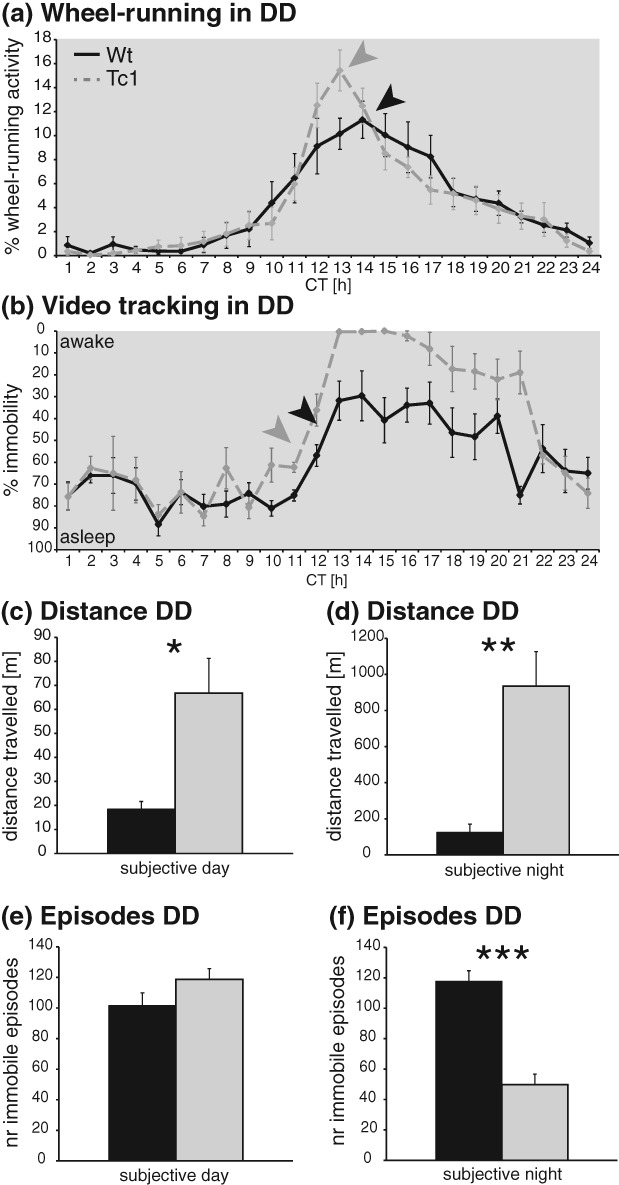
Activities of Tc1 and control mice under DD conditions. (a) Wheel-running: percentage of activity for wild-type (black) and Tc1 (grey) animals during constant darkness (DD). (b) Video-tracking: percentage of immobility for wild-type and Tc1 animals during DD. For some data points, all Tc1 animals showed 0% immobility. (c–f) Video-tracking. (c) Distance travelled during subjective day. (d) Distance travelled during subjective night. (e) Number of immobile episodes during subjective day. (f) Number of immobile episodes during subjective night. *P < 0.05; **P < 0.005; ***P < 0.002.
The results of the video-tracking in DD were similar to those described for LD conditions. At the beginning of the subjective night, Tc1 mice maintained their state of sustained wakefulness (displayed as 0% immobility) compared with littermate controls, although the length of this period was shortened to 3 h of intense activity. This increased wakefulness in Tc1 animals shows a non-significant advanced trend of 1–2 h relative to that of wild types (latency to first immobile episode in subjective night, wild-type 67.94 min, Tc1 185.40 min, F1,9 = 1.338, P = 0.277). Wild-type littermates exhibited a level of immobility of about 30% at this time, again decreasing towards the end of the subjective night (Fig. 3b). Distance travelled by Tc1 animals was significantly higher not only during subjective night (F1,108 = 58.09; P < 0.001, Fig. 3d) but also during the subjective day (F1,108 = 10.02; P = 0.002, Fig. 3c). The latter effect is likely related to the advanced phase of activity seen in Tc1 animals. Finally, by comparing the number of immobile episodes in DD conditions, no significant differences between genotypes were detected during the subjective day whereas during the subjective night mutant animals exhibited a significantly lower number of immobile episodes (F1,108 = 44.68; P < 0.001, Fig. 3f).
