Abstract
Recent reviews (Feachem et al.; Alonso et al.) have concluded that in order to have a sustainable impact on the global burden of malaria, it is essential that we knowingly reduce the global incidence of infected persons. To achieve this we must reduce the basic reproductive rate of the parasites to < 1 in diverse epidemiological settings. This can be achieved by impacting combinations of the following parameters: the number of mosquitoes relative to the number of persons, the mosquito/human biting rate, the proportion of mosquitoes carrying infectious sporozoites, the daily survival rate of the infectious mosquito and the ability of malaria-infected persons to infect mosquito vectors.
This paper focuses on our understanding of parasite biology underpinning the last of these terms: infection of the mosquito. The article attempts to highlight central issues that require further study to assist in the discovery of useful transmission-blocking measures.
Introduction
Recent reviews (Feachem et al., 2010; Alonso et al., 2011) have concluded that in order to have a sustainable impact on the global burden of malaria, it is essential that we knowingly reduce the global incidence of infected persons. To achieve this we must reduce the basic reproductive rate of the parasites to < 1 in diverse epidemiological settings. This can be achieved by impacting combinations of the following parameters: the number of mosquitoes relative to the number of persons, the mosquito/human biting rate, the proportion of mosquitoes carrying infectious sporozoites, the daily survival rate of the infectious mosquito and the ability of malaria-infected persons to infect mosquito vectors.
This paper focuses on our understanding of parasite biology underpinning the last of these terms: infection of the mosquito (Fig. 1). The article attempts to highlight central issues that require further study to assist in the discovery of useful transmission-blocking measures.
Figure 1.
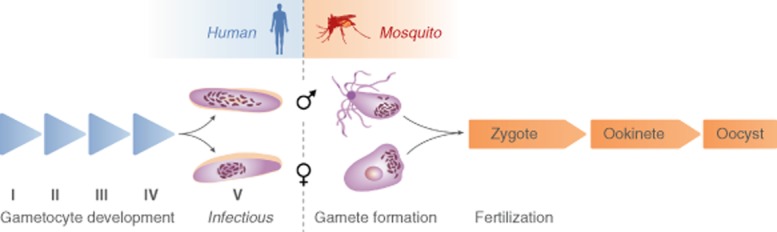
| I | II | III | IV | V | Gamete formation | Fertilization | Ookinete | Oocyst | |
|---|---|---|---|---|---|---|---|---|---|
| Light microscopy1881- | (Hawking et al., 1971; Carter and Miller, 1979) | (Laveran, 1881) | (McCallum, 1897) | (Ross, 1897) | |||||
| Induction mechanisms1926- | (Sinton et al., 1926; Kafsack et al., 2014; Sinha et al., 2014) | (Billker et al., 1998) | |||||||
| Electron microscopy1965- | (Sinden et al., 1978) | (Garnham et al., 1967) | (Sinden et al., 1976) | (Mehlhorn et al., 1980) | (Garnham, 1965) | ||||
| Nuclear organization1973- | (Janse et al., 1986b) | (Sinden et al., 1976) | (Sinden and Hartley, 1985) | (Canning and Sinden, 1973) | |||||
| Transcriptome2000- | (Lanfrancotti et al., 2007) | (Hayward et al., 2000) | (Hall et al., 2005) | ||||||
| Translation repression1990- | (Paton et al., 1993; Guerreiro et al., 2014) | (Mair et al., 2010) | |||||||
| Proteins first identified1974- | (Silvestrini et al., 2005) | (Vermeulen et al., 1985) | (Kumar and Carter, 1984) | (Liu et al., 2008) | Vermeulen et al., 1985) | (Krotoski et al., 1974) | |||
| Proteome2005- | (Khan et al., 2005) | (Talman et al., 2014) | (Hall et al., 2005) | ||||||
| Metabolome2014- | (Lamour et al., 2014) | ||||||||
From vertebrate host to insect vector
Noting the apparent differences in the sexual biology of malaria parasites in the subgenera Laverania and Plasmodium, where comment or data specifically refers to just one subgenus it will be stated explicitly, and where it is felt by the author to apply to both subgenera it will not be stated.
Transmission from host to vector is the exclusive role of the intra-erythrocytic gametocytes. The development of which can be divided into induction, maturation and gamete formation. The often extended periods of gametocyte induction raises the question whether any generalization can be made as to a physical site where gametocytogenesis is triggered. Rarely it is appreciated that there is evidence that merozoites released directly from the liver schizont can produce gametocytes (Suhrbier et al., 1987; Sinden et al., 1990), and that in the ‘ancestral’ haemoproteids all the merozoites released from the tissue schizonts make gametocytes, i.e. induction is not necessarily a consequence of prior blood infection. Past research (Smalley et al., 1981) showed the young falciparum gametocytes were found in the bone marrow. Trager and Gill (1992) recognized the eminent logic of the slow-growing gametocytes of this species (subgenus Laverania), initiating their protracted development in hematopoietic tissues. These observations have been elegantly extended using both molecular techniques (Aguilar et al., 2014; Joice et al., 2014) and in vitro systems (Farfour et al., 2012; Tiburcio et al., 2012; Peatey et al., 2013). The latter illustrate how both the rigidity of the red blood cell (RBC) infected by the elongated immature P. falciparum (Pf) gametocyte, and surfin expression on the infected RBC (iRBC), might correlate with retention of the immature gametocyte in the complex extravascular environment of the bone marrow. The former attribute is unlikely to apply to the spherical parasites of the subgenus Plasmodium that lack the elongate sub-pellicular cytoskeleton of Laverania. When considering induction of gametocytogenesis in the peripheral blood, current in vitro evidence suggests that in P. falciparum induction occurs in the trophozoite of the asexual generation preceding gametocyte formation, and that the merozoites from any one committed schizont are predetermined to be either male or female (i.e. multiple fate ‘decisions’ have already been made prior to merozoite formation) (Bruce et al., 1994). A marker of this commitment has recently been described as Pf10_0164 (ETRAMP 10.3) (Brancucci, unpubl.). In the rodent malaria parasite P. berghei gametocyte determination possibly occurs following invasion by the merozoite (Mons et al., 1985). Myriad potential external inducers of gametocytogenesis have been described, all of which may be broadly classified as ‘stress related’, but molecular characterization of any consensus induction pathway still eludes us (Sinton, 1926; Bhasin and Trager, 1984; Dyer and Day, 2000; Carter et al., 2013). Future experiments to understand sexual/asexual differentiation should at least entertain the hypothesis that sexual development is the ancestral – and therefore default – pathway.
Not knowing the molecular properties of the inducers, it is unsurprising that we do not know the receptors initiating the signalling pathways for gametocytogenesis. Things we do know however are that the down-regulation of the histone deacetylase gene pfHda2, the heterochromatin protein PfHP1 and the ABC transporter-encoding gene gabcg2 increase the proportion of gametocytes in bloodstage infections (Alano, 2015; Brancucci et al., 2014; Coleman et al., 2014; Tran et al., 2014). Perhaps the closest we have come to understanding these pathways is through the production and characterization of mutations resulting in the loss of gametocyte formation. The genetic regulation of gametocytogenesis was first appreciated in studies correlating the loss of chromosomal integrity (notably P. falciparum chromosome 9) with the loss of sexual potential (Birago et al., 1982; Janse et al., 1989; Day et al., 1993; Ikadai et al., 2013), but it was transposon-mediated mutagenesis (Ikadai et al., 2013) that identified 16 loci, nine of whose disruption led to loss of any identifiable (stage I) gametocytes [this group includes a member of the ap2 family (PF13_0097)] and seven loci essential to formation of stage II gametocytes. Of these 16 ‘mutagenized’ loci only five were successfully complemented. Perhaps, unsurprisingly, the former nine loci were classifiable as genes encoding regulators/signalling moieties, and the latter seven included genes with potential roles in the formation of the extensive cytoskeletal structures defining Pf gametocyte morphology. These conclusions are consistent with parallel observations (Young et al., 2005) that noted up-regulation of 246 gametocyte-specific gene classes in the mature gametocytes – shown to include kinase/phosphatase enzymes (Guttery et al., 2012b; Brochet et al., 2014). Genes that have been considered of particular note include pfgdv1 (Eksi et al., 2012), pfgig, pfgly and the plasma membrane transporter NPT1 (Baker, 2010; Boisson et al., 2011). Elegant recent studies have confirmed a pivotal role for ap2-g in gametocytogenesis (Kafsack et al., 2014; Sinha et al., 2014); transcription of ap2-g is regulated by PfHP1-H3K9me3 histone modification (Kafsack et al., 2014). Pathways regulating gametocytogenesis have been reviewed (Morahan and Garcia-Bustos, 2014); these authors suggest ap2g may regulate pfgdv1, thence pfnek4 and nek2 – genes that are additionally regulated by extracellular factors via cyclic adenosine monophosphate-mediated pathways. Interestingly, the protein phosphatase PPM2 (see Table 1) has been shown to have a positive role in the determination of sex ratio (female bias) (Guttery et al., 2014). Have we identified all the key regulator(s) of sexual differentiation, certainly not, but we are now developing the tools for doing so.
Table 1.
Suggested stages of Plasmodium development in the mosquito vector at which identified protein phosphatases and protein kinases are active
| Female sex allocation | Male gamete exflagellation | Zygote/ookinete differentiation | Ookinete movement | Ookinete invasion | Oocyst development | Sporogony | |
|---|---|---|---|---|---|---|---|
| Protein phosphatases | PPM2 | PPM1 | PPKL PPM2 |
PPKL | SHLP1 | PPM5 | PTPLA |
| Protein kinases | – | SRPK CDPK4 MAP2 |
NEK2 NEK4 GAK PK7 |
CDPK3 | CDPK3 | GAK PK7 |
CDLK |
CDLK, cyclin-dependent-like kinase; CDPK3,4, calcium-dependent protein kinase; GAK, cyclin g-associated kinase; MAP2, mitogen-activated protein kinase; NEK2, NEK4, NimA-related kinase; PK7, protein kinase; PPKL, protein phosphatase containing an N-terminal β-propeller formed by kelch-like motifs; PPM1, PPM2, PPM5, metallo-dependent protein phosphatases; PTPLA, protein tyrosine phosphatase-like A; SHLP1, Shewanella-like protein phosphatase; SRPK, SR protein kinase.
