Abstract
The Reelin-signaling pathway regulates neuronal positioning during embryonic development. Reelin, the extracellular matrix protein missing in reeler mutants, is secreted by neurons in laminae I, II and V, binds to Vldl and Apoe2 receptors on nearby neurons, and tyrosine phosphorylates the adaptor protein Disabled-1 (Dab1), which activates downstream signaling. We previously reported that reeler and dab1 mutants had significantly reduced mechanical and increased heat nociception. Here we extend our analysis to chemical, visceral, and cold pain and importantly, used Fos expression to relate positioning errors in mutant mouse dorsal horn to changes in neuronal activity. We found that noxious mechanical stimulation-induced Fos expression is reduced in reeler and dab1 laminae I–II, compared to wild type mice. Additionally, mutants had fewer Fos-immunoreactive neurons in the lateral reticulated area of the deep dorsal horn than wild type mice, a finding that correlates with a 50% reduction and subsequent mispositioning of the large Dab1-positive cells in the mutant lateral reticulated area. Furthermore, several of these Dab1 cells expressed Fos in wild type mice but rarely in reeler mutants. By contrast, paralleling the behavioral observations, noxious heat stimulation evoked significantly greater Fos expression in laminae I–II of reeler and dab1 mutants. We then used the formalin test to show that chemical nociception is reduced in reeler and dab1 mutants and that there is a corresponding decrease in formalin-induced Fos expression. Finally, neither visceral pain nor cold pain sensitivity differed between wild type and mutant mice. As differences in the nociceptor distribution within reeler and dab1 mutant dorsal horn were not detected, these differential effects observed on distinct pain modalities suggest that dorsal horn circuits are organized along modality-specific lines.
Keywords: reeler, disabled-1, superficial dorsal horn, lateral spinal nucleus, neuronal migration, pain modalities
The reeler gene is a naturally occurring autosomal recessive mutation identified and cloned by D’Arcangelo et al. (1995). The protein missing in these mice, Reelin, is a large secreted extracellular matrix-type molecule that is highly expressed during embryonic development and at lower levels in postnatal and adult mice (Rice et al., 1998, D’Arcangelo et al., 1999, Niu et al., 2004). Reelin binding to the Apolipoprotein E receptor 2 (Apoer2) and the Very low-density lipoprotein receptor (Vldlr) leads to tyrosine phosphorylation of the adaptor protein Disabled-1 (Dab1; D’Arcangelo et al., 1999, Hiesberger et al., 1999) by the Src family of non-receptor tyrosine kinases (Arnaud et al., 2003, Bock and Herz, 2003). Downstream signaling events activated by Dab1 phosphorylation influence embryonic neuronal positioning, postnatal dendritic development and synaptic plasticity in the adult (Howell et al., 1997, Niu et al., 2004, Matsuki et al., 2008). As mice with mutations in Reelin, both Apoer2 and Vldlr, or Dab1 are phenotypically indistinguishable in their ‘reeling’ gait and neuronal migration defects, these mutants provide strong genetic evidence that the Reelin-signaling pathway is required for correct neuronal positioning (Trommsdorff et al., 1999, Rice and Curran, 2001).
The classic migratory defects in reeler mutants occur in laminated structures of the cerebral and cerebellar cortices and in the hippocampal formation (Goffinet, 1984). Reelin signaling also affects less laminated CNS structures, including the spinal cord, where it functions in a cell-specific manner (Phelps et al., 2002). That is, many neurons in reeler spinal cord are located correctly but others are aberrantly positioned. Prominent migratory errors in reeler spinal cord occur among sympathetic and parasympathetic preganglionic neurons (Yip et al., 2000, Phelps et al., 2002). Incorrectly positioned neurons are identified by their high Dab1 protein expression in reeler dorsal horn, specifically in laminae I, II and V and in the lateral spinal nucleus (LSN; Villeda et al., 2006), areas involved in the transmission of ‘pain’ signals (Menétrey et al., 1980, Burstein et al., 1990b, Craig, 2003, Olave and Maxwell, 2004, Basbaum et al., 2009, Pinto et al., 2010). Importantly, there is a behavioral correlate of these positioning errors. Thus, reeler mice exhibit a significant reduction in mechanical sensitivity and an increased thermal sensitivity/heat hyperalgesia (Villeda et al., 2006). As the same alterations of pain processing occur in dab1 mutants (Akopians et al., 2008), we concluded that the Reelin-signaling pathway is an essential contributor to the normal development of ‘pain’ circuits. The fact that there is differential processing of heat and mechanical pain messages in these mutants is of particular interest, and emphasizes the specificity of the circuits affected by these positioning defects.
To delineate further the modality specificity of the alterations in acute pain processing in reeler and dab1 mutant mice, we extended the analysis to chemical, noxious cold, and visceral pain, using behavioral and Fos expression studies. We report that despite presenting with an unusual combination of increased thermal (heat) sensitivity and reduced mechanical and chemical responsiveness, Reelin signaling pathway mutants behave normally in tests of responsiveness to noxious cold and visceral stimulation.
