SUMMARY
Protein 4.1R (4.1R) is the prototypical member of protein 4.1 superfamily comprising protein 4.1 family (4.1R, 4.1B, 4.1G and 4.1N) and ERM family (ezrin, radixin and meosin). These proteins in general serve as adaptors between membrane and the cytoskeketon. Here we show that 4.1R expressed in the gastric epithelial cells associates with adherens junction protein β-catenin. Biochemical examination of 4.1R-deficient stomach epithelia revealed a selective reduction of β-catenin which is accompanied by a weaker linkage of E-cadherin to the cytoskeleton. In addition, organization of actin cytoskeleton was altered in 4.1R-deficient cells. Moreover, histological examination revealed that cell-cell contacts are impaired and gastric glands are disorganized in 4.1R null stomach epithelia. These results demonstrate an important and previously unidentified role of 4.1R in linking cadherin/catenin complex to the cytoskeleton through its direct interaction with β-catenin and in regulating the integrity of adherens junction.
Keywords: 4.1R, β-catenin, E-cadherin, adherens junction, actin cytoskeleton, epithelial cell
INTRODUCTION
4.1R is the prototypical member of the 4.1 superfamily, comprising protein 4.1 family 4.1R[1], 4.1B [2], 4.1G [3], and 4.1N [4] and those of the ERM family ezrin [5], radixin [6] and moesin [7]. All have in common a structure comprising of two conserved domains: an N-terminal membrane binding domain associating with various membrane proteins, and an actin-binding domain interacting with actin. Thus these proteins in general serve as a bridge between membrane proteins and the actin cytoskeleton.
4.1R was first discovered in red blood cells where its function has been well studied. In both human and mouse red blood cells 4.1R was shown to stabilize the spectrin-actin based membrane skeletal network [8, 9] and anchor it to the overlying lipid bilayer through interactions with the cytoplasmic domains of several transmembrane proteins [10, 11]. Many subsequent studies revealed that 4.1R is expressed in a variety of nucleated cells and is located in several different sub-cellular compartments [12-15], from which its multifunctional character was inferred. Thus it has been shown that 4.1R is expressed in epithelial cells of kidney and in the Madin-Darby canine kidney cell line [16, 17], implying a role in the physiology of epithelial cells, which, however, was not further explored.
Epithelial cells are polarized cells with three domains: apical domain, basal domain and lateral domain. The apical domain is exposed to the lumen or external environment. The basal domain is associated with a basal lamina that separates the epithelium from underlying connective tissue. The lateral domain faces neighboring epithelial cells that are attached to each other by junctional complexes and adhesion molecules. There are two major junctional complexes on the lateral membrane: tight junctions and adherens junctions. The tight junction is a specialized membrane domain at the apical extremes of lateral membrane of the polarized epithelial cells, representing the primary barrier against paracellular transport of solutes [18]. Adherens junctions (AJs) are cadherin/catenin-containing adhesive structures, located below the tight junctions on the bilateral membrane.
Cadherin-mediated AJs play important roles in the formation and maintenance of intercellular adhesive interactions [19, 20]. In stomach epithelia, E-cadherin, the archetypic member of the cadherin family, forms the core of the cadherin/catenin complex. E-cadherin physically links neighboring cells together through the homophilic interaction of its extracellular domain [21]. Cadherin adhesion complexes interact with the actin cytoskeleton in a complex fashion that is still poorly understood. The current view is that E-cadherin is linked to the actin cytoskeleton by its direct interaction with β-catenin which in turn binds to the actin binding protein α-catenin [22, 23]. In addition, there are a group of molecules that associated either constitutively or transiently with the complex and have the potential to affect the complex. This includes p120 catenin [24], IQGAP [25] and maybe others yet unidentified.
In the present study, we have examined the role of 4.1R in epithelial cells by examining stomach epithelia of normal and 4.1R-deficient mice. Our results demonstrated that 4.1R is a component of adherens junctions and that lack of 4.1R resulted in reduction of β-catenin which in turn led to weakened attachment of E-cadherin to actin cytoskeleton and the subsequent impaired cell-cell contact.