Despite long-established protocols for the culture of P. falciparum gametocytes en masse (Ponnudurai et al., 1983; Sinden et al., 1984) and the detailed descriptions of the complex morphological changes occurring in the skeletal, cytoplasmic and nuclear organization of the parasites (Sinden et al., 1978; Dixon et al., 2012; Hliscs et al., 2015) and in the organization of the mitochondrion and apicoplast (Okamoto et al., 2008), we remain disturbingly ignorant of the molecular profile of the maturing gametocyte. Transcriptomic and proteomic studies (Hall et al., 2005; Khan et al., 2005; Tao et al., 2014) have revealed, for example, 174 male- and 258 female-enriched proteins in mature (dimorphic/separable) P. falciparum gametocyte preparations. The gametocyte metabolomes are incompletely described (Lamour et al., 2014); indirect observations, e.g. drug sensitivity, currently suggest the immature gametocytes (stages I–III) are more readily killed by inhibitors of RNA and protein synthesis (Sinden and Smalley, 1979) and schizonticides (e.g. chloroquine) than the mature (stage IV–V) gametocytes (Smalley, 1977; Sinden, 1982; Targett et al., 2001), an observation previously linked to the substantial reduction in protein synthesis/ribosome population seen in the more mature forms (Sinden et al., 1976; 1978,). To date one of the most interesting of the unexplored aspects of gametocyte biology lies in protein translation for two reasons: first, the mature female gametocyte (which retains a ‘normal’ ribosome population) accumulates a reported 169–731 species of translationally repressed gene transcripts (Mair et al., 2006; Guerreiro et al., 2014), which may be localized in cytoplasmic ‘granules’ (Thompson and Sinden, 1994; Vervenne et al., 1994) where they are presumably complexed with the translational regulators DOZI and CITH (Mair et al., 2006; 2010,; Guerreiro et al., 2014); but what is often overlooked is the evidence that the structure of the ribosome population itself changes as the parasite moves from vertebrate to insect host – the population transitions from A to S-form ribosomes in which different alleles encoding the SsuRNAs 30 and 31 are expressed (Waters et al., 1989). Why these changes occur and whether they have any relevance to the significant role of translational regulation in gametogenesis has yet to be tested.
Structural studies have shown that the mitochondrion enlarges significantly throughout gametocyte maturation (Aikawa et al., 1969; Sinden et al., 1976; Jensen, 1979; Okamoto et al., 2008). The seminal early electron microscopy studies of Aikawa et al. (1969) demonstrated beyond question that the mitochondrion (at the time considered to be numerous organelles) of the gametocytes was morphologically distinguished from those of the asexual bloodstages by the presence of prominent tubular cristae (Aikawa et al., 1969); subsequent studies revealed that in the ookinete and oocyst stages the cristae were even more prominently developed. Jim Jensen (1979) was the first to suggest that the multiple profiles of mitochondria seen in the gametocytes were in fact derived from a single network-like organelle. By contrast the apicoplast remains comparatively small and closely associated with the nucleus. Recent application of elegant whole-cell confocal studies combined with the use of organelle-specific fluorochrome-tagged markers has emphasized how the mitochondrion and apicoplast subsequently proliferate in the oocyst (Stanway et al., 2009). Recent metabolomic studies (Lamour et al., 2014) suggest that oxidative – and lipid – metabolism may differ significantly between the asexual and sexual bloodstage parasites. Recognizing the female gametocyte provides the majority of the biomass of the zygote/ookinete, this expansion of energy provision is understandable, but the male gametocyte forms eight microgametes devoid of both plastids, suggesting in the male cell that it performs a vital function during gametocytogenesis or during the dramatic events of gamete formation in the mosquito? It has been shown that the apicoplast provides isoprenoids essential for gametocytogenesis (Wiley et al., 2013), and up-regulation of glyoxalase provides defence against oxidative stress (Okamoto et al., 2008) that has recently been shown to be a component of the mosquitoes' responses to malarial infection (Shrinet et al., 2014). Up-regulation of genes encoding type II fatty acid, and 15 of 16 TCA enzymes in the mature gametocyte are also consistent with the cytological observations. In marked contrast it is the enzymes of the glycolytic pathway that are among the most abundant proteins in the free swimming microgamete (Wass et al., 2012; Talman et al., 2014). The male gamete lacks both plastids (Sinden et al., 1976; Okamoto et al., 2008); as a consequence their inheritance is maternal (Creasey et al., 1993), and the male gametes rely entirely on hexose import and glycolysis for energy to drive their vigorous swimming (Slavic et al., 2011; Talman et al., 2014). Reflecting on this body of data, it is interesting to note that some of the antimalarial drugs, e.g. atovaquone with known mitochondrial targets such as the cytochrome B1 complex and adenosine triphosphate (ATP) binding cassette transporter (Fry and Pudney, 1992; Rijpma et al., 2014), are exquisitely active against transmission of parasites from vertebrate to mosquito hosts, and the ookinete in particular (Fowler et al., 1995; Delves et al., 2012).
An outstanding dilemma in the understanding of gametocyte biology is whether the pattern of metabolism revealed by the studies on slow maturing/long-lived gametocytes of the subgenus Laverania (Smalley and Sinden, 1977; Eichner et al., 2001; Bousema and Drakeley, 2011) is representative of the subgenus Plasmodium. Evidence suggesting this may be the case stems from the observations that purified populations of viable mature infectious gametocytes of the rodent malaria parasites, like those of P. falciparum, can be prepared by treating mixed bloodstage infections with schizonticides (Beetsma et al., 1998; Rodrıguez et al., 2002).
The impact of gametocytes on the biology of host/parasite–vector interactions has for decades provoked intriguing discussion and experimentation. ‘Is the infectious host less responsive to mosquito bites?’ (Rossignol et al., 1985); ‘Is it more attractive to mosquitoes?’ (Batista et al., 2014); ‘Is the sporozoite-infected mosquito more likely to probe?’ (Anderson et al., 1999). At the cellular level the oft-raised question whether mature (infectious) gametocytes are preferentially retained in the capillaries of the skin where they would be accessible to the mosquito remains an interesting but still unresolved question. The potential now to use late gametocyte promoters to regulate luciferase expression in the mature gametocyte, combined with IVIS technology, might provide the quickest method of resolving this question. Should gametocytes exhibit this tropism, it would seriously impact the methodologies required to describe the infectious reservoir. However, recognizing the now widely acknowledged fact that ∼ 80% of persons infected with P. falciparum will also be gametocyte positive (Muirhead-Thomson and Mercier, 1952a,b,; Muirhead-Thomson, 1957; Bousema and Drakeley, 2011) does raise the question as to how any further knowledge, beyond correlating peripheral gametocytaemia with the probability of infecting mosquitoes (Churcher et al., 2013), is going to change any control strategy (Bousema et al., 2012) – which must surely treat every infected individual as if they were infectious to the vector.
Sexual development in mosquito
For this author, the events of malaria microgametogenesis (exflagellation) in the mosquito vector remain one of the most beautifully orchestrated and dramatic developmental transformations in eukaryote biology.
Induction of exflagellation
Our understanding of the induction of gametogenesis at the molecular level has advanced little since its discovery in 1998 (Billker et al., 1998). We know two conditions must prevail: a fall in temperature of > 5°C (Roller and Desser, 1973; Sinden and Croll, 1975), suggesting an as yet undescribed role for heat shock proteins in protein remodelling, and the presence of elevated levels of the mosquito waste product xanthurenic acid (XA) (Billker et al., 1998). The fall in temperature alone may trigger the secretion of the osmiophilic bodies leading to RBC breakdown (see below).
In marked contrast the downstream pathways regulating the component events of gamete formation, namely – escape from the RBC and molecular remodelling of the gamete surfaces and exclusively in the male cell – genome replication, genome separation and axoneme assembly, are now extensively although still incompletely characterized. Current models of the signalling cascade regulating gametogenesis have been outlined (Baker, 2010; Morahan and Garcia-Bustos, 2014; Sinha et al., 2014). Although prior data suggested raised extracellular pH (∼pH 8.0) is a useful laboratory ‘inducer’ (Nijhout and Carter, 1978), the overall pH of the bloodmeal only rises by some 0.2 pH units in the 24 h following bloodmeal ingestion (Billker et al., 1997), suggesting that a transient rise in intracellular pH may form part of the signalling cascade. Studies on the role of gametocyte ion pumps (Kawamoto et al., 1992; 1993,; Kawamoto, 1993) nonetheless suggested that the cytoplasmic pH of the gametes might change both rapidly and significantly.
Activation (directly or indirectly) of guanylyl cyclase by XA produces cGMP that is in turn converted to guanidine 5 monophosphate by phosphodiesterase (knockout of pdeδ ablates exflagellation). cGMP activates PKG (McRobert et al., 2008) that in turn maintains elevated levels of cytosolic Ca2+ (see below) (Brochet et al., 2014), which regulates rounding up (i.e. increase in volume = water uptake?) of the cells and the expression of at least some translationally repressed surface proteins. Deletion of pbcax, however, prevents the onward development of the rounded female cell into an ookinete (Guttery et al., 2013).
XA additionally stimulates PIPLC to produce PIP2 and IP3; these in turn give rise to elevated cytoplasmic calcium just 10 s after activation (Billker et al., 2004; Sebastian et al., 2012) which, through CDPK4, results in DNA replication and axoneme assembly in the male cell, and translation release in the female. Downstream of CDPK4, and mediated by NEK1 and NEK3, MAP2 and SPRK regulate the motility of the axonemes and cytokinesis of the microgametocyte (Tewari et al., 2010). The writer is amused by the observation that a viagra-like molecule (Zaprinast) can obviate the need for XA in male gamete release (McRobert et al., 2008).