EXPERIMENTAL PROCEDURES
Animals
The reeler (B6C3Fe-ala-Relnlrl, The Jackson Laboratory, Bar Harbor, ME), and dab1 (BALB/cByJ dab1−/−, generous gift from Dr. B. Howell, Syracuse, NY and Dr. J. Cooper, Seattle, WA) mice were generated and maintained at UCLA or transferred to UCSF. Genotyping was conducted as reported by D’Arcangelo et al. (D’Arcangelo et al., 1996) or Brich et al. (Brich et al., 2003).
Behavioral analyses
Experiments were performed following the guidelines of the International Association for the Study of Pain with approval of the University of California, San Francisco Institutional Animal Care and Use Committee or the Chancellor’s Animal Research Committee at UCLA. Mice were housed in a 12 h light/dark cycle at 21°C. Comparisons were made between reeler or dab1 mice and their wild type littermates.
Mechanical stimulation. 15 min after sodium pentobarbital (50 mg/kg) injection, we applied a modified alligator clip to the left hind paw at the base of the toes every 3 min for a 20 sec duration, for a total of 5 stimulus presentations.
Thermal (heat) stimulation. Mice were anesthetized using sodium pentobarbital (50 mg/kg) 15 min prior to the stimulation. The left hindpaw was dipped into a 50°C water bath for 3 sec per min, for a total of 10 min.
Formalin test. Mice were placed in Plexiglas testing chambers and allowed to acclimate to the environment for 30 min. Next 10 μl of 2% formaldehyde diluted in saline was injected subcutaneously into the plantar surface of one hindpaw. The duration of licking (nocifensive behavior) of the injected paw was recorded in 5 min intervals for one hour after the injection (Tjolsen et al., 1992).
Double cold plate test. In this test, the temperature of one aluminum plate is controlled with fluid circulation (from room temperature to 5°C), and the immediately adjacent plate set at room temperature (Bautista et al., 2007). Half the animals were placed initially on the room temperature plate and the others on the colder plate. Animals typically explore both plates. We recorded the time that the animal spent with both hindpaws in contact with the room temperature plate versus the colder plate.
Visceral pain test. Acetic acid was administrated intraperitoneally (100μl/10g body weight of 0.6% (v/v) in saline) and the evoked stretching responses were counted for the next 20 minutes.
Tissue preparation and immunohistochemistry
One hour after the heat or mechanical stimulus, mice were deeply anesthetized with sodium pentobarbital (100 mg/kg) and directly perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), ph 7.4. Alternately, after chemical and visceral stimuli, mice were anesthetized with 2.5% Avertin, then perfused transcardially with the fixative described above. The spinal cord was dissected out and post-fixed in 4% paraformaldehyde/PB for 3–4 hours at 4°C and then cryoprotected in 30% sucrose/PB overnight at 4°C. L4/L5 spinal cord sections (for mice in the heat, mechanical, and formalin studies) or thoracic spinal cord sections (for the visceral pain stimulus) were immunostained for Fos as previously described (Presley et al., 1990). Digitized images of 6–10 heavily Fos-labeled sections per animal were captured using both bright and darkfield illumination. Using the darkfield image as a guide, a line was drawn at the border between the relatively dark substantia gelatinosa (lamina II) and the more lucent or opaque lamina III. Fos immunoreactive profiles were counted in laminae I–II, III–IV, V–VI, and the lateral spinal nucleus as delineated in figure 1. Most Fos-positive cells found in the lateral reticulated part of the neck of the dorsal horn, i.e., the area which corresponds to the thin strands of lateral gray matter intermixed with longitudinal axon bundles (Menétrey et al., 1982), were included in laminae V–VI counts. Finally, to determine the distribution of Dab1-labeled cells in the reeler wild type and mutant lateral reticulated area and LSN of lumbar segments L4/5, we used a Dab1 antiserum (B3, 1:5000; gift of Dr. Brian Howell, Syracuse, NY) with standard avidin-biotin methods (Villeda et al., 2006, Akopians et al., 2008).
Fig. 1.
Schematic of dorsal horn lamination and the area of the lateral spinal nucleus (LSN) used for Fos analysis. DLF, dorsolateral funiculus; DF, dorsal funiculus.
Statistical Analyses
Mean number of Fos-positive cells were compared by genotype and dorsal horn area using a 2 × 4 repeated measure two way analysis of variance (ANOVA) model. In this analysis the dorsal horn area is the repeated within group factor and genotype is the between group factor. An unstructured covariance matrix was conservatively assumed allowing for variance heterogeneity. Using this ANOVA model, post hoc mean comparisons for a given outcome were judged significant using the Fisher least significant difference criterion. We compared the mean number of Dab1-immunoreactive neurons with a 2 × 2 repeated measures ANOVA and the Fisher least significant difference criterion. We used t tests to compare the mean number of Fos-immunoreactive neurons and body stretches in wild type and mutant mice. Calculations were carried out using JMP 10 (SAS Inc, Cary NC) and Sigma Plot 12.0. (Systat Software Inc, San Jose, CA).