MATERIALS AND METHODS
Generation and Use of 4.1R Knockout Mice
The generation of 4.1R knockout mice has been described previously [9]. The mice were backcrossed onto C57BL/6 background and inbred for more than 20 generations. All the mice were maintained at the animal facility of New York Blood Center under specific pathogen-free conditions, according to institutional guidelines. Animal protocols were reviewed and approved by our Institutional Animal Care and Use Committee.
Generation of Anti-4.1R Antibodies
Four polyclonal antibodies against 4.1R were raised in rabbit or goat using His-tagged recombinant exon13, synthetic exon17b peptide (SVNGIRTEEVATVTKGPST), exon18 peptide (KSEIPTKDVPIVHTETK) and exon19 peptide (TDDNSGDLDPGVLLTAQTITSETPSSTTTTQITK) as antigens. Exon 13 is a constitutive exon and exon17b, exon18 and exon19 are alternative exons. The sequences of these peptides are unique to 4.1R and do not share any homology with 4.1G, 4.1N and 4.1B. The antibodies were affinity purified on Sulfolink Coupling Gel (Pierce Biotechonology, Inc.). Antibody specificity was examined by Western blotting using recombinant 4.1R, 4.1G, 4.1N and 4.1B. The 4.1R antibodies did not recognize other members of the protein 4.1 family.
Preparation of Recombinant Proteins
The sub-cloning and expression of His-tagged full length 4.1R 80 kDa and the 10 kDa spectrin-actin binding domain of 4.1R were described previously [26, 27]. The His-tagged 30 kDa membrane binding domain of 4.1R (also called FERM domain) was cloned into the pET31b(+) vector (Novagene, Madison, MI) using NsiI and XhoI sites upstream and downstream respectively. His-tagged 16 kDa and 22/24 kDa fragments of 4.1R were sub-cloned into pET28b(+) vector (Novagene, Madison, MI) using NcoI and XhoI. β-catenin was sub-cloned into pGEX-4T-2 vector using BamHI and SalI cloning sites. α-catenin and cytoplasmic domain of E-cadherin (a.a 733~884) was sub-cloned into pGEX-4T-2 vector using EcoRI and SalI cloning sites. The templates used for the amplification of β-catenin, α-catenin and E-cadherin were from Addgene, GeneCopoeia or Origene respectively. The fidelity of all constructs was confirmed by DNA sequencing. The cDNA was transformed into E. coli BL21 (DE3) for protein expression. The His-tagged 4.1R and its domains were purified by Nickle column and the GST-tagged β-catenin, α-catenin and cytoplasmic domain of E-cadherin were purified by glutathione sepharose-4B affinity column.
GST Pull-Down Assay
To examine the binding of 4.1R or its domains to β-catenin, α-catenin or E-cadherin, GST, GST-tagged β-catenin, α-catenin or cytoplasmic domain of E-cadherin was coupled to glutathione sepharose-4B beads at room temperature for 30 min. Beads were pelleted and washed. His-tagged 4.1R or its domain was added to the beads in a total volume of 100 μl. The final concentrations of both coupled protein and the protein in solution were 1 μM. The mixture was incubated for 1 hr at room temperature, pelleted, washed and eluted with 10% SDS. The pellet was analyzed by 15% SDS-PAGE, and protein brought down was detected by Western blotting, using anti-His antibody.