Introduction of an acute LP in the dark phase of the LD cycle should rapidly induce sleep in mice (Lupi et al. 2008; Muindi et al. 2013). Light-induced immobility-defined sleep was less pronounced for Tc1 animals during wheel-running (not significant, Fig. S1, Supporting information) and significantly delayed by around 20 min in Tc1 animals compared with that in their wild-type littermate controls in the video-tracking approach (Fig. 4a). This was confirmed as a significantly longer latency to the first occurrence of immobility-defined sleep (F1,9 = 66.17; P < 0.001, Fig. 4b). Analysis of the total amount of immobility-defined sleep and activity across the duration of the LP showed significant differences between genotypes with Tc1 animals travelling a greater distance (F1,9 = 33.40; P < 0.001, Fig. 4c) and spending less time asleep (F1,9 = 7.92; P = 0.020, Fig. 4d). Furthermore, comparing immobility between control littermates and Tc1 mutants in pre- and post-LP periods (darkness) showed much higher levels of activity for the Tc1 carrier group (Fig. 4a). This was consistent with the results of the 24-h LD and DD recordings (Figs. 3).
Figure 4.
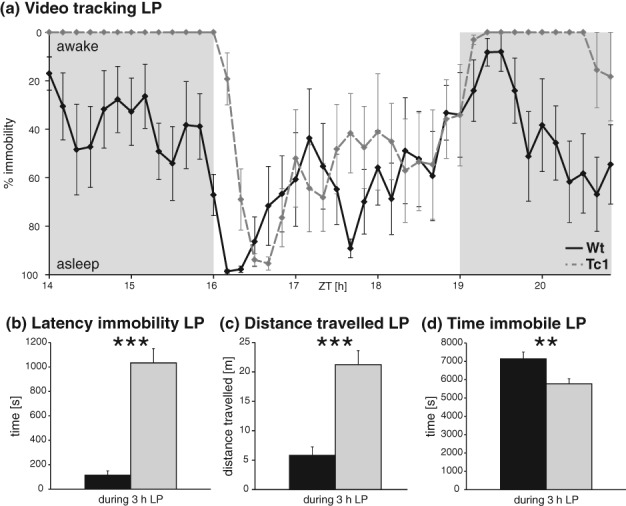
Activity suppression in Tc1 and control mice following a 3-h light pulse. (a–d) Video-tracking. (a) Percentage of immobility for wild-type (black) and Tc1 (grey) animals before, during and after the light pulse (LP, unshaded segment of graph). For some data points, all Tc1 animals showed 0% immobility. (b) Latency for first immobile episode during LP. (c) Distance travelled during LP. (d) Time immobile during LP. *P < 0.05; **P < 0.005; ***P < 0.002.
Discussion
Using two diverse and complementary methodologies, conventional circadian wheel-running analysis and a novel validated video-tracking system that defines sleep based upon periods of immobility (Fisher et al. 2012), we have been able to gain new insights into the general activity, circadian function and sleep-related behaviours of the Tc1 mutant mouse line. Estimating periods of sleep based on immobility is a useful approach to assess sleep/wake behaviour in mice as it is a non-invasive technique that is both faster and more flexible for high-throughput analysis. It is also much more appropriate for mouse lines like Tc1, which show deficits in skilled motor function (Galante et al. 2009), a compromised health status and reduced survival rates after surgery. Moreover, as video-tracking results are so closely correlated with EEG data (Fisher et al. 2012), this approach has significant advantages over the use of more invasive tethered or telemetric EEG recording approaches as mutant animals may be unduly affected by anaesthesia and surgical procedures. In contrast, however, video-based assessment of sleep-like behaviour may be confounded by motor-skills differences in mutant mice and this possibility should be considered in studies such as this.