Escape from the RBC
Both male and female cells increase in volume (Sinden and Croll, 1975); thus, stressing the host cell, the males additionally release very motile gametes that undoubtedly assist the disruption of a weakened host cell (Sologub et al., 2011; Deligianni et al., 2013; Wirth et al., 2014). Escape is mediated by the secretion of the osmiophilic bodies (more abundant in the sessile female; Sinden et al., 1976). The biogenesis and lytic function of the osmiophilic bodies is dependent on the following moieties Pfg377: exclusively in females (Alano et al., 1995; Olivieri et al., 2014), MDV-1/PEG3 (Lanfrancotti et al., 2007; Lal et al., 2009; Ponzi et al., 2009), pbGEST (Talman et al., 2011) cysteine and aspartic protease, and a perforin-like protein 2 (Wirth et al., 2014).
Genome replication in male gametocyte
The ploidy and replication of the gametocyte genome has been a controversial topic, resolved in large part by the studies of Cornelissen and Janse in the 1980s, who, using cytochemical techniques, showed that mature gametocytes like the merozoite are haploid, and that the male cell replicated its DNA three times in brief period (∼10 min) between induction and microgamete release – exflagellation (Janse et al., 1986a,b,; Cornelissen, 1988). This replication is cdpk4 dependent (Guttery et al., 2012a). In cdc20 and map2 knockout lines subsequent mitotic separation of the replicated genomes in the male cells is blocked after metaphase (Guttery et al., 2012a). The incredible speed of replication suggested that thousands of replication forks are distributed across the 14 chromosomes. DNA polymerase-α mediates replication, a process sensitive to aphidicolin but not mitomycin C (Janse et al., 1986a). Electron microscopy revealed three conventional mitotic divisions within the single large male nucleus at ∼ 3, 8 and 15 min following activation (conventional with the no exception that at no point do the chromosomes condense! – a fact that might correlate with the very rapid replication of the genomes). Although this observation is entirely consistent with the absence of histone 1 from the genome, it also raises fascinating questions as to how the initial 14, and final 112, filamentous chromosomes (whose telomeres are likely embedded in the nuclear envelope) can be moved by the kinetochores on the spindle microtubules with such speed and precision without breaking. The female gamete like the originating immature female gametocyte is haploid, but the intervening nuclear events are unclear; data suggested that the DNA content may rise above 1°C (Cornelissen, 1988) and descriptions of spindle-like structures in the developing (putative) macrogametocyte are either erroneous or suggest the female genome is somehow modified (Sinden et al., 1978).
Axoneme assembly in male gametocyte
The cytoplasm of the mature microgametocyte contains large quantities of tubulin, which upon activation rapidly polymerize into microtubules. Polymerization begins with the de novo assembly of eight basal bodies (apparently as two conventionally orientated orthogonal tetrads). Basal body structure/patterning and function requires SAS6 expression (Marques et al., 2015). Axoneme polymerization, being intracytoplasmic, is independent of intra-flagellar transport and consequentially very rapid; all eight axonemes reaching 14 μm in length in just 10 min, when formed, lay coiled around the persistent envelope of the single nucleus. Axonemes are largely of conventional 9 + 2 design (Sinden et al., 2010). The formation of the central pair is severely, although incompletely, disrupted when the armadillo repeat protein PF16 is deleted (Straschil et al., 2010). At the onset of exflagellation, the axonemes become motile and swim basal body first out of the parental cell. The prior attachment of each basal body to a mitotic spindle pole ‘ensures’ each basal body drags a haploid genome into the gamete; the gamete then swims through the dense mosquito bloodmeal using alternating periods of fast and slow rotary ‘ambidextrous’ sinusoidal waves (Sinden and Croll, 1975; Wilson et al., 2013). Microgamete motility is entirely dependent on the import of hexose from the bloodmeal (Slavic et al., 2011).
Protein remodelling of the gamete surfaces
The surface proteins of the male and female gametes differ both from each other and from that of the parental gametocytes. Sexual differences between gametes, e.g. expression of P48/45, P230 and HAP2 in male and P47 and the LAP/CCCP proteins in female (van Schaijk et al., 2006; Raine et al., 2007; Liu et al., 2008), may reflect functions in fertilization, and differences between gametocyte and gametes may indicate functions related to gamete survival in the challenging (both immunologically and enzymatically) environment of the bloodmeal. Expression of some 169–731 proteins in the female gamete, including the surface proteins P25 and P28 on the fertilized zygote, is dependent on the release of their mRNA from translation repression (Mair et al., 2006; 2010,; Guerreiro et al., 2014), a process in part regulated by CDPK1 (reviewed by Morahan and Garcia-Bustos, 2014). Early defence of the gametes/zygote by expression of a complement regulator factor H-like protein (Simon et al., 2013) inactivates complement ingested in the bloodmeal (Grotendorst and Carter, 1987; Margos et al., 2001; Simon et al., 2013); however, the gametes have no defence against antibody attack targeted against proteins secreted onto the gamete surface (e.g. P45/48; P230), making these molecules excellent targets for ‘contraceptive’ vaccines (Carter et al., 2000; Sauerwein, 2007). P230 is reportedly responsible for the attachment of the male gametes to RBCs in the bloodmeal (Templeton et al., 1998), although what possible advantage this is to the parasite escapes this writer.
Fertilization
Fertilization of malaria gametes was first recognized in 1897 (McCallum, 1897) and described at the ultrastructural level some 40 years ago (Sinden et al., 1976; Aikawa et al., 1984). Gamete recognition and binding is mediated through GPI-anchored P47 on female interacting with P230, complexed to GPI-anchored P48/45 on the male cell. The ‘ambidextrous’ ability of P230 to bind both male- and female-specific moieties may explain the formation of intimately bound same-sex and mixed-sex clusters of gametes/gametocytes in vitro (Janse et al., 1985; Sinden and Hartley, 1985; Sinden et al., 1985). The fusion of the gamete membranes is now known to be mediated by male-specific HAP2/CSC1 (Liu et al., 2008; Mori et al., 2010) and can be severely disrupted by anti-HAP2 antibodies (Blagborough and Sinden, 2009; Miura et al., 2013). Fusion of the gamete nuclei occurs within 1 h of plasma membrane fusion (Aikawa et al., 1984), and is preceded by the de-condensation of the chromosomes in the male nucleus. There is substantial transcriptional up-regulation of the zygote genome via AP2O (Yuda et al., 2009; Akinosoglou et al., 2015). We do not yet have definitive evidence as to when expression of male-inherited genes begins, but zygote development reportedly can progress in the absence of RNA polymerase II mediated transcription (Guerreiro et al., 2014).
Within the mosquito the parasite is from the time of zygote formation until budding of daughter sporozoites from the oocyst a polyploid cell, but cytological study shows that the genetic organization of the parasite, following fertilization, is immediately returned to a haploid state, within a single nucleus, by a conventional two-step meiotic division producing four haploid genomes in the ookinete (Janse et al., 1986b; Sinden, 1991). Thus, any one oocyst has the potential to contain four discrete and potentially recombinant genotypes; this has been confirmed experimentally (Ranfordcartwright et al., 1991).
It must be remembered that the process of gamete production and fertilization occurs in an environment composed essentially of unaltered host blood to which the standing components of the mosquito gut have been added, among which the trypsin-like proteases are significant factor (Muller et al., 1993). The introduction of proteolytic enzymes to the bloodmeal degrades vertebrate complement in the first 3 h (Margos et al., 2001), a change that is intriguingly mirrored by the short-lived initial defence of the parasite against complement (see above) – a truly fascinating interplay between host, vector and parasite.
Host antibodies persist, at potentially lethal concentrations, in the bloodmeal for at least 24 h, and can escape through the midgut epithelium into the haemocoele (possibly enhanced by the rupture of the epithelium by the invading ookinetes; Han and Barillas-Mury, 2002). Convincing evidence has been provided that cytokines, which can be present at significant levels in the hosts' blood especially following synchronous schizogony (Motard et al., 1990), can, when present in the bloodmeal, significantly suppress both gametogenesis and oocyst infection (Dearsly et al., 1990; Naotunne et al., 1993). The duration for which active host cytokines persist in the bloodmeal is however not known. Interesting data suggesting a dialogue between vertebrate insulin and mosquito receptors (Pakpour et al., 2012) raise the fascinating possibility that the antiparasitic activity of mosquito may be both directly and indirectly mediated.
The mosquito places both physical and immune barriers in the path of the parasite. The physical barrier to infection/invasion offered by the peritrophic matrix (PTM) was revealed when inhibitors of parasite chitinase (e.g. allosamidin) were shown to decrease oocyst numbers (Shahabuddin et al., 1993; 1995,; Shahabuddin and Kaslow, 1994a,b,; Zieler et al., 1999; 2000,). Humoral insect defence mechanisms however remain potent inhibitors when the ookinete crosses the epithelial layer (James, 1928; Dimopoulos, 2003; Osta et al., 2004; Christophides, 2005; Povelones et al., 2011; Shrinet et al., 2014).