Immunoblot Analysis
Total protein from gastric epithelium was prepared as follows: adult mice were killed and the stomachs rapidly removed and flushed with PBS containing protease inhibitor cocktail (Sigma-Aldrich). Gastric corpus mucosa was extracted and homogenized by sonication in 0.32M sucrose, 0.01M HEPES (pH 7.4), 2mM EDTA, 1mM DTT, and protease inhibitor cocktail. The homogenate was spun at 900g for 5 min to remove whole cells and debris. After centrifugation, the supernatant protein (20 μg samples) were analyzed on an 8% SDS-PAGE gel, and transferred to nitrocellulose membrane (Bio-Rad). The membranes were probed with rabbit anti-4.1R exon13, exon17b, exon18, exon19, rabbit polyclonal anti-β-catenin (1:2000, eBioscience), rabbit anti-α-catenin (1:2000, abcam), mouse anti-p120ctn (1:50,00, Sigma-Aldrich), rat anti-E-cadherin (1:2500, Zymed), rabbit anti-ZO1 (1:5000, abcam), rabbit anti-occludin (1:1000, Zymed) or rabbit anti-GAPDH (1:200,000, Sigma-Aldrich) antibodies, followed by HRP-conjugated goat anti-rabbit or anti-rat IgG (Jackson Immuno Research). The film was developed using Renaissance chemiluminescence detection kit (Pierce Biotechnology, Inc.).
Co-Immunoprecipitation
Gastric mucosa from wild-type or 4.1R-/- mice was lysed in ice-cold buffer containing 50 mM HEPES (pH 8.3), 420 mM KCl, 0.1% NP-40, 1mM EDTA, 1mM DTT and protease inhibitor cocktail for 30 min. After centrifugation at 16,000g at 4°C for 10 min, the supernatant was collected and protein concentration was estimated by the Bradford method with BSA as standard. 250 μg of extract was incubated with 2.5 μg of goat anti-4.1R exon13 polyclonal antibody or pre-immune IgG in 500 μl Co-IP buffer (Activemotif) at 4°C for 1 hour with rotation. The immunoprecipitate was isolated on immobilized Protein G agarose beads (Pierce) and separated by 10% SDS-PAGE followed by transfer to nitrocellulose membrane. The membrane was probed with rabbit anti-4.1R exon13 antibody and rabbit anti-β-catenin antibody.
Histology
Adult mice were anesthetized with pentobarbital and perfused through the heart with 10 ml of 4% paraformaldehyde (PFA) in PBS (pH7.4) to fix tissues in vivo. Then a strip of stomach tissue along the line of greater curvature from the cardia to the pylorus was dissected, further fixed in 10% neutral buffered-formalin for 24 hours and embedded in paraffin with cut surface flat for horizontal sectioning. 4 μm-thick sections were subjected to hematoxylin and eosin (H&E) staining.
Immunohistochemistry
Paraffin-embedded tissue sections, 4 μm thick, were dewaxed in xylene and rehydrated in descending concentrations of ethanol. Antigen retrieval was achieved by boiling the sections in 10 mM citrate buffer for 20 min. Sections were incubated overnight at 4°C with the following primary polyclonal antibodies: rabbit anti-4.1R exon13 (1:50), rabbit anti-β-catenin (1:40), and rat anti-E-cadherin (1:50). After thorough washing with TBS-T (0.05M Tris, pH7.4, 0.15M NaCl, 0.1% Tween 20), the sections were treated with horseradish peroxides (HRP) conjugated secondary antibodies (DakoCytomation Inc.) for 40 min at room temperature and developed with the liquid diaminobenzidine (DAB)+ substrate chromogen system (DakoCytomation). The nucleus was counterstained with hematoxylin (DakoCytomation) and images were acquired with a Leica DM 2000 microscope (Leica Microsystems Inc.).
Detergent Extractability of Transmembrane Proteins
The scraped gastric mucosa were immersed in 200μl buffer containing 5 mM sodium phosphate (pH7.0), 150 mM NaCl, 0.5% Triton X-100, mixed well and incubated on ice for 1 hour. After centrifugation at 14,000rpm for 10 min, the supernatants were separated, and the pellets were re-suspended in 200μl the above buffer. Then, both the supernatants and pellets were subjected to Western blotting analysis with rat anti-E-cadherin antibody (Zymed) or rabbit anti-occludin antibody (Zymed).