The use of multiple tests in this study has allowed us to measure disturbances in general activity and motor function. Wheel-running is a non-invasive test that has been used traditionally to measure circadian activity as well as phasic responses to LD conditions. The majority of studies suggest no alterations in circadian locomotor activity for Ts65Dn animals during a 12:12-h LD cycle (Martinez-Cue et al. 2002; Reeves et al. 1995) or in constant lighting conditions (Ruby et al. 2010). Surprisingly, however, Ts65Dn mice exhibit a hyperactive phenotype visible predominantly in the early hours of the dark phase that is not evident during the light phase (Escorihuela et al. 1995; Martinez-Cue et al. 2013). Moreover, a single independent study found that Ts65Dn animals exhibit a significant advanced phase in activity onset of approximately 4 h compared with wild-type mice under a 12:12-h LD cycle (Stewart et al. 2007). Overall, we detected only very subtle circadian phase-associated disturbances in Tc1 animals using this behavioural assessment. However, we did detect a reduction in the average wheel-running activity of Tc1 mutant mice during the dark phase of the 12:12-h LD cycle. Motor disabilities are a common symptom in Down syndrome patients (Spano et al. 1999). In earlier studies, Tc1 mice were shown to have impairments in skilled motor functions although general movement, gait, grip strength and other simple motor functions were unaffected (Galante et al. 2009), and this is reflected in our study where mice are more active while engaging in less wheel-running activity. This distinction is not unprecedented as we have shown in an earlier study using principal component analysis that wheel-running performance is independent of locomotor activity (Mandillo et al. 2014). Of course, we cannot discount the fact that Tc1 animals were less motivated to run on wheels. Additional studies testing animal motivation would need to be carried out to comment on whether this might contribute to the low wheel-running we found.
In contrast to the wheel-running study, Tc1 mice express a hyperlocomotor activity in the home cage as assessed using video-tracking. Hyperlocomotion had been previously recorded in Tc1 mice when they were assessed over short intervals in the open field test (Galante et al. 2009), but this is the first instance where consistent levels of hyperactivity have been recorded over long intervals in the home cage. This suggests that increased activity in Tc1 animals is not only precipitated by introducing mutant animals to a novel environment, as in the open field, but is expressed as an unprovoked behaviour in the familiar surroundings of the home cage. Galante et al. (2009) suggest that hyperactivity may be associated with a deficit in hippocampal function in Tc1 animals. Interestingly, impulsivity and hyperactivity are frequently reported for Down syndrome patients (Ekstein et al. 2011).
Our data also highlight the influence of LD phases on Tc1 activity disturbances. Hyperactivity in Tc1 animals is most evident at night, while the duration of this hyperactive phase is shortened when they are maintained in DD. Similar observations have been noted for Ts65Dn animals. For example, activity levels have been significantly higher during the dark phase in LD compared with those in DD or LL for Ts65Dn animals when compared with wild types in an actimetry study (Ruby et al. 2010). Also, Ts65Dn mice tested under white and red light conditions in an open field arena only showed a significantly increased number of line crossings under white light conditions when compared with control animals (Escorihuela et al. 1995). Light at night-time also highlights significant differences between Tc1 animals and littermate controls. Analysis of the LP data emphasizes how changing environmental lighting conditions can modulate the behaviour of Tc1 animals as evidenced in the delayed onset and reduction of light-induced sleep for the duration of the LP.