RESULTS
Decreased noxious mechanical stimulation-evoked Fos expression in reeler and dab1 mutants
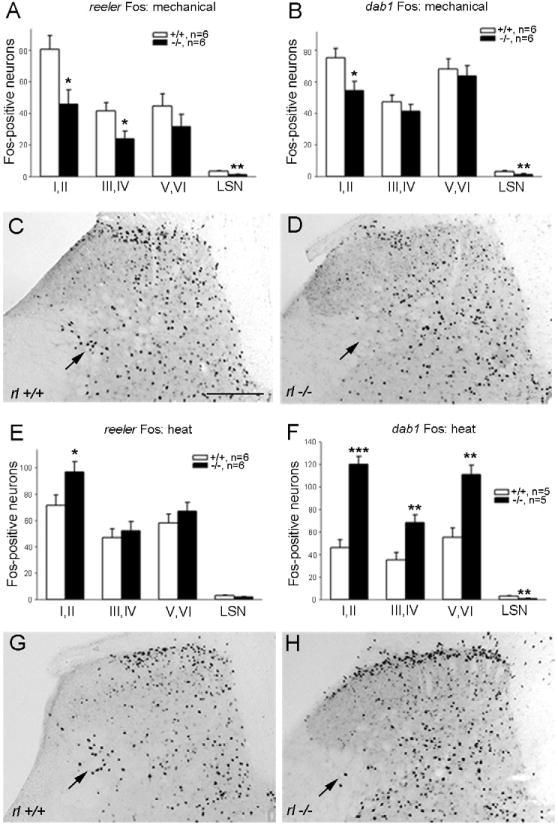
As noted above, neuronal positioning errors are found in the dorsal horn of reeler and dab1 mutant mice and both lines of mice have pronounced mechanical insensitivity, i.e., much higher mechanical thresholds using the von Frey test (up-down method; Chaplan et al., 1994, Villeda et al., 2006, Akopians et al., 2008). Here we document the pattern and magnitude of Fos expression induced by noxious mechanical stimulation. Consistent with the behavioral observations, we found that reeler mutants have significantly reduced Fos expression in laminae I–II and III–IV, compared to their wild type controls (Fig. 2A). We found no differences in the total number of Fos-immunoreactive neurons in laminae V–VI (Fig. 2A), although greater Fos expression was evident in the lateral part of the deep dorsal horn in wild type than in reeler mutant mice (Fig. 2C, D, arrows). We also analyzed Fos expression in the LSN, the neurons of which convey nociceptive signals rostrally and which are reduced in number in reeler mutants (Villeda et al., 2006). Although the total number of Fos-immunoreactive neurons in the LSN is small (mean of 3.3 vs. 1.1 neurons per section in wild type and mutant mice, respectively), this difference was significant (p<0.003).
Fig. 2.
Fos expression in the dorsal horn following mechanical (A–D) and thermal (heat, E–H) stimulation. A, B: Compared to wild type controls, the number of Fos-immunoreactive cells stimulated by mechanical pinch is significantly decreased in laminae I–II, III–IV, and the LSN in reeler (A) and in laminae I–II and the LSN of dab1 mutant mice (B). C, D: Following mechanical stimulation, more Fos-expressing cells are present in wild type laminae I–II and the lateral reticulated area (C, arrow) than in reeler mutants (D, arrow). E, F: Following heat stimulation, reeler mutants (E) showed a significantly elevated Fos response in laminae I–II and dab1 mutants (F) displayed a pronounced increase in Fos throughout the dorsal horn compared to wild type mice. G, H: Heat stimulation elevates Fos-immunoreactive cells in reeler laminae I–II and the lateral reticulated area (at arrows) versus wild type mice. Significant differences between wild type and mutants at *p<0.05; **p<0.01; ***p<0.001. Error bars reflect SEM. Scale bar C, D, G, H = 200 μm.
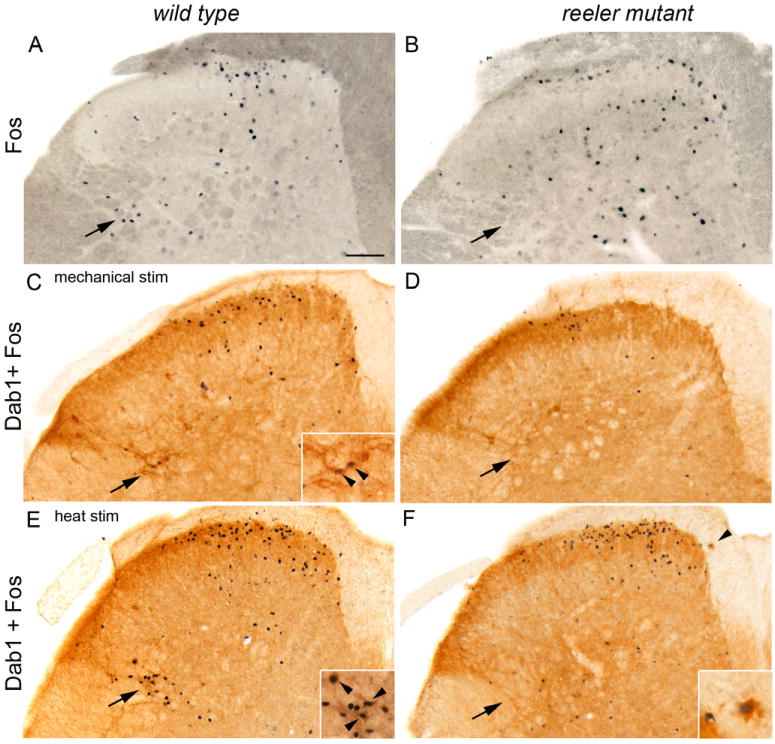
To determine if Fos-immunoreactive neurons are located within the lateral reticulated area of the neck of the dorsal horn, we visualized, without tissue dehydration, the prominent axon bundles and cellular bridges that characterize this region. Figures 3A and B illustrate that after mechanical stimulation we recorded greater numbers of Fos-immunoreactive neurons among the axon bundles in the lateral reticulated area of wild type mice than in reeler mutants (arrows).