The ookinete
Notwithstanding the critical genetic events of meiosis (Sinden, 1985; Sinden and Hartley, 1985) – recently shown to be regulated by NIMA kinases, NEK2 and 4 (Reininger et al., 2005; 2009; 2012,,) and reviewed in Morahan and Garcia-Bustos (2014) – the key role of differentiation of the zygote into the ookinete is to produce a polarized motile cell that can escape the increasingly potent digestive enzymes of the bloodmeal to which the ookinete is susceptible (Gass, 1977; Gass and Yeates, 1979). This differentiation is both fertilization dependent and reliant upon PK7, PPKL (protein phosphatase with Kelch-like domains) and GA kinase activities (Guttery et al., 2012b; Philip et al., 2012; Morahan and Garcia-Bustos, 2014). Although formation of the single ookinete from each zygote in very large part mirrors the assembly of both the merozoite and the sporozoite from their respective ‘schizonts’, we know less of the cellular events. Although the assembly and cytoskeletal polarity of apicomplexan ‘zoites’ is clearly determined by the position of a single nuclear spindle plaque/microtubule organizing centre (Dubremetz, 1975), it is challenging to understand how/whether the ookinete nucleus, which contains four recombinant haploid genomes/spindle plaques, similarly dictates the polar assembly of the single ookinete cytoskeleton (unless polarity was determined by the early polarization of the zygote nucleus and formation of the perinuclear array of cytoplasmic microtubules; Aikawa et al., 1984). The functions of the elaborate, and ookinete-specific, anterior apical collar, beyond providing apparent rigidity to the ookinete penetrative apparatus, have yet to be determined. The functions of those components driving motility are better but still incompletely understood. Conventional thinking suggests motility is dependent on CDPK3, Ca++ and cGMP signalling pathways (Siden-Kiamos and Louis, 2008; Moon et al., 2009; Guttery et al., 2012b), controlling the activity of the extensively described actin-myosin glideosome motor (Raibaud et al., 2001; Opitz and Soldati, 2002; Kan et al., 2014) that is linked to transmembrane surface adhesins such as CTRP, a large protein composed of seven thrombospondin and six von Willebrand factor A domains, of which only the proximal A domains appear to be essential to locomotion (Ramakrishnan et al., 2011). The validity of the conventional glideosome model has recently been challenged by controversial data from Toxoplasma suggesting neither actin nor myosin is essential for locomotion (Andenmatten et al., 2012). The spiral architecture of the microtubular ookinete skeleton dictates both the overall shape of the ookinete and the direction of movement (essentially a left-handed corkscrew; Vlachou et al., 2004; Kan et al., 2014), which may be optimal to drive the parasite through the particulate and increasingly ‘viscous’ bloodmeal.
Whether the movement of the ookinete is in any way directed to the midgut epithelium is not known, but interaction with the complex layers of the midgut epithelium is certainly not understood. The epithelium is composed of three layers: the chitinous PTM, which is secreted at the time of bloodmeal ingestion (Shao et al., 2001); the tubular network (Zieler et al., 2000); and the cell epithelium monolayer. Traversal of the PTM (if formed) is assisted by the secretion of one or more chitinase enzymes, one secreted as a pro-enzyme that is reportedly activated by the mosquito trypsins (Shahabuddin et al., 1993). Addition of the chitinase inhibitor allosamidin to bloodmeals containing P. gallinaceum or P. falciparum reduced oocyst numbers (Shahabuddin et al., 1993), and in P. berghei knockout of chitinase 1 reduces infectivity significantly (Dessens et al., 2001).
Microscopic evidence has been interpreted as suggesting that ookinetes preferentially invade/lyse epithelial cells at the posterior of the midgut, and close to intercellular junctions. Early work suggested that the ‘preferential’ oocyst distribution at the posterior midgut was simply due to the sedimentation of the iRBC in the bloodmeal during the prolonged period when the bloodfed female mosquitoes rest ‘head-up’ after the feed. The validity of this early observation has, however, been questioned (Kan et al., 2014). Studies in the 1990s showing that ookinetes in the midgut wall frequently co-located with v-ATPase activity led workers to suggest that ookinetes invaded a specific subclass of epithelial cell, which they termed the ‘Ross Cell’ (Shahabuddin, 2002). The study, however, failed to recognize that v-ATPase might be up-regulated following, and not before, invasion. To some, the most compelling data suggesting there is a specific epithelial ligand bound by the ookinete comes from antibody blockade studies where anti-P25, -P28 (Gozar et al., 1998; Miura et al., 2013), enolase (through interaction with host plasminogen; Ghosh et al., 2011) and AgAPN1 antibodies (Armistead et al., 2013) can inhibit invasion. However, it must be recognized that coating abundant surface moieties with antibodies does not necessarily identify specific ligands. Other molecules reducing ookinete invasion by unknown mechanisms when added to the bloodmeal include PLA2 (Rodrigues et al., 2008), bee venom (Moreira et al., 2002) and the peptides SHIVA (Boisbouvier et al., 1998), and SM1 (Fang et al., 2011). Early data (Rosales-Ronquillo and Silverman, 1974) strongly suggested that ookinetes can recognize and lyse RBC – exactly the same mechanisms now believed to mediate midgut invasion, it is thus tempting to speculate that the host molecules inducing secretion of the lytic contents of the abundant micronemes are shared between the RBC and mosquito epithelial cells (and are perhaps somewhat less specific than commonly hypothesized). In our efforts to develop ever improved transmission-blocking vaccines, we must recognize we know too little of the molecular architecture and immunogenic potential of the ookinete surface.
Twelve to 36 h following ookinete–epithelium interaction, the secretions from micronemes lyse the host cell and the ookinete migrates into the resulting ‘cadaver’. Should many ookinetes invade in the same locality epithelial destruction is considerable (Ecker et al., 2007), with the consequent opportunity of transfer of bloodmeal contents to the haemocoele, significant parasite density-dependent mosquito death may occur at this time. The writer is fascinated by the question of how the ookinete escapes, in a directed manner, the sac of the lysed midgut cell, particularly noting the very different molecular profiles presented by the cytoplasmic membrane surfaces potentially encountered. The ‘time bomb’ theory of midgut invasion (Han and Barillas-Mury, 2002) suggests the lysed cells will be ejected from the epithelium by a draw-string repair mechanism, involving substantial reorganization of the epithelial–cell actin network (Shiao et al., 2006); thus, escape from the lysed cell is essential for ‘infection’ to be a success. We do not yet understand what, on reaching the luminal/midgut face of the basal lamina, induces the emergent ookinete to become immotile and begin differentiation into an oocyst, but it is tempting to suggest that the molecular recognition of the collagen-rich basal lamina is a regulatory factor. Note, however, that the ookinete is very motile in Matrigel® (Corning Life Sciences), an artificial intercellular matrix rich in (mouse) laminin, enactin and collagen (Moon et al., 2009).
Irrespective of the extent of the damage caused by the ookinete to the epithelial cells, the mosquito mounts a significant response to the ookinete and developing oocyst within the epithelial layer. Current evidence in a rapidly changing area of study suggests that, in a nitration-dependent event (Molina-Cruz et al., 2008), the mosquito thioester (complement-like) molecule TEP-1 forms a complex with LRIM1 and APL1 and the ookinete surface, causing the lethal deposition of melanin on the parasites' membrane systems (Sinden and Garnham, 1973; Fraiture et al., 2009; Povelones et al., 2011). The presence of the protein Pfs47 on the parasite surface reportedly reduces this nitration-dependent attack mechanism (Molina-Cruz et al., 2013). Other PKC-mediated antimalarial immune response pathways have been identified (Pakpour et al., 2013).
Although infection of the mosquito is theoretically ‘completed’ following the induction of sporogony in the ookinete under the basal lamina, the parasite nonetheless undergoes further unique and poorly understood developmental steps that intrigue the writer, topmost being the restructuring of its ribosome population. In the early 1990s pioneering work in the McCutchan laboratory recognized that the sSUrRNA from sporozoites (S) and asexual blood stages (A) differed (Zhu et al., 1990; Waters, 1994). Subsequently, it was demonstrated that shortly after fertilization a third form (O) of the 18S RNA is expressed to be replaced in the late oocyst by the S form (Li et al., 1997). It is interesting to note that the small ribosomal subunit protein S1 has the highest transcript abundance in the gametocyte and S2 in the ookinete; whether this is related to the skeletal 18S rRNA changes is unclear. What biological critical advantage is gained from these fundamental and fascinating changes in ribosome design are still unresolved.
The future?
Over the past 50 years the advances in our understanding of malaria cell biology have been truly amazing, what of the next 50 years? Ongoing metabolomic studies are opening a broader understanding of parasite function and might be expected to play a central role in future drug development programmes. Image resolution does not need to improve on that of the electron microscope (at the cellular level) and X-ray crystallography (at the molecular level), but without doubt the new abilities to determine in live or un-fixed specimens, the exact position of identified molecules in complex molecular/cellular machines will be utterly fascinating (Wong et al., 2014). This combined with in vivo single-cell imaging has the potential to transform our understanding of these amazing cells.
Recognizing the paucity of technologies available to cell biologists in the 1970s, it is somewhat bizarre to believe that it is almost to be taken for granted that we will have at our disposal fluorescent-tagged constructs, and knockouts of every parasite gene of interest, but to be provocative, if we are to make significant steps in our understanding of the parasites molecular machines perhaps we should already consider the construction of appropriately tagged protein libraries for the ultrastructural localization of molecules in situ by cryo-tomography. As ever we will be dependent on emerging technologies, such as single-cell ‘omics’, but perhaps we should think more of the difficult areas of lipid and carbohydrate biology and the roles of small nucleic acids in parasite biology.
It is now clear that in our efforts to reduce the impact of these potent pathogens, the sexual and early sporogonic development of malarial parasites offer unique opportunities to attack vulnerable bottleneck populations with both drugs (Ruecker et al., 2014) and ‘simple’ antibody-based vaccines (Miura et al., 2013). It should therefore be a collective priority to understand: first, the contrasting metabolic organizations of the quiescent (and comparatively schizonticide insensitive) mature gametocyte and the diverse pathways mediating gamete, zygote and ookinete development; and second, how the molecular composition and architecture of the exposed surfaces of the gametes, zygotes and ookinetes define the immunological vulnerability of both fertilization and zygote development. We live in exciting times with ever new and powerful technologies at our disposal; we must however accept our responsibility to those infected by these formidable pathogens that perhaps the most important task in front of us is to ensure the considerable knowledge we have already gathered and will continue to gather is translated into useful intervention measures, a process which of itself provides academic challenges and rewards every bit as demanding as those of basic research.
Acknowledgments
The author would like to thank the reviewers and the editor for their very constructive and detailed critique of the original manuscript; any remaining errors/omissions in the text are entirely my responsibility. The author perceives no conflicts of interest.