Down syndrome patients suffer from sleep disturbances such as increased daytime sleepiness, prolonged sleep latency at night, reduced amount and number of bouts of rapid eye movement (REM) sleep and sleep fragmentation (Carter et al. 2009; Diomedi et al. 1999; Grubar et al. 1986; Hamaguchi et al. 1989; Levanon et al. 1999). Sleep apnoea is thought to play a major role in causing sleep abnormalities in Down syndrome patients (Marcus et al. 1991) but is not the only cause (Levanon et al. 1999). In developing mouse models for Down syndrome, it is important that these sleep disturbances can be reflected accurately. Circadian and sleep/wake-related behaviour in the Ts65Dn mouse model shows similarities to Down syndrome patients with clear disturbances in EEG parameters showing decreased non-rapid eye movement (NREM) sleep and NREM bout durations associated with increased wakefulness during the light phase (Colas et al. 2008). Although not accompanied by EEG recordings, Tc1 animals exhibit a lower percentage of estimated sleep and delays in the onset of light-induced sleep in our study. Nevertheless, traits like sleep fragmentation and longer latencies for sleep onset in patients (Breslin et al. 2011; Carter et al. 2009) are reflected in delayed latency for light-induced sleep (LP) and a higher amount of immobile episodes in Tc1 animals. Conversely, major sleep disturbances are not found in Ts1Cje (Duchon et al. 2011), although they do show a delay in sleep rebound (Colas et al. 2008). These findings have prompted this group to make some assumptions on the contribution of loci to the sleep disturbance phenotype, suggesting that they should not be triplicated in Ts1Cje. In particular, they noted that APP transgenic mice show consistent sleep disturbances in multiple studies (Colas et al. 2004). However, recent findings in Tc1 mice that the final coding exon of APP is rearranged with no human APP protein detectable would argue that loci other than APP contribute to the sleep phenotypes in Down syndrome mutant models (Reinholdt et al. 2011).
Although not fully investigated in Tc1 mice, data from human studies and from other mouse models would suggest that rest/activity and rhythm disturbances may arise as a consequence of either generalized synaptic deficits or disturbances in particular brain circuitries. MRI scans of individual Down syndrome patients have recorded numerous brain anomalies including changes in size of cerebellum, frontal, temporal and occipital cortical lobes and hippocampus (Roubertoux & Kerdelhue 2006), while alterations in cortical lamination, dendritic branching and numbers of synapses have also been recorded (Roizen & Patterson 2003). Aside from these general structural deficits, disturbances in cholinergic (Roizen & Patterson 2003) and serotonergic function (Seidl et al. 1999) identified in Down syndrome patients would seem most likely to affect activity/sleep parameters. More specifically, deterioration in cholinergic basal forebrain neuronal function may be causative. Most data on neural correlates from mouse models have come from the Ts65Dn line. In line with the human MRI data, dendritic spine density is lower in hippocampus (Belichenko et al. 2004) and cortical pyramidal cells of environmentally enriched mutant animals (Dierssen et al. 2003). Disturbances in many neurochemical circuits are also evident in this model. Although there is no consensus as to when disturbances in particular circuits contribute to the numerous behavioural phenotypes (Granholm et al. 2000; Hunter et al. 2004; Seidl et al. 1999; Seo & Isacson 2005), it is possible, for example, that subtle effects in cholinergic neurons from early adulthood may be affecting rest/activity patterns in mouse models. Finally, although neuroendocrine function is disturbed in Down syndrome (Roubertoux & Kerdelhue 2006), there is no specific data from mouse work, suggesting that hypothalamic dysfunction contributes to the rest/activity or sleep fragmentation phenotypes seen in mouse models. Future studies into hypothalamic function in Tc1 mice may help in clarifying its contribution to rest/activity and sleep disturbances.
Sleep disturbances may contribute to cognitive dysfunctions in Down syndrome patients, while individuals with high ratings of sleep disruption have greater difficulties with executive functions (Chen et al. 2013). In a study using optogenetics tools to disrupt sleep in mice, sleep fragmentation in itself can impair mouse performance in an object recognition task without affecting the overall amount or intensity of sleep (Rolls et al. 2011). In general, poor sleep seems to impair memory consolidation (Brown et al. 2012; Stickgold 1998) further exacerbating cognitive impairments in Down syndrome patients and warranting further investigation in mouse models. Tc1 mice display a number of these additional traits with deficits evident in a number of learning and memory paradigms (Morice et al. 2008; O'Doherty et al. 2005).