Fig. 3.
Fos and Dab1 expression in adult lumbar dorsal horn after noxious mechanical (A–D) or heat (E–F) stimulation. A, B: More Fos-labeled nuclei are found in between the dark gray axon bundles of the lateral reticulated area (arrows) in wild type (A) than in reeler mutant (B) mice. C, D: Large Dab1-labeled cells are more common in the lateral reticulated area (C, arrow) of wild type than in reeler mutants (D). Two Dab1 neurons express mechanically-induced Fos (C, enlarged in inset at arrowheads) in the wild type lateral reticulated area, but not in reeler mutants (D, arrow). E, F: After noxious heat stimulation several Dab-labeled neurons express Fos (E, enlarged in inset at arrowheads) in the wild type lateral reticulated area. An ectopic Dab1-labeled cell (F, arrowhead) is outside of the lamina I border in the dorsal funiculus of a reeler mutant and expresses Fos (enlarged in inset). Scale bar A–F = 100 μm.
To confirm that it was the loss of the Reelin-Dab1 signaling pathway that reduced Fos expression in reeler mutants, we tested the mechanical responsiveness of the dab1 mutants. We found that the dab1 mutants also have decreased noxious mechanical stimulus-induced Fos expression compared to their wild type controls (Fig. 2B). This was true for laminae I–II and the LSN, but we found no differences in laminae III–VI.
Enhanced noxious heat-evoked Fos expression in reeler and dab1 mutants
A second nociceptive consequence of deletion of the Reelin-signaling pathway is a greater sensitivity in the Hargreaves radiant heat test (Villeda et al., 2006, Akopians et al., 2008). Consistent with these findings, we now show that the reeler mutants have significantly greater Fos expression in laminae I–II of lumbar dorsal horn. The number of Fos-immunoreactive neurons in laminae III–IV, V–VI and in the LSN did not differ between genotypes (Fig. 2E, G, H), although there were more Fos-positive neurons in the lateralmost part of the reticulated area in wild type mice than in reeler mutants (Fig. 2G, H, arrows).
The dab1 mutants also show a heightened response to heat stimulation in the Hargreaves test (Akopians et al., 2008). Now we show that dab1 mutants also have increased heat-induced Fos expression compared to their control counterparts in the superficial (laminae I–II) and deep dorsal horns (laminae III–VI, Fig. 2F). By contrast, the number of Fos-labeled neurons in the LSN of dab1 mutants is reduced compared to wild type mice. These somewhat paradoxical results may be explained by our earlier findings that ~50% of the LSN neurons at lumbar levels are mispositioned, most likely to other areas of the dab1 mutant dorsal horn (Akopians et al., 2008).
Lateral reticulated area: Fos expression in Dab1-containing neurons of reeler wild type, but not the mutant
As mispositioned neurons in reeler mutants contain high levels of Dab1 protein, we examined the distribution of Dab1 cells in reeler wild type and mutant lateral reticulated area at L4/L5 segmental levels. Figure 3C illustrates several prominent Dab1-immunoreactive neurons (arrow) wedged between the lateralmost white matter bundles in a wild type mouse; other Dab1-positive neurons are located further laterally and dorsally, in the LSN. We detected fewer Dab1 cells in the lateral reticulated area of reeler mutants (Fig. 3D, arrow). On average, wild type mice had almost twice the number of Dab1 neurons in the lateral reticulated area (mean + SEM; wild type 4.2 + 0.25 cells/hemisection; mutant 2.1 + 0.28; p<0.0001) and LSN (wild type 2.2 +0.25; mutant 1.3 + 0.28; p<0.04) compared to reeler mutants. On the other hand, reeler mutants have ectopically located Dab1-positive cells along the outer (dorsal) border of lamina I and at the medial border adjacent to the dorsal columns (Fig. 3F, arrowhead). These findings suggest that some projection neurons in the lateral reticulated area express Dab1 and require the presence of the Reelin-signaling pathway for correct positioning in the deep dorsal horn.
We next asked if the prominent Dab1-labeled neurons in the lateral reticulated area expressed Fos following noxious mechanical and heat stimulation. Interestingly, we observed Fos-immunoreactive Dab1 cells in the wild type lateral reticulated area (Fig. 3C, E, arrowheads on insets) following both noxious stimuli but they were rarely found in reeler mutants (Fig. 3D, F), consistent with our hypothesis that some neurons in the lateral reticulated area are mispositioned. Several ectopically positioned Dab1 neurons expressed Fos (Fig. 3F at arrowhead, enlarged in inset) in reeler mutants following noxious heat stimulation.