References
- Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cisteró P, et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood. 2014;123:959–966. doi: 10.1182/blood-2013-08-520767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M, Huff CG. Sprinz H. Comparative fine structure study of the gametocytes of avian, reptilian and mammalian malaria parasites. J Ultrastruct Res. 1969;26:316–331. doi: 10.1016/s0022-5320(69)80010-9. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Carter R, Ito Y. Nijhout M. New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. J Protozool. 1984;31:403–413. doi: 10.1111/j.1550-7408.1984.tb02987.x. [DOI] [PubMed] [Google Scholar]
- Akinosoglou KA, Bushell ES, Ukegbu CV, Schlegelmilch T, Cho JS, Redmond S, et al. Characterization of Plasmodium developmental transcriptomes in Anopheles gambiae midgut reveals novel regulators of malaria transmission. Cell Microbiol. 2015;17:254–268. doi: 10.1111/cmi.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano P. The sound of sexual commitment breaks the silencing of malaria parasites. Trends Parasitol. 2014;30:509–510. doi: 10.1016/j.pt.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Alano P, Read D, Bruce M, Aikawa M, Kaido T, Tegoshi T, et al. COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol Biochem Parasitol. 1995;74:143–156. doi: 10.1016/0166-6851(95)02491-3. [DOI] [PubMed] [Google Scholar]
- Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andenmatten N, Egarter S, Jackson AJ, Jullien N, Herman JP. Meissner M. Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nat Methods. 2012;10:125–127. doi: 10.1038/nmeth.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Koella JC. Hurd H. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc R Soc Lond B. 1999;266:1729–1733. doi: 10.1098/rspb.1999.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead JS, Morlais I, Mathias DK, Jardim JG, Joy J, Fridman A, et al. Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of falciparum and vivax malaria. Infect Immun. 2013;82:818–829. doi: 10.1128/IAI.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista E, Costa E. Silva A. Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasit Vect. 2014;7:251. doi: 10.1186/1756-3305-7-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetsma AL, van de Viel TJJM, Sauerwein RW. Eling WMC. Plasmodium berghei ANKA: purification of large numbers of infectious gametocytes. Exp Parasitol. 1998;88:69–72. doi: 10.1006/expr.1998.4203. [DOI] [PubMed] [Google Scholar]
- Bhasin VK. Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984;33:534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Billker O, Shaw MK, Margos G. Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology. 1997;115:1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B. Brinkman V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Birago C, Bucci A, Dore E, Frontali C. Zenobi P. Mosquito infectivity is directly related to the proportion of repetitive DNA in Plasmodium berghei. Mol Biochem Parasitol. 1982;6:1–12. doi: 10.1016/0166-6851(82)90048-2. [DOI] [PubMed] [Google Scholar]
- Blagborough AM. Sinden RE. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine. 2009;27:5187–5194. doi: 10.1016/j.vaccine.2009.06.069. [DOI] [PubMed] [Google Scholar]
- Boisbouvier J, Prochnika-Chalufour A, Nieto AR, Torres JA, Nanard N, Rodriguez MH, et al. Structural information on a cercropin-like synthetic peptide, Shiva 3 toxic to the sporogonic development of Plasmodium berghei. Eur J Biochem. 1998;257:263–273. doi: 10.1046/j.1432-1327.1998.2570263.x. [DOI] [PubMed] [Google Scholar]
- Boisson B, Lacroix C, Bischoff E, Gueirard P, Bargieri DY, Franke-Fayard B, et al. The novel putative transporter NPT1 plays a critical role in early stages of Plasmodium berghei sexual development. Mol Microbiol. 2011;81:1343–1357. doi: 10.1111/j.1365-2958.2011.07767.x. [DOI] [PubMed] [Google Scholar]
- Bousema T. Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:1–35. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci NM, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, et al. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. 2014;16:165–176. doi: 10.1016/j.chom.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Brochet M, Collins MO, Smith TK, Thompson E, Sebastian S, Volkmann K, et al. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca2 signals at key decision points in the life cycle of malaria parasites. PLoS Biol. 2014;12:e1001806. doi: 10.1371/journal.pbio.1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MC, Carter RN, Nakamura K, Aikawa M. Carter R. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen pfs16. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Canning EU. Sinden RE. The organization of the ookinete and observations on nuclear division in oocysts of Plasmodium berghei. Parasitology. 1973;67:29–40. doi: 10.1017/s0031182000046266. [DOI] [PubMed] [Google Scholar]
- Carter LM, Kafsack BFC, Llinás M, Mideo N, Pollitt LC. Reece SE. Stress and sex in malaria parasites: why does commitment vary? Evol, Med Public Health. 2013;2013:135–147. doi: 10.1093/emph/eot011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ. 1979;57:37–52. [PMC free article] [PubMed] [Google Scholar]
- Carter R, Mendis KN, Miller LH, Molineaux L. Saul A. Malaria transmission-blocking vaccines – how can their development be supported? Nat Med. 2000:241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- Christophides G. Transgenic mosquitoes and malaria transmission. Cell Microbiol. 2005;7:325–333. doi: 10.1111/j.1462-5822.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouedraogo AL. Basanez MG. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife. 2013;2:e00626. doi: 10.7554/eLife.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BI, Skillman KM, Jiang RH, Childs LM, Altenhofen LM, Ganter M, et al. A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion. Cell Host Microbe. 2014;16:177–186. doi: 10.1016/j.chom.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen AWCA. Sex determination and sexual differentiation in malaria parasites. Biol Rev. 1988;63:379–394. doi: 10.1111/j.1469-185x.1988.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Creasey AM, Ranfordcartwright LC, Moore DJ, Williamson DH, Wilson RJM, Walliker D. Carter R. Uniparental inheritance of the mitochondrial gene cytochrome-b in Plasmodium falciparum. Curr Genet. 1993;23:360–364. doi: 10.1007/BF00310900. [DOI] [PubMed] [Google Scholar]
- Day KP, Karamalis F, Thompson J, Barnes DA, Peterson C, Brown H, et al. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-Megabase region of chromosome-9. Proc Natl Acad Sci U S A. 1993;90:8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearsly AL, Sinden RE. Self I. Sexual development in malarial parasites: gametocyte production, fertility and infectivity to the mosquito vector. Parasitology. 1990;100:359–368. doi: 10.1017/s0031182000078628. [DOI] [PubMed] [Google Scholar]
- Deligianni E, Morgan RN, Bertuccini L, Wirth CC, Silmon de Monerri NC, Spanos L, et al. A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell Microbiol. 2013;15:1438–1455. doi: 10.1111/cmi.12131. [DOI] [PubMed] [Google Scholar]
- Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, et al. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens JT, Mendoza J, Claudianos C, Vinetz JM, Khater E, Hassard S, et al. Knockout of the rodent malaria parasite chitinase PbCHT1 reduces infectivity to mosquitoes. Infect Immun. 2001;69:4041–4047. doi: 10.1128/IAI.69.6.4041-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G. Insect immunity and its implication in mosquito-malaria interactions. Cell Microbiol. 2003;5:3–14. doi: 10.1046/j.1462-5822.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- Dixon MWA, Dearnley MK, Hanssen E, Gilberger T. Tilley L. Shape-shifting gametocytes: how and why does P. falciparum go banana-shaped? Trends Parasitol. 2012;28:471–478. doi: 10.1016/j.pt.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Dubremetz JF. La genese des merozoites chez la Coccidie Eimeria necatrix. Etude ultrastructurale. J Protozool. 1975;22:71–84. doi: 10.1111/j.1550-7408.1975.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Dyer M. Day KP. Commitment to gametocytogenesis in Plasmodium falciparum. Parasitol Today. 2000;16:102–107. doi: 10.1016/s0169-4758(99)01608-7. [DOI] [PubMed] [Google Scholar]
- Ecker A, Pinto SB, Baker KW, Kafatos FC. Sinden RE. Plasmodium berghei: Plasmodium perforin-like protein 5 is required for mosquito midgut invasion in Anopheles stephensi. Exp Parasitol. 2007;116:504–508. doi: 10.1016/j.exppara.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, Diebner HH, Molineux L, Collins WE, Jeffery GM. Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans R Soc Trop Med Hyg. 2001;95:497–501. doi: 10.1016/s0035-9203(01)90016-1. [DOI] [PubMed] [Google Scholar]
- Eksi S, Morahan BJ, Haile Y, Furuya T, Jiang H, Ali O, et al. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog. 2012;8:e1002964. doi: 10.1371/journal.ppat.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Vega-Rodríguez J, Ghosh AK, Jacobs-Lorena M, Kang A. St Leger RJ. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. 2011;331:1074–1077. doi: 10.1126/science.1199115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfour E, Charlotte F, Settegrana C, Miyara M. Buffet P. The extravascular compartment of the bone. Malar J. 2012;11:285. doi: 10.1186/1475-2875-11-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem RGA, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, et al. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler RE, Sinden RE. Pudney M. Inhibitory activity of the anti-malarial atovaquone (566C80) against ookinetes, oocysts, and sporozoites of Plasmodium berghei. J Parasitol. 1995;81:452–458. [PubMed] [Google Scholar]
- Fraiture M, Baxter RHG, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, et al. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Fry M. Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquin one (566C80) Biochem Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- Garnham PCC. The structure of the early sporogonic stages of Plasmodium berghei. Ann Soc Belg Med Trop. 1965;45:259–265. [PubMed] [Google Scholar]
- Garnham PCC, Bird RG. Baker JR. Electron microscope studies of motile stages of malaria parasites. V. Exflagellation in plasmodium, hepatocystis and leucocytozoon. Trans R Soc Trop Med Hyg. 1967;61:58–68. doi: 10.1016/0035-9203(67)90054-5. [DOI] [PubMed] [Google Scholar]