Immunofluorescence
Mice were perfused with 4% PFA as described above, the stomach tissues were dissected and embedded in optimal cutting compound (OCT) (Sakura Finetek U.S.A.), and snap-frozen in liquid nitrogen. 6 μm-thick cryosections were cut in a cryostat, further fixed and treated with 4% PFA and 0.1% Triton X-100 for 20 minutes at room temperature, and blocked with 10% horse serum and 1% BSA for 1 hour at room temperature. The sections were incubated overnight at 4°C with goat polyclonal anti-4.1R exon13 antibody (1:100), followed by Alexa 488 conjugated donkey anti-goat IgGs (Molecular Probes). After blocking again, the rabbit anti-β-catenin antibody (1:100) or rabbit anti-ZO1 antibody (1:200) were applied, followed by Alexa 594 conjugated donkey anti-rabbit IgGs (Molecular Probes). Sections were mounted and observed with a Nikon Eclipse E600 epifluorescence microscope (Nikon Inc., Japan).
Phalloidin staining
Frozen sections were fixed with 4% PFA and blocked with 1% BSA for 20 minutes at room temperature, then stained with 1 unit Texas Red-X phalloidin (Molecular Probes) for 40 minutes at room temperature. After washing 3 times with PBS, the sections were mounted in fluorescence mounting media containing DAPI (Vector) and observed under Nikon Eclipse E600 fluorescence microscope (Nikon Inc., Japan).
RESULTS
Expression and Localization of 4.1R in Gastric Epithelium
It has been shown that 4.1R is expressed in epithelial cell lines. But the role of 4.1R in epithelial biology in situ remains unexplored. As the first step to study the role of 4.1R in epithelial cell biology, we examined the expression and localization of 4.1R in the epithelial cells of various tissues. We found that 4.1R is widely expressed and localized at the lateral membrane of various epithelial cells (our unpublished observation) and it is strongly expressed in the epithelial cells of stomach. Thus the present study is focused on the gastric epithelia. We first performed Western blotting analysis using four anti-4.1R antibodies which target the constitutive exon 13 and the alternative exons 17b, 18 and 19. Fig 1A shows that all antibodies revealed a ~100 kDa band in gastric epithelial cells of 4.1R+/+ mice, but not in those of 4.1R-/- mice. Anti-exon 13 antibody detected an additional ~80 kDa band which was not recognized by anti-exon 17b, exon 18 and exon 19, implying that the ~80 kDa band does not contain these three alternative exons. Immunohistochemical staining using anti-exon 13 showed that 4.1R is expressed in the entire layer of stomach epithelium, including surface mucous cells, mucous neck cells, parietal cells and basal chief cells (Fig 1B). From longitudinal (Fig 1C) and transverse sections (Fig 1D) it can be seen that 4.1R is predominantly located at the bilateral membrane with very weak staining in the cytosol. No staining was observed in 4.1R-/- gastric epithelial cells (Fig 1E).
Fig 1. Expression and Localization of Protein 4.1R on Gastric Epithelia.
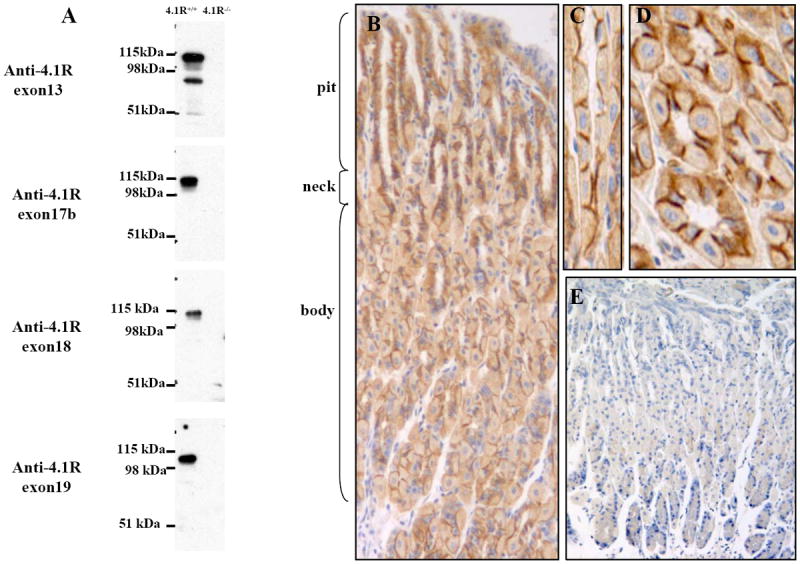
(A) Western blotting analysis of 4.1R from gastric mucosa. Total cell lysates from wild-type and 4.1R-/-mice mucosa were probed with antibodies against 4.1R exon13, exon17b, exon18 and exon19, while anti-exon 13 antibody detected an ~80 kDa and a ~100 kDa band, the other three antibodies only detected the ~100 kDa band. (B-E) Immunolocalization of 4.1R on gastric epithelia. 4.1R is expressed in all layers of gastric epithelia, with strong staining in the middle part of the gastric glands, where parietal cells predominate (B, 20x). Part of the neck region was observed under higher magnification (C, longitudinal section; D, transverse section, 40x). Note positive signal mainly on the bilateral membrane of the cells. No staining was observed in corresponding sections from 4.1R-/- mice (E, 20x).