Both reeler and dab1 mutants show reduced nocifensive behavior and Fos expression after hindpaw formalin
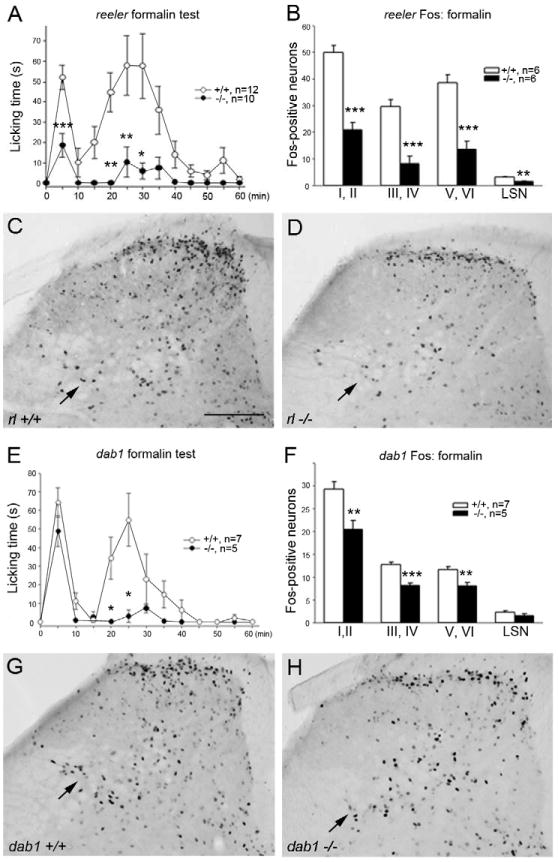
Intraplantar formalin induced the typical biphasic pattern of licking (Tjolsen et al., 1992) in both reeler and dab1 wild type control mice (Fig. 4A, E). However, in reeler mutants both the first (0–10 min) and second phases (11–60 min) of behavior were significantly decreased (Fig. 4A). The response to formalin in the dab1 mutants was also reduced, but interestingly, only during the second phase of behavior (Fig. 4E). Consistent with the behavior observed, formalin-induced Fos expression was significantly reduced in laminae I through VI of the spinal cord, in both reeler and dab1 mutants (Fig. 4C, D). The magnitude of the reduction was comparable in all regions of the dorsal horn (Fig. 4B, F) but there appears to be an especially striking reduction of Fos expression in the lateral reticulated area of the reeler mutant mice (Fig. 4C, D; arrows).
Fig. 4.

Formalin-evoked nocifensive behavior and Fos expression in reeler (A–D) and dab1 (E–H) mice. Formalin was injected into the hindpaw and the duration of licking/biting the injected paw was measured in 5-min intervals for 1 hr. A, E: Compared to wild type mice, reeler mutants had reduced nocifensive behavior in phases I and II (A), whereas in dab1 mutants, a decrease was seen only in phase II (E). B, F: Fewer Fos immunoreactive cells were found in reeler (B) and dab1 (F) mutant dorsal horn laminae than in wild type mice. Fos expressing neurons were decreased in the reeler lateral spinal nucleus (LSN), but not dab1 mutants. C, D, G, H: Representative sections illustrate distribution of Fos immunoreactive neurons in wild type (C, G), reeler (D) and dab1 mutant (H) mice. Significant differences between wild type and mutant mice, *p<0.05; **p<0.01; ***p<0.001. Scale bar C, D, G, H = 200 μm.
Neither reeler nor dab1 mutants differ from wild type mice in their cold pain responsiveness
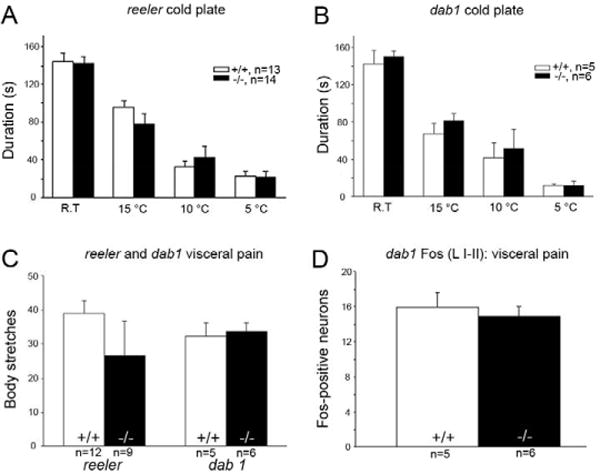
To determine whether the altered heat responses generalized to a cold thermal stimulus, we also examined responsiveness on the two-platform cold plate preference assay (Bautista et al., 2007). Figure 5A and B show that reeler and dab1 mutants do not differ from their respective controls in their responsiveness to noxious cold stimulation (15°C, 10°C and 5°C).
Fig. 5.
Cold and visceral pain responsiveness is unchanged in reeler and dab1 mutants. A, B: Regardless of temperature, wild type, reeler and dab1 mutants spent equivalent time on the coldest plate (compared to the adjacent plate). C: Number of abdominal stretches induced by i.p. acetic acid did not differ between wild type, reeler, or dab1 mutant mice. D: The number of Fos immunoreactive cells induced by acetic acid in the thoracic superficial dorsal horn did not differ between wild type and dab1 mutant mice. p>0.05 for all comparisons; error bars reflect SEM.