- Gass RF. Influences of blood digestion on the development of Plasmodium gallinaceum (Brumpt) in the midgut of Aedes aegypti (L.) Acta Trop. 1977;34:127–140. [PubMed] [Google Scholar]
- Gass RF. Yeates RA. In vitro damaged of cultured ookinetes of Plasmodium gallinaceum by digestive proteinases from susceptible Aedes aegypti. Acta Trop. 1979;36:243–252. [PubMed] [Google Scholar]
- Ghosh AK, Coppens I, Gardsvoll H, Ploug M. Jacobs-Lorena M. Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. PNAS. 2011;108:17153–17158. doi: 10.1073/pnas.1103657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozar MMG, Price VL. Kaslow DC. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission- blocking antibodies in mice. Infect Immun. 1998;66:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst CA. Carter R. Complement effects on the infectivity of Plasmodium gallinaceum to Aedes aegypti mosquitoes. II. Changes in sensitivity to complement-like factors during zygote development. J Parasitol. 1987;73:980–984. [PubMed] [Google Scholar]
- Guerreiro A, Deligianni E, Santos JM, Silva PA, Louis C, Pain A, et al. Genome-wide RIP-Chip analysis of translational repressor-bound mRNAs in the Plasmodium gametocyte. Genome Biol. 2014;15:493. doi: 10.1186/s13059-014-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Ferguson DJP, Poulin B, Xu Z, Straschil U, Klop O, et al. A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development. PLoS Pathog. 2012a;8:e1002554. doi: 10.1371/journal.ppat.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Poulin B, Ferguson DJ, Szoor B, Wickstead B, Carroll PL, et al. A unique protein phosphatase with kelch-like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion. PLoS Pathog. 2012b;8:e1002948. doi: 10.1371/journal.ppat.1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Pittman JK, Frenal K, Poulin B, McFarlane LR, Slavic K, et al. The Plasmodium berghei Ca2+/H+ exchanger, PbCAX, is essential for tolerance to environmental Ca2+ during sexual development. PLoS Pathog. 2013;9:e1003191. doi: 10.1371/journal.ppat.1003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Poulin B, Ramaprasad A, Wall RJ, Ferguson DJP, Brady D, et al. Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe. 2014;16:128–140. doi: 10.1016/j.chom.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- Han YS. Barillas-Mury C. Implications of time bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol. 2002;32:1311–1316. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Hawking F, Wilson ME. Gammage K. Evidence for the cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Hayward RE, DeRisi JL, Alfadhli S, Kaslow DC, Brown PO. Rathod PK. Shotgun DNA microarrays and stage-specific gene expression in Plasmodium falciparum malaria. Mol Microbiol. 2000;35:6–14. doi: 10.1046/j.1365-2958.2000.01730.x. [DOI] [PubMed] [Google Scholar]
- Hliscs M, Millet C, Dixon MW, Siden-Kiamos I, McMillan P. Tilley L. Organisation and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell Microbiol. 2015;17:207–225. doi: 10.1111/cmi.12359. [DOI] [PubMed] [Google Scholar]
- Ikadai H, Shaw Saliba K, Kanzok SM, McLean KJ, Tanaka TQ, Cao J, et al. Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. PNAS. 2013;110:E1676–E1684. doi: 10.1073/pnas.1217712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SP. Ross's ‘black spores’ in the stomach and salivary glands of malaria-infected mosquitoes. Trans R Soc of Trop Med Hyg. 1928;XXI(4):258. [Google Scholar]
- Janse CJ, Rouwenhorst RJ, van der Klooster PFJ, van der Kaay HJ. Overdulve JP. Development of Plasmodium berghei ookinetes in the midgut of Anopheles atroparvus mosquitoes and in vitro. Parasitology. 1985;91:219–225. doi: 10.1017/s0031182000057322. [DOI] [PubMed] [Google Scholar]
- Janse CJ, van der Klooster PF, van der Kaay HJ, van der Ploeg M. Overdulve JP. Mitomycin-C is an unreliable inhibitor for study of DNA synthesis in Plasmodium. Mol Biochem Parasitol. 1986a;21:33–36. doi: 10.1016/0166-6851(86)90076-9. [DOI] [PubMed] [Google Scholar]
- Janse CJ, van der Klooster PFJ, van der Kaay HJ, van der Ploeg M. Overdulve JP. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1986b;80:154–157. doi: 10.1016/0035-9203(86)90219-1. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Boorsma EG, Ramesar J, van Vianen P, van der Meer R, Zenobi P, et al. Plasmodium berghei: gametocyte production, DNA content, and chromosome-size polymorphisms during asexual multiplication in vivo. Exp Parasitol. 1989;68:274–282. doi: 10.1016/0014-4894(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Jensen JB. Observations on gametogenesis in Plasmodium falciparum from continuous culture. J Protozool. 1979;26:129–132. doi: 10.1111/j.1550-7408.1979.tb02748.x. [DOI] [PubMed] [Google Scholar]
- Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med. 2014;6:244re245. doi: 10.1126/scitranslmed.3008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Tan YH, Angrisano F, Hanssen E, Rogers KL, Whitehead L, et al. Quantitative analysis of Plasmodium ookinete motion in three-dimensions suggests a critical role for cell shape in the biomechanics of malaria parasite gliding motility. Cell Microbiol. 2014;16:734–750. doi: 10.1111/cmi.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto F. Ionic regulation and signal transduction system involved in the induction of gametogenesis in malaria parasites. Ann N Y Acad Sci. 1993;707:431–434. doi: 10.1111/j.1749-6632.1993.tb38090.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto F, Kido N, Hanaichi T, Djamgoz MBA. Sinden RE. Gamete development in Plasmodium berghei regulated by ionic exchange mechanisms. Parasitol Res. 1992;78:277–284. doi: 10.1007/BF00937084. [DOI] [PubMed] [Google Scholar]
- Kawamoto F, Fujioka H, Murakami RI, Syafruddin, Hagiwara M, Ishikawa T. Hidaka H. The roles of Ca2+/calmodulin-dependent and cGMP-dependent pathways in gametogenesis of a rodent malaria parasite, Plasmodium berghei. Eur J Cell Biol. 1993;60:101–107. [PubMed] [Google Scholar]
- Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M. Waters AP. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Krotoski WA, Omar MS. Jumper JR. Immunofluorescent staining of plasmodial oocysts in the mosquito. J Parasitol. 1974;60:344–347. [PubMed] [Google Scholar]
- Kumar N. Carter R. Biosynthesis of the target antigens of antibodies blocking transmission of Plasmodium falciparum. Mol Biochem Parasitol. 1984;13:333–342. doi: 10.1016/0166-6851(84)90124-5. [DOI] [PubMed] [Google Scholar]
- Lal K, Delves MJ, Bromley E, Wastling JM, Tomley FM. Sinden RE. Plasmodium male development gene-1 (mdv-1) is important for female sexual development and identifies a polarised plasma membrane during zygote development. Int J Parasitol. 2009;39:755–761. doi: 10.1016/j.ijpara.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Lamour S, Straschil U, Saric J. Delves M. Changes in metabolic phenotypes of Plasmodium falciparum in vitro cultures during gametocyte development. Malar J. 2014;13:468. doi: 10.1186/1475-2875-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfrancotti A, Bertuccini L, Silvestrini F. Alano P. Plasmodium falciparum: mRNA co-expression and protein co-localisation of two gene products upregulated in early gametocytes. Exp Parasitol. 2007;116:497–503. doi: 10.1016/j.exppara.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Laveran MA. De la nature parasitaire des accidents de l'impaludisme. Comptes Rendues de la Societe de Biologie Paris. 1881;93:627–630. [Google Scholar]
- Li J, Gutell RR, Damberger SH, Wirtz RA, Kissinger JC, Rogers MJ, et al. Regulation and trafficking of three distinct 18 S ribosomal RNAs during development of the malaria parasite. J Mol Biol. 1997;269:203–213. doi: 10.1006/jmbi.1997.1038. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tewari R, Ning J, Blagborough A, Garbom S, Pei J, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum WG. On the flagellated form of the malarial parasite. Lancet. 1897;2:1240–1241. [Google Scholar]
- McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, et al. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008;6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair G, Braks JAM, Garver LS, Wiegant JCAG, Hall N, Dirks RW, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair GR, Lasonder E, Garver LS, Franke-Fayard BM, Carret CK, Wiegant JC, et al. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010;6:e1000767. doi: 10.1371/journal.ppat.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Navarette S, Butcher G, Davies A, Willers C, Sinden RE. Lachmann PJ. Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect Immun. 2001;69:5064–5071. doi: 10.1128/IAI.69.8.5064-5071.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques SR, Ramakrishnan C, Carzaniga R, Blagborough AM, Delves MJ, Talman AM. Sinden RE. An essential role of the basal body protein SAS-6 in Plasmodium male gamete development and malaria transmission. Cell Microbiol. 2015;17:191–206. doi: 10.1111/cmi.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Peters W. Haberkorn A. The formation of kinetes and oocyst in Plasmodium gallinaceum (Haemosporidia) and considerations on phylogenetic relationships between Haemosporidia, Piroplasmida and other Coccidia. Protistologica. 1980;16:135–154. [Google Scholar]
- Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun. 2013;81:4377–4382. doi: 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Guttierrez G. Barillas-Mury C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons B, Janse CJ, Boorsma EG. Van der Kay HJ. Synchronized erythrocytic schizogony and gametocytogenesis of Plasmodium berghei in vivo and in vitro. Parasitology. 1985;91:423–430. doi: 10.1017/s0031182000062673. [DOI] [PubMed] [Google Scholar]
- Moon RW, Taylor CJ, Bex C, Schepers R, Goulding D, Janse CJ, et al. A cyclic GMP signalling module that regulates gliding motility in a malaria parasite. PLoS Pathog. 2009;5:e1000599. doi: 10.1371/journal.ppat.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan B. Garcia-Bustos J. Kinase signalling in Plasmodium sexual stages and interventions to stop malaria transmission. Mol Biochem Parasitol. 2014;193:23–32. doi: 10.1016/j.molbiopara.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, et al. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- Mori T, Hirai M, Kuroiwa T. Miyagishima SY. The functional domain of GCS1-based gamete fusion resides in the amino terminus in plant and parasite species. PLoS ONE. 2010;5:e15957. doi: 10.1371/journal.pone.0015957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motard A, Baccam D. Landau I. Temporary loss of Plasmodium gametocytes infectivity during schizogony. Ann Parasitol Hum Comp. 1990;65:218–220. [Google Scholar]