The investigation of sleep and rhythm-related disturbances in mouse models of Down syndrome shows consistently abnormal parameters, although the contribution of different loci on Hsa21 remains to be clarified. Nevertheless, the continued use of diverse phenotyping tools in different mouse models will be invaluable in furthering our understanding of sleep disturbances in Down syndrome patients.
Acknowledgments
The authors thank Prof. E.M.C. Fisher and Prof. V.L.J. Tybulewicz for providing the Tc1 mouse line for this study. The work was supported by the Medical Research Council (P.M.N.), the Wellcome Trust (R.G.F. and S.N.P.) and BBSRC (S.N.P.). S.P.F. was supported by a Knoop Junior Research Fellowship (St Cross, Oxford). The authors declare no conflicts of interest.
Supporting Information
Figure S1: Representative actograms focusing on wheel-running in response to a light pulse. Representative actograms for two Tc1 mice and a wild-type control mouse showing wheel-running activity in response to an acute light pulse. Shaded regions indicate where lights are on. Wheel-running behaviour was quite variable, and no significant differences were found.
References
- Banks GT. Nolan PM. Assessment of circadian and light-entrainable parameters in mice using wheel-running activity. Curr Protoc Mouse Biol. 2011;1:369–381. doi: 10.1002/9780470942390.mo110123. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A. Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- Breslin JH, Edgin JO, Bootzin RR, Goodwin JL. Nadel L. Parental report of sleep problems in Down syndrome. J Intellect Disabil Res. 2011;55:1086–1091. doi: 10.1111/j.1365-2788.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, Mckenna JT, Strecker RE. Mccarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M, Mccaughey E, Annaz D. Hill CM. Sleep problems in a Down syndrome population. Arch Dis Child. 2009;94:308–310. doi: 10.1136/adc.2008.146845. [DOI] [PubMed] [Google Scholar]
- Chen CC, Spano G. Edgin JO. The impact of sleep disruption on executive function in Down syndrome. Res Dev Disabil. 2013;34:2033–2039. doi: 10.1016/j.ridd.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Churchill SS, Kieckhefer GM, Landis CA. Ward TM. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960–2010. Sleep Med Rev. 2012;16:477–488. doi: 10.1016/j.smrv.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas D, London J, Gharib A, Cespuglio R. Sarda N. Sleep-wake architecture in mouse models for Down syndrome. Neurobiol Dis. 2004;16:291–299. doi: 10.1016/j.nbd.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Colas D, Valletta JS, Takimoto-Kimura R, Nishino S, Fujiki N, Mobley WC. Mignot E. Sleep and EEG features in genetic models of Down syndrome. Neurobiol Dis. 2008;30:1–7. doi: 10.1016/j.nbd.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SE, Wiltshire T. Reeves RH. Physical mapping of the evolutionary boundary between human chromosomes 21 and 22 on mouse chromosome 10. Genomics. 1998;50:109–111. doi: 10.1006/geno.1998.5312. [DOI] [PubMed] [Google Scholar]
- Dierssen M, Benavides-Piccione R, Martinez-Cue C, Estivill X, Florez J, Elston GN. Defelipe J. Alterations of neocortical pyramidal cell phenotype in the Ts65Dn mouse model of Down syndrome: effects of environmental enrichment. Cereb Cortex. 2003;13:758–764. doi: 10.1093/cercor/13.7.758. [DOI] [PubMed] [Google Scholar]