Neither reeler nor dab1 mutants differ from wild type mice in the acetic acid test of visceral pain responsiveness
To determine the involvement of the Reelin-signaling pathway in the transmission of visceral pain messages, we next monitored the number of abdominal stretches after intraperitoneal injection of acetic acid. As illustrated in figure 5C neither reeler nor dab1 mutants differed in the number of stretches compared to mice in the wild type groups. And consistent with the lack of behavioral differences, we found equivalent Fos expression in laminae I–II in dab1 wild type and mutant mice (Fig. 5D).
DISCUSSION
The present results greatly extend our previous findings (Villeda et al., 2006, Akopians et al., 2008) of an important contribution of the Reelin-Dab1 signaling pathway to nociceptive processing. The reeler and dab1 mutants respond in an opposing manner to heat versus both mechanical and chemical (formalin) stimuli. The mutant mice are hyperesponsive to thermal (heat), but much less sensitive to mechanical and chemical stimulation. Using Fos as a marker of activity of spinal cord neurons (Morgan and Curran, 1989, Presley et al., 1990), we now show that the behavioral phenotype in response to heat and mechanical or formalin stimulation almost certainly is a manifestation, respectively, of increased and decreased activity of dorsal horn nociceptive circuits. These findings provide support for our hypothesis that there is a causal relationship between laminae I–II positioning errors in animals with Reelin signaling abnormalities and alterations in the processing of nociceptive messages. As we have not detected changes in primary afferent nociceptors in adult reeler or dab1 mutant dorsal root ganglion nor in their termination patterns in the superficial dorsal horn (Villeda et al., 2006, Akopians et al., 2008), the changes in Fos expression that we recorded strongly suggest that it is the neuronal connections between primary afferents and projection neurons or interneurons that are altered in the mutant superficial dorsal horn.
Our results also bear directly on the question of modality segregation of nociceptor circuits. In other words, the differential effects on heat, mechanical and chemical nociceptive processing suggest that the modality segregation that we recently described at the level of the primary afferent nociceptor (Cavanaugh et al., 2009) is maintained, at least at the level of the initial connectivity of the afferents with spinal nociceptive circuitry. We conclude that the reeler and dab1 mutations naturally segregate circuits that process noxious heat, mechanical, and chemical signals at the level of the superficial dorsal horn. By contrast, we did not find changes in the processing of cold or visceral pain messages, which suggests independence of the circuits that transmit these modalities of pain from those for which abnormalities occur in the mutant mice.
Positioning errors in the LSN and lateral reticulated area contribute to nociceptive abnormalities in reeler and dab1 mutants
Using Fos expression as a marker of activity, we found that intense heat and mechanical stimulation activated fewer LSN neurons in reeler and dab1 mutants than in their respective controls. This result is consistent with our present findings of fewer Dab1-positive LSN neurons in reeler mutants and with previous reports that reeler and dab1 mutants have fewer total neurons in the LSN (Villeda et al., 2006, Akopians et al., 2008). The “missing” neurons are likely displaced to other areas, including laminae I–II. The LSN neurons (in wild type mice) almost certainly correspond to those originally described in the dorsal lateral funiculus by Gwyn and Waldron (1968) and which subsequent studies demonstrated project widely to the brainstem, hypothalamus and thalamus (Menétrey et al., 1982, Burstein et al., 1990a, Burstein et al., 1990b). Taken together with more recent studies reporting that LSN neurons both receive and integrate nociceptive information (Olave and Maxwell, 2004, Pinto et al., 2010), we conclude that the LSN positioning errors in Reelin signaling pathway mutants likely contribute to the abnormalities in pain processing.
We also recorded a striking reduction of noxious stimulus (heat, mechanical and formalin)-evoked Fos expression in cells located in the lateral reticulated area of the deep dorsal horn, adjacent to the dorsolateral funiculus. And in a remarkable correspondence, we found that prominent Dab1-immunoreactive neurons are common in the lateral reticulated area of wild type mice, but they are reduced in number and presumably mispositioned in reeler mutants. Furthermore, mechanical and noxious heat stimulation induced Fos in a subset of these large Dab1 neurons, but only in wild type mice. Although the physiology of these Dab1-positive neurons is unknown, based on their size and location they likely correspond to the nociresponsive neurons described by Menétrey et al. (1980). As many neurons in this region project to thalamic targets, as do the majority of lateral lamina V neurons (Burstein et al., 1990b, Willis et al., 2001, Craig, 2003, Basbaum et al., 2009), their incorrect positioning in mutants probably contributes to reduced pain responsiveness. Because the Reelin-signaling pathway is required for postnatal dendritic development (Niu et al., 2004, Matsuki et al., 2008), mispositioned Dab1-labeled lamina V neurons would likely have stunted dendrites and, in addition, altered synaptic function in adults (Herz and Chen, 2006; Forster et al., 2010).