- Muirhead-Thomson RC. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae. Am J Trop Med Hyg. 1957;6:971–979. doi: 10.4269/ajtmh.1957.6.971. [DOI] [PubMed] [Google Scholar]
- Muirhead-Thomson RC. Mercier EC. Factors in malaria transmission by Anopheles albimanus in Jamaica. Part I. Ann Trop Med Parasitol. 1952a;46:103–116. doi: 10.1080/00034983.1952.11685512. [DOI] [PubMed] [Google Scholar]
- Muirhead-Thomson RC. Mercier EC. Factors in Malaria transmission by Anopheles albimanus in Jamaica. Part II. Ann Trop Med Parasitol. 1952b;46:201–213. doi: 10.1080/00034983.1952.11685523. [DOI] [PubMed] [Google Scholar]
- Muller HM, Crampton JM, Della Torre A, Sinden RE. Crisanti A. Members of a trypsin gene family in Anopheles gambiae are induced in the gut by bloodmeal. EMBO J. 1993;12:2891–2900. doi: 10.1002/j.1460-2075.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naotunne TD, Karunaweera ND, Mendis KN. Carter R. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology. 1993;78:555–562. [PMC free article] [PubMed] [Google Scholar]
- Nijhout MM. Carter R. Gamete development in malarial parasites: bicarbonate-dependent stimulation by pH in vitro. Parasitology. 1978;76:39–53. doi: 10.1017/s0031182000047375. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Spurck TP, Goodman CD. McFadden GI. Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryot Cell. 2008;8:128–132. doi: 10.1128/EC.00267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A, Bertuccini L, Deligianni E, Franke-Fayard B, Curra C, Siden-Kiamos I, et al. Distinct properties of the egress-related osmiophilic bodies in male and female gametocytes of the rodent malaria parasite Plasmodium berghei. Cell Microbiol. 2014 doi: 10.1111/cmi.12370. . doi: 10.1111/cmi.12370. [DOI] [PubMed] [Google Scholar]
- Opitz C. Soldati D. ‘The glideosom’: a dynamic complex powering gliding motion and host cell invasion by Toxoplasma gondii. Mol Microbiol. 2002;45:597–604. doi: 10.1046/j.1365-2958.2002.03056.x. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK. Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA. Luckhart S. Ingested human insulin inhibits the mosquito NF-ΚB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80:2141–2149. doi: 10.1128/IAI.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakpour N, Camp L, Smithers HM, Wang B, Tu Z, Nadler SA. Luckhart S. Protein kinase C-dependent signaling controls the midgut epithelial barrier to malaria parasite infection in anopheline mosquitoes. PLoS ONE. 2013;8:e76535. doi: 10.1371/journal.pone.0076535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton MG, Barker GC, Matsuoka H, Ramesar J, Janse CJ, Waters AP. Sinden RE. Structure and expression of a conserved and post-transcriptionally regulated gene encoding a surface protein of the sexual stages from malaria parasite Plasmodium berghei. Mol Biochem Parasitol. 1993;59:263–275. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- Peatey CL, Watson JA, Trenholme KR, Brown CL, Nielson L, Guenther M, et al. Enhanced gametocyte formation in erythrocyte progenitor cells: a site-specific adaptation by Plasmodium falciparum. J Infect Dis. 2013;208:1170–1174. doi: 10.1093/infdis/jit309. [DOI] [PubMed] [Google Scholar]
- Philip N, Vaikkinen HJ, Tetley L. Waters AP. A unique kelch domain phosphatase in Plasmodium regulates ookinete morphology, motility and invasion. PLoS ONE. 2012;7:e44617. doi: 10.1371/journal.pone.0044617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AHW. Meuwissen JHET. An automated large-scale culture system of Plasmodium falciparum using tangential flow filtration for medium change. Parasitology. 1983;87:439–445. doi: 10.1017/s0031182000082962. [DOI] [PubMed] [Google Scholar]
- Ponzi M, Siden-Kiamos I, Bertuccinin L, Curra C, Kroeze H, Camarda G, et al. Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by MDV-1/PEG3 protein. Cell Microbiol. 2009;11:1272–1288. doi: 10.1111/j.1462-5822.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- Povelones M, Upton LM, Sala KA. Christophides GK. Structure-function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3-like protein TEP1. PLoS Pathog. 2011;7:e1002023. doi: 10.1371/journal.ppat.1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud A, Lupetti P, Paul REL, Mercati D, Brey PT, Sinden RE, et al. Cryofracture electron microscopy of the ookinete pellicle of Plasmodium gallinaceum reveals the existence of novel pores in the alveolar membranes. J Struct Biol. 2001;135:47–57. doi: 10.1006/jsbi.2001.4396. [DOI] [PubMed] [Google Scholar]
- Raine JD, Ecker A, Mendoza J, Tewari R, Stanway RR. Sinden RE. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog. 2007;3:e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan C, Dessens JT, Armson R, Pinto SB, Talman AM, Blagborough AM. Sinden RE. Vital functions of the malarial ookinete protein, CTRP, reside in the A domains. Int J Parasitol. 2011;41:1029–1039. doi: 10.1016/j.ijpara.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranfordcartwright LC, Balfe P, Carter R. Walliker D. Genetic hybrids of Plasmodium falciparum identified by amplification of genomic DNA from single oocysts. Mol Biochem Parasitol. 1991;49:239–244. doi: 10.1016/0166-6851(91)90067-g. [DOI] [PubMed] [Google Scholar]
- Reininger L, Billker O, Tewari R, Mukhopadhyay A, Fennell C, Dorin-Semblat D, et al. A nima-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J Biol Chem. 2005;280:31957–31964. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- Reininger L, Tewari R, Fennell C, Holland Z, Goldring D, Ranford-Cartwright L, et al. An essential role for the Plasmodium nek-2 nima-related protein kinase in the sexual development of malaria parasites. J Biol Chem. 2009;284:20858–20868. doi: 10.1074/jbc.M109.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L, Garcia M, Tomlins A, Muller S. Doerig C. The Plasmodium falciparum, Nima-related kinase Pfnek-4: a marker for asexual parasites committed to sexual differentiation. Malar J. 2012;11:250. doi: 10.1186/1475-2875-11-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpma S, van den Heuvel J, van der Velden M, Sauerwein R, Russel F. Koenderink J. Atovaquone and quinine anti-malarials inhibit ATP binding cassette transporter activity. Malar J. 2014;13:359. doi: 10.1186/1475-2875-13-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues FG, Santos MN, de Carvalho TXT, Rocha BC, Riehle MA, Pimenta PFP, et al. Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol Biol. 2008;17:175–183. doi: 10.1111/j.1365-2583.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrıguez MC, Margos G, Compton H, Ku M, Lanz H, Rodríguez MH. Sinden RE. Plasmodium berghei: routine production of pure gametocytes, extracellular gametes, zygotes, and ookinetes. Exp Parasitol. 2002;101:73–76. doi: 10.1016/s0014-4894(02)00035-8. [DOI] [PubMed] [Google Scholar]
- Roller NR. Desser SS. The effect of temperature, age and density of gametocytes, and changes in gas composition on exflagellation of Leucocytozoon simondi. Can J Zool. 1973;51:577–587. doi: 10.1139/z73-084. [DOI] [PubMed] [Google Scholar]
- Rosales-Ronquillo MC. Silverman PH. In vitro ookinete development of the rodent malarial parasite, Plasmodium berghei. J Parasitol. 1974;60:819–824. [PubMed] [Google Scholar]
- Ross R. On some peculiar pigmented cells found in two mosquitoes fed on malarial blood. Br Med J. 1897;18:1786–1788. doi: 10.1136/bmj.2.1929.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol PA, Ribeiro JMC, Jungery M, Turell MJ, Spielman A. Bailey CL. Enhanced mosquito blood-finding success on parasitaemic hosts: evidence for vector-parasite mutualism. Proc Nat Acadf Sci U S A. 1985;82:7725–7727. doi: 10.1073/pnas.82.22.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, et al. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother. 2014;58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwein RW. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect. 2007;9:792–795. doi: 10.1016/j.micinf.2007.02.011. [DOI] [PubMed] [Google Scholar]
- van Schaijk BCL, van Dijk MR, van de Vegte-Bolmer M, van Gemert GJ, van Dooren MW, Eksi S, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol. 2006;149:216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Brochet M, Collins M-Á, Schwach F, Jones M-Á, Goulding D, et al. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe. 2012;12:9–19. doi: 10.1016/j.chom.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabuddin M. Do Plasmodium ookinetes invade a specific cell type in the mosquito midgut? Trends Parasitol. 2002;18:157–161. doi: 10.1016/s1471-4922(01)02219-x. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M. Kaslow DC. Biology of the development of Plasmodium in the mosquito midgut: a molecular and cellular view. Bull l'Institut Pasteur. 1994a;92:119–132. [Google Scholar]
- Shahabuddin M. Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp Parasitol. 1994b;79:85–88. doi: 10.1006/expr.1994.1066. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M, Toyoshima T, Aikawa M. Kaslow DC. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci U S A. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabuddin M, Kaidoh T, Aikawa M. Kaslow DC. Plasmodium gallinaceum: mosquito peritrophic matrix and the parasite-vector compatibility. Exp Parasitol. 1995;81:386–393. doi: 10.1006/expr.1995.1129. [DOI] [PubMed] [Google Scholar]
- Shao L, Devenport M. Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. Arch Insect Biochem Physiol. 2001;47:119–125. doi: 10.1002/arch.1042. [DOI] [PubMed] [Google Scholar]
- Shiao SH, Whitten MMA, Zachary D, HoffmannJ A. Levashina EA. Fz2 and Cdc42 mediate melanization and actin polymerization but are dispensable for Plasmodium killing in the mosquito midgut. PLoS Pathog. 2006;2:1152–1164. doi: 10.1371/journal.ppat.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrinet J, Nandal UK, Adak T, Bhatnagar RK. Sunil S. Inference of the oxidative stress network in Anopheles stephensi upon Plasmodium infection. PLoS ONE. 2014;9:e114461. doi: 10.1371/journal.pone.0114461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden-Kiamos I. Louis C. Intracellular calcium levels in the Plasmodium berghei ookinete. Parasitology. 2008;135:1355–1362. doi: 10.1017/S0031182008004939. [DOI] [PubMed] [Google Scholar]