- Diomedi M, Curatolo P, Scalise A, Placidi F, Caretto F. Gigli GL. Sleep abnormalities in mentally retarded autistic subjects: Down's syndrome with mental retardation and normal subjects. Brain Dev. 1999;21:548–553. doi: 10.1016/s0387-7604(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Duchon A, Raveau M, Chevalier C, Nalesso V, Sharp AJ. Herault Y. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: relevance for modeling Down syndrome. Mamm Genome. 2011;22:674–684. doi: 10.1007/s00335-011-9356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstein S, Glick B, Weill M, Kay B. Berger I. Down syndrome and attention-deficit/hyperactivity disorder (ADHD) J Child Neurol. 2011;26:1290–1295. doi: 10.1177/0883073811405201. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, Tobena A. Florez J. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–146. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- Fernandez F. Edgin JO. Poor sleep as a precursor to cognitive decline in Down syndrome: a hypothesis. J Alzheimers Dis Parkinsonism. 2013;3:124–135. doi: 10.4172/2161-0460.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SP, Godinho SI, Pothecary CA, Hankins MW, Foster RG. Peirson SN. Rapid assessment of sleep-wake behavior in mice. J Biol Rhythms. 2012;27:48–58. doi: 10.1177/0748730411431550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante M, Jani H, Vanes L, Daniel H, Fisher EM, Tybulewicz VL, Bliss TV. Morice E. Impairments in motor coordination without major changes in cerebellar plasticity in the Tc1 mouse model of Down syndrome. Hum Mol Genet. 2009;18:1449–1463. doi: 10.1093/hmg/ddp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli GL, Grubar JC, Colognola RM, Amata MT, Pollicina C, Ferri R, Musumeci SA. Bergonzi P. Butoctamide hydrogen succinate and intensive learning sessions: effects on night sleep of Down's syndrome patients. Sleep. 1987;10:563–569. doi: 10.1093/sleep/10.6.563. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA. Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down's syndrome. Exp Neurol. 2000;161:647–663. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- Grubar JC, Gigli GL, Colognola RM, Ferri R, Musumeci SA. Bergonzi P. Sleep patterns of Down's syndrome children: effects of butoctamide hydrogen succinate (BAHS) administration. Psychopharmacology (Berl) 1986;90:119–122. doi: 10.1007/BF00172882. [DOI] [PubMed] [Google Scholar]
- Hamaguchi H, Hashimoto T, Mori K. Tayama M. Sleep in the Down syndrome. Brain Dev. 1989;11:399–406. doi: 10.1016/s0387-7604(89)80024-5. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bachman D. Granholm AC. Minocycline prevents cholinergic loss in a mouse model of Down's syndrome. Ann Neurol. 2004;56:675–688. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- Lejeune J, Turpin R. Gautier M. Chromosomic diagnosis of mongolism. Arch Fr Pediatr. 1959;16:962–963. [PubMed] [Google Scholar]
- Levanon A, Tarasiuk A. Tal A. Sleep characteristics in children with Down syndrome. J Pediatr. 1999;134:755–760. doi: 10.1016/s0022-3476(99)70293-3. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S. Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Malt EA, Dahl RC, Haugsand TM, Ulvestad IH, Emilsen NM, Hansen B, Cardenas YE, Skold RO, Thorsen AT. Davidsen EM. Health and disease in adults with Down syndrome. Tidsskr Nor Laegeforen. 2013;133:290–294. doi: 10.4045/tidsskr.12.0390. [DOI] [PubMed] [Google Scholar]
- Mandillo S, Heise I, Garbugino L, Tocchini-Valentini GP, Giuliani A, Wells S. Nolan PM. Early motor deficits in mouse disease models are reliably uncovered using an automated home cage wheel-running system: a cross-laboratory validation. Dis Model Mech. 2014;7:397–407. doi: 10.1242/dmm.013946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CL, Keens TG, Bautista DB, von Pechmann WS. Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88:132–139. [PubMed] [Google Scholar]