Large lateral lamina V neurons respond to both thermal and mechanical noxious stimulation (Menétrey et al., 1980, Craig, 2003), as do the Dab1 lamina V neurons that co-express Fos. It was, therefore, surprising that the loss of Reelin signaling only reduced mechanical pain responsiveness. An interesting possibility is that heightened thermal (heat) pain responsiveness reflects additional positioning errors, for example, one that places nociresponsive neurons of the LSN in a circuit that is more directly, and perhaps selectively, engaged by noxious heat responsive, TRPV1-expressing primary afferent nociceptors. To date we have documented a polysynaptic connection from nociceptors to neurons of the LSN, but we did not specifically address the connectivity of the TRPV1 subsets of nociceptors (Braz and Basbaum, 2009). The fact that heat and mechanical (and chemical) pain processing can be influenced in opposite directions, of course, supports the view that despite the presence of polymodal neurons in the dorsal horn, there may be considerable behaviorally relevant modality segregation of heat and mechanical pain processing beyond the primary afferent nociceptors. That cold and visceral pain responsiveness are unchanged in the mutant mice strongly supports that view.
Genetic background of the reeler and dab1 mutants does not influence their mechanical and heat pain processing abnormalities
A major conclusion of the present analysis is that the functional changes in the nociceptive circuits in both the reeler and dab1 mutants can be attributed to the loss of a common Reelin-signaling pathway. Because the sensitivity of nociceptive testing varies in different inbred mouse strains (Mogil et al., 1999), it is significant that we found comparable functional changes in three mutant mouse lines that are each on a different genetic background (Villeda et al., 2006, Akopians et al., 2008): reeler (B6C3Fe; Jackson Laboratory; Falconer, 1951), dab1 (BALB/cByJ; Howell et al., 1997, Brich et al., 2003) and scrambler (dab1 hypomorph; C3HeB/FeJ x DC/Le; Sweet et al., 1996). These mice also differ as to how the mutation arose: reeler and scrambler are spontaneous mutations (Falconer, 1951, Sweet et al., 1996) whereas dab1 is a targeted mutation (129Sv x C57BL/6; Howell et al., 1997) that was crossed into a BABL/cByJ background to enhance mutant survival (Brich et al., 2003). Furthermore, in preliminary studies of the reeler Orleans variant (129/Sv x BALB/c), an independent and spontaneously derived mutant that synthesizes a truncated Reelin protein that is not secreted (Takahara et al., 1996, de Bergeyck et al., 1997), we observed the same mechanical and heat abnormalities (unpublished data). What is common in these mice is the loss of the Reelin-Dab1 signaling pathway, without the confounding effects of poor mutant survival that occur in dab1 mutants generated by targeted gene disruptions on a 129Sv x C57Bl/6 background (Brich et al., 2003). By contrast, there is no deficit in acute pain processing in mice with single Reelin receptor mutations (Akopians et al., 2008) in apoer2 (B6;129S7; Trommsdorff et al., 1999) or vldlr (B6;129S6; Frykman et al., 1995), or in mice with mutations in the cytoplasmic tail of the apoer2 variants bred on a vldlr−/− background (deltaex19 and apoer2 stop; Beffert et al., 2005, Beffert et al., 2006). The fact that the apoer2-exon 19 variant engages a different downstream pathway (Beffert et al., 2005) provides further support for our hypothesis that the Reelin-Dab1signaling pathway is required for normal nociceptive processing. This finding argues that the segregation of functional pain processing arises from the common developmental defects associated with loss of Reelin-Dab1 signaling.
Highlights.
Enhanced heat pain processing in reeler & dab1 mutant versus wild type dorsal horn
Decreased mechanical/chemical pain processing in dorsal horn of reeler & dab1 mutants
Lateral reticulated area abnormalities contribute to mutant pain phenotype
Preserved cold and visceral pain processing suggest modality specific circuits
Loss of Reelin signaling segregates heat and mechanical/chemical pain circuits
Acknowledgments
This work was supported by the National Science Foundation (IOB-0518714 and IOB-0924143 to PEP) and the National Institute of Health (NS14627 and DA29204 to AIB). We thank Autumn Abadesco and Marianne Cilluffo for excellent technical assistance.