- Silvestrini F, Bozdech Z, Lanfrancotti A, Di Guilio E, Bultrini E, Picci L, et al. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:100–110. doi: 10.1016/j.molbiopara.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Simon N, Lasonder E, Scheuermayer M, Kuehn A, Tews S, Fischer R, et al. Malaria parasites co-opt human factor H to prevent complement-mediated lysis in the mosquito midgut. Cell Host Microbe. 2013;13:29–41. doi: 10.1016/j.chom.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Gametocytogenesis of Plasmodium falciparum in vitro: ultrastructural observations on the lethal action of chloroquine. Ann Trop Med Parasitol. 1982;76:15–23. doi: 10.1080/00034983.1982.11687500. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Gametocytogenesis in Plasmodium spp., and observations on the meiotic division. Ann Soc Belg Med Trop. 1985;65:21–33. [PubMed] [Google Scholar]
- Sinden RE. Mitosis and meiosis in malarial parasites. Acta Leiden. 1991;60:19–27. [PubMed] [Google Scholar]
- Sinden RE. Croll NA. Cytology and kinetics of microgametogenesis and fertilization of Plasmodium yoelii nigeriensis. Parasitology. 1975;70:53–65. doi: 10.1017/s0031182000048861. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Garnham PCC. A comparative study of the ultrastructure of Plasmodium sporozoites within the oocyst and salivary glands, with particular reference to the incidence of the micropore. Trans R Soc Trop Med Hyg. 1973;67:631–637. doi: 10.1016/0035-9203(73)90031-x. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Hartley RH. Identification of the meiotic division of malarial parasites. J Protozool. 1985;32:742–744. doi: 10.1111/j.1550-7408.1985.tb03113.x. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Smalley ME. Gametocytogenesis of Plasmodium falciparum in vitro: the cell cycle. Parasitology. 1979;79:277–296. doi: 10.1017/s003118200005335x. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Canning EU. Spain B. Gametogenesis and fertilization in Plasmodium yoelii nigeriensis: a transmission electron microscope study. Proc R Soc. 1976;193:55–76. doi: 10.1098/rspb.1976.0031. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Canning EU, Bray RS. Smalley ME. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B. 1978;201:375–399. doi: 10.1098/rspb.1978.0051. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Ponnudurai T, Smits MA, Simm AM. Meuwissen JHET. Gametocytogenesis of Plasmodium falciparum in vitro: a simple technique for the routine culture of pure capacitated gametocytes en masse. Parasitology. 1984;88:239–247. [PubMed] [Google Scholar]
- Sinden RE, Hartley R. Winger L. The development of Plasmodium ookinetes in vitro: an ultra-structural study including a description of the meiotic division. Parasitology. 1985;91:227–244. doi: 10.1017/s0031182000057334. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Suhrbier A, Davies C, Fleck S, Hodivala K. Nicholas JC. The development and routine application of high-density exoerythrocytic-stage cultures of Plasmodium berghei. Bull World Health Organ. 1990;68:115–125. [PMC free article] [PubMed] [Google Scholar]
- Sinden RE, Talman A, Marques SR, Wass MN. Sternberg MJE. The flagellum in malarial parasites. Curr Opin Microbiol. 2010;13:491–500. doi: 10.1016/j.mib.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton JA. Studies in malaria, with special reference to treatment. Part IV. The occurrence of sexual forms of Plasmodium falciparum in the peripheral circulation. Indian J Med Res. 1926;XIII:895–916. [Google Scholar]
- Sinton JA, Baily JD. Chand D. Studies in malaria with special reference to treatment. IV. The occurrence of sexual forms of P. falciparum in the peripheral circulation. Indian J Med Res. 1926;XIII:895–902. [Google Scholar]
- Slavic K, Delves MJ, Prudencio M, Talman AM, Straschil U, Derbyshire ET, et al. Use of a selective inhibitor to define the chemotherapeutic potential of the plasmodial hexose transporter in different stages of the parasite's life cycle. Antimicrob Agents Chemother. 2011;55:2824–2830. doi: 10.1128/AAC.01739-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley ME. Plasmodium falciparum gametocytes: the effect of chloroquine on their development. Trans R Soc Trop Med Hyg. 1977;71:526–529. doi: 10.1016/0035-9203(77)90149-3. [DOI] [PubMed] [Google Scholar]
- Smalley ME. Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74:1–8. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- Smalley ME, Abdalla S. Brown J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg. 1981;75:103–105. doi: 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- Sologub L, Kuehn A, Kern S, Przyborski J, Schillig R. Pradel G. Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell Microbiol. 2011;13:897–912. doi: 10.1111/j.1462-5822.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- Stanway RR, Witt T, Zobiak B, Aepfelbacher M. Heussler VT. GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol Cell. 2009;101:415–435. doi: 10.1042/BC20080202. [DOI] [PubMed] [Google Scholar]
- Straschil U, Talman AM, Ferguson DJ, Bunting KA, Xu Z, Bailes E, et al. The Armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes. PLoS ONE. 2010;5:e12901. doi: 10.1371/journal.pone.0012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhrbier A, Janse CJ, Mons B, Fleck SL, Nicholas J, Davies CS. Sinden RE. The complete development in vitro of the vertebrate stages of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1987;82:907–910. doi: 10.1016/0035-9203(87)90346-4. [DOI] [PubMed] [Google Scholar]
- Talman A, Prieto J, Marques S, Ubaida-Mohien C, Lawniczak M, Wass M, et al. Proteomic analysis of the Plasmodium male gamete reveals the key role for glycolysis in flagellar motility. Malar J. 2014;13:315. doi: 10.1186/1475-2875-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman AM, Lacroix C, Marques SR, Blagborough AM, Carzaniga R, Ménard R. Sinden RE. PbGEST mediates malaria transmission to both mosquito and vertebrate host. Mol Microbiol. 2011;82:462–474. doi: 10.1111/j.1365-2958.2011.07823.x. [DOI] [PubMed] [Google Scholar]
- Tao D, Ubaida-Mohien C, Mathias DK, King JG, Pastrana-Mena R, Tripathi A, et al. Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol Cell Proteomics. 2014;13:2705–2724. doi: 10.1074/mcp.M114.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Keister DB, Muratova O, Procter JL. Kaslow DC. Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. J Exp Med. 1998;187:1599–1609. doi: 10.1084/jem.187.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, et al. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8:377–387. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Sinden RE. In situ detection of Pbs21 mRNA during sexual development of Plasmodium berghei. Mol Biochem Parasitol. 1994;68:189–196. doi: 10.1016/0166-6851(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Tiburcio M, Niang M, Deplaine G, Perrot S, Bischoff E, Ndour PA, et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood. 2012;119:e172–e180. doi: 10.1182/blood-2012-03-414557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W. Gill GS. Enhanced gametocyte formation in young erythrocytes by Plasmodium falciparum in vitro. J Protozool. 1992;39:429–432. doi: 10.1111/j.1550-7408.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Tran PN, Brown SH, Mitchell TW, Matuschewski K, McMillan PJ, Kirk K, et al. A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nat Commun. 2014;5:4773. doi: 10.1038/ncomms5773. [DOI] [PubMed] [Google Scholar]
- Vermeulen AN, Ponnudurai T, Beckers PJA, Verhave JP, Smits MA. Meuwissen JHET. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervenne RA, Dirks RW, Ramesar J, Waters AP. Janse CJ. Differential expression in blood stages of the gene coding for the 21-kilodalton surface protein of ookinetes of Plasmodium berghei as detected by RNA in situ hybridisation. Mol Biochem Parasitol. 1994;68:259–266. doi: 10.1016/0166-6851(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Zimmermann T, Cantera R, Janse CJ, Waters AP. Kafatos FC. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell Microbiol. 2004;6:671–685. doi: 10.1111/j.1462-5822.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- Wass MN, Stanway R, Blagborough AM, Lal K, Prieto JH, Raine D, et al. Proteomic analysis of Plasmodium in the mosquito: progress and pitfalls. Parasitology. 2012;139:1131–1145. doi: 10.1017/S0031182012000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AP. The ribosomal RNA genes of Plasmodium. Adv Parasitol. 1994;34:33–79. doi: 10.1016/s0065-308x(08)60136-0. [DOI] [PubMed] [Google Scholar]
- Waters AP, Syin C. McCutchan TF. Development regulation of stage-specific ribosome populations in plasmodium. Nature. 1989;342:438–440. doi: 10.1038/342438a0. [DOI] [PubMed] [Google Scholar]
- Wiley JD, Merino EF, Krai PM, McLean KJ, Tripathi AK, Vega-Rodríguez J, et al. Isoprenoid precursor biosynthesis is the essential metabolic role of the apicoplast during gametocytogenesis in Plasmodium falciparum. Eukaryotic Cell. 2013 doi: 10.1128/EC.00198-14. pii.EC.00198-00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LG, Carter LM. Reece SE. High-speed holographic microscopy of malaria parasites reveals ambidextrous flagellar waveforms. Proc Natl Acad Sci U S A. 2014;110:18769–18774. doi: 10.1073/pnas.1309934110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, et al. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol. 2014;16:709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Bai XC, Brown A, Fernandez IS, Hanssen E, Condron M, et al. Cryo-EM structure of the Plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. eLife. 2014;3 doi: 10.7554/eLife.03080. . doi: 10.7554/elife.03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, Waters AP, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol Microbiol. 2009;71:1402–1414. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- Zhu JD, Waters AP, Appiah A, McCutchan TF, Lal AA. Hollingdale MR. Stage-specific ribosomal RNA expression switches during sporozoite invasion of hepatocytes. J Biol Chem. 1990;265:12740–12744. [PubMed] [Google Scholar]
- Zieler H, Nawrocki JP. Shahabuddin M. Plasmodium gallinaceum ookinetes adhere specifically to the midgut epithelium of Aedes aegypti by interaction with a carbohydrate ligand. J Exp Biol. 1999;202:485–495. doi: 10.1242/jeb.202.5.485. [DOI] [PubMed] [Google Scholar]
- Zieler H, Garon CF, Fischer ER. Shahabuddin M. A tubular network associated with the brush-border surface of Aedes aegypti midgut: implications for pathogen transmission by mosquitoes. J Exp Biol. 2000;203:1599–1611. doi: 10.1242/jeb.203.10.1599. [DOI] [PubMed] [Google Scholar]