- Martinez-Cue C, Baamonde C, Lumbreras M, Paz J, Davisson MT, Schmidt C, Dierssen M. Florez J. Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behav Brain Res. 2002;134:185–200. doi: 10.1016/s0166-4328(02)00026-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Cue C, Martinez P, Rueda N, Vidal R, Garcia S, Vidal V, Corrales A, Montero JA, Pazos A, Florez J, Gasser R, Thomas AW, Honer M, Knoflach F, Trejo JL, Wettstein JG. Hernandez MC. Reducing gabaa alpha5 receptor-mediated inhibition rescues functional and neuromorphological deficits in a mouse model of Down syndrome. J Neurosci. 2013;33:3953–3966. doi: 10.1523/JNEUROSCI.1203-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice E, Andreae LC, Cooke SF, Vanes L, Fisher EM, Tybulewicz VL. Bliss TV. Preservation of long-term memory and synaptic plasticity despite short-term impairments in the Tc1 mouse model of Down syndrome. Learn Mem. 2008;15:492–500. doi: 10.1101/lm.969608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muindi F, Zeitzer JM, Colas D. Heller HC. The acute effects of light on murine sleep during the dark phase: importance of melanopsin for maintenance of light-induced sleep. Eur J Neurosci. 2013;37:1727–1736. doi: 10.1111/ejn.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, Sesay A, Modino S, Vanes L, Hernandez D, Linehan JM, Sharpe PT, Brandner S, Bliss TV, Henderson DJ, Nizetic D, Tybulewicz VL. Fisher EM. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science. 2005;309:2033–2037. doi: 10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von Smith R, Kay T, Lian J, Svenson K. Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- Pletcher MT, Wiltshire T, Cabin DE, Villanueva M. Reeves RH. Use of comparative physical and sequence mapping to annotate mouse chromosome 16 and human chromosome 21. Genomics. 2001;74:45–54. doi: 10.1006/geno.2001.6533. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT. Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Reinholdt LG, Ding Y, Gilbert GJ, Czechanski A, Solzak JP, Roper RJ, Johnson MT, Donahue LR, Lutz C. Davisson MT. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome. 2011;22:685–691. doi: 10.1007/s00335-011-9357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizen NJ. Patterson D. Down's syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC. De Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubertoux PL. Kerdelhue B. Trisomy 21: from chromosomes to mental retardation. Behav Genet. 2006;36:346–354. doi: 10.1007/s10519-006-9052-0. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Fernandez F, Zhang P, Klima J, Heller HC. Garner CC. Circadian locomotor rhythms are normal in Ts65Dn “Down syndrome” mice and unaffected by pentylenetetrazole. J Biol Rhythms. 2010;25:63–66. doi: 10.1177/0748730409356202. [DOI] [PubMed] [Google Scholar]
- Seidl R, Kaehler ST, Prast H, Singewald N, Cairns N, Gratzer M. Lubec G. Serotonin (5-ht) in brains of adult patients with Down syndrome. J Neural Transm Suppl. 1999;57:221–232. doi: 10.1007/978-3-7091-6380-1_14. [DOI] [PubMed] [Google Scholar]
- Seo H. Isacson O. Abnormal app, cholinergic and cognitive function in Ts65Dn down's model mice. Exp Neurol. 2005;193:469–480. doi: 10.1016/j.expneurol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Spano M, Mercuri E, Rando T, Panto T, Gagliano A, Henderson S. Guzzetta F. Motor and perceptual-motor competence in children with Down syndrome: variation in performance with age. Eur J Paediatr Neurol. 1999;3:7–13. doi: 10.1053/ejpn.1999.0173. [DOI] [PubMed] [Google Scholar]
- Stewart LS, Persinger MA, Cortez MA. Snead OC., 3rd Chronobiometry of behavioral activity in the Ts65Dn model of Down syndrome. Behav Genet. 2007;37:388–398. doi: 10.1007/s10519-006-9119-y. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep: off-line memory reprocessing. Trends Cogn Sci. 1998;2:484–492. doi: 10.1016/s1364-6613(98)01258-3. [DOI] [PubMed] [Google Scholar]
- Yu T, Li Z, Jia Z, et al. A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Hum Mol Genet. 2010;19:2780–2791. doi: 10.1093/hmg/ddq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Representative actograms focusing on wheel-running in response to a light pulse. Representative actograms for two Tc1 mice and a wild-type control mouse showing wheel-running activity in response to an acute light pulse. Shaded regions indicate where lights are on. Wheel-running behaviour was quite variable, and no significant differences were found.