Abbreviations
- ANOVA
analysis of variance
- Apoer2
Apolipoprotein E receptor 2
- Dab1
Disabled-1
- DF
dorsal funiculus
- DLF
dorsolateral funiculus
- LSN
lateral spinal nucleus
- PB
phosphate buffer
- TRPV1
Transient receptor potential V1
- Vldlr
Very low-density lipoprotein receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akopians AL, Babayan AH, Beffert U, Herz J, Basbaum AI, Phelps PE. Contribution of the Reelin signaling pathways to nociceptive processing. Eur J Neuroscience. 2008;27:523–537. doi: 10.1111/j.1460-9568.2008.06056.x. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Current Biology. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Beffert U, Nematollah-Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biology. 2006;16:2446–2452. doi: 10.1016/j.cub.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates Src family tyrosine kinases in neurons. Current Biology. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163:1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brich J, Shie F-S, Howell BW, Li R, Tus K, Wakeland EK, Jin L-W, Mumby M, Churchill G, Herz J, Cooper JA. Genetic modulation of Tau phosphorylation in the mouse. J Neuroscience. 2003;23:187–192. doi: 10.1523/JNEUROSCI.23-01-00187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Cliffer K, Giesler G., Jr Cells of origin of the spinohypothalamic tract in the rat. J Comp Neurol. 1990a;291:329–344. doi: 10.1002/cne.902910302. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado R, Giesler G., Jr The cells of origin of the spinothalamic tract of the rat: a quantitative reexamination. Brain Res. 1990b;511:329–337. doi: 10.1016/0006-8993(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: Labeled lines versus convergence in central processing. Annu Rev Neuroscience. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Curran T. Detection of the reelin breakpoint in reeler mice. Mol Brain Res. 1996;39:234–236. doi: 10.1016/0169-328x(96)00046-0. [DOI] [PubMed] [Google Scholar]
- de Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet AM, Miyata T, Ogawa M, Mikoshiba K. A truncated Reelin protein is produced but not secreted in the Orleans reeler mutation. Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Two new mutants, trembler and reeler, with neurological actions in the house mouse. J Genet. 1951;50:192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010;31:1511–1518. doi: 10.1111/j.1460-9568.2010.07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykman P, Brown M, Yamamoto T, Goldstein J, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci USA. 1995;92:8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet AM. Events governing organization of postmigratory neurons: Studies on brain development in normal and reeler mice. Brain Res Reviews. 1984;7:261–296. doi: 10.1016/0165-0173(84)90013-4. [DOI] [PubMed] [Google Scholar]
- Gwyn DG, Waldron HA. A nucleus in the dorsolateral funiculus of the spinal cord of the rat. Brain Res. 1968;10:342–351. doi: 10.1016/0006-8993(68)90204-7. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nature Reviews Neuroscience. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Pramatarova A, Howell BW. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Science. 2008;121:1869–1875. doi: 10.1242/jcs.027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menétrey D, Chaouch A, Besson JM. Location and properties of dorsal horn neurons at origin of spinoreticular tract in lumbar enlargement of the rat. J Neurophysiol. 1980;44:862–877. doi: 10.1152/jn.1980.44.5.862. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Chaouch A, Binder D, Besson JM. The origin of the spinomesencephalic tract in the rat: an anatomical study using the retrograde transport of horseradish peroxidase. J Comp Neurol. 1982;206:193–207. doi: 10.1002/cne.902060208. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: Role of cellular immediate-early genes. TINS. 1989;12:159–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Olave MJ, Maxwell DJ. Axon terminals possessing 2c-adrenergic receptors densely innervate neurons in the rat lateral spinal nucleus which respond to noxious stimulation. Neuroscience. 2004;126:391–403. doi: 10.1016/j.neuroscience.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Rich R, Dupuy-Davies S, Rios Y, Wong T. Evidence for a cell-specific action of Reelin in the spinal cord. Dev Biol. 2002;244:180–198. doi: 10.1006/dbio.2002.0580. [DOI] [PubMed] [Google Scholar]
- Pinto V, Szucs P, Lima D, Safronov BV. Multisegmental A – and C-fiber input to neurons in lamina I and the lateral spinal nucleus. J Neuroscience. 2010;30:2384–2395. doi: 10.1523/JNEUROSCI.3445-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley RW, Menétrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked fos protein-like immunoreactivity in the rat spinal cord. J Neuroscience. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the Reelin signaling pathway in central nervous system development. In: Cowan WM, et al., editors. Annu Rev Neuroscience. Vol. 24. Annual Reviews; Palo Alto: 2001. pp. 1005–1039. [DOI] [PubMed] [Google Scholar]
- Rice DS, Sheldon M, D’Arcangelo G, Nakajima K, Goldowitz D, Curran T. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- Sweet HO, Bronson RT, Johnson KR, Cook SA, Davisson MT. Scrambler, a new neurological mutation of the mouse with abnormalities of neuronal migration. Mamm Genome. 1996;7:798–802. doi: 10.1007/s003359900240. [DOI] [PubMed] [Google Scholar]
- Takahara T, Ohsumi T, Kuromitsu J, Shibata K, Sasaki N, Okazaki Y, Shibata H, Sato S, Yoshiki A, Kusakabe M, Muramatsu M, Ueki M, Okuda K, Hayashizaki Y. Dysfunction of the Orleans reeler gene arising from exon skipping due to transposition of a full-length copy of an active L1 sequence into the skipped exon. Hum Mol Genetics. 1996;5:989–993. doi: 10.1093/hmg/5.7.989. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Akopians AL, Babayan AH, Basbaum AI, Phelps PE. Absence of Reelin results in altered nociception and aberrant neuronal positioning in the dorsal spinal cord. Neuroscience. 2006;139:1385–1396. doi: 10.1016/j.neuroscience.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Zhang X, Honda CN, Giesler GJ., Jr Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain. 2001;92:267–276. doi: 10.1016/s0304-3959(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Yip JW, Yip YP, Nakajima K, Capriotti C. Reelin controls position of autonomic neurons in the spinal cord. Proc Natl Acad Sci U S A. 2000;97:8612–8616. doi: 10.1073/pnas.150040497. [DOI] [PMC free article] [PubMed] [Google Scholar]