Association of 4.1R with Adherens Junction Protein β-catenin
The bilateral localization of 4.1R suggests its association with adherens junction. To examine whether 4.1R is a component of adherens junction, we performed immunofluorescence double staining of 4.1R and β-catenin, the hallmark of adherens junctions. Fig 2A revealed almost identical staining pattern for 4.1R and β-catenin and merged image demonstrated that 4.1R co-localized with β-catenin. No co-localization was observed between 4.1R and tight junction protein ZO-1 (Fig 2B). These findings suggest that 4.1R is a component of adherens junction. To confirm the association between 4.1R and β-catenin, we performed co-immuoprecipitation assay. As Fig 2C shows, β-catenin was co-precipitated with 4.1R from a gastric mucosa lysate by anti-4.1R antibody but not by pre-immune IgG. We further performed in vitro GST pull-down binding assay. As shown in Fig 2D, 4.1R bound to the GST-tagged β-catenin, but not to GST-tagged α-catenin, cytoplasmic domain of E-cadherin or GST alone. Furthermore, only the 30 kDa FERM domain, but not other domains of 4.1R binds to the GST-conjugated β-catenin (Fig 2E). These results demonstrate that the FERM domain of 4.1R specifically interacts with β-catenin.
Fig 2. Association of 4.1R with β-catenin.
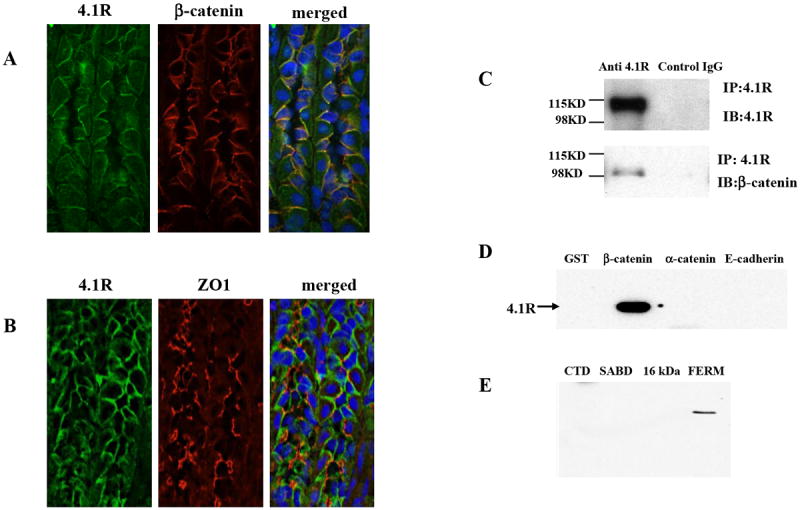
(A) Co-localization of 4.1R with β-catenin. Frozen sections from wild-type stomach were co-stained with goat anti-4.1R exon13 antibody (green) and rabbit anti-β-catenin antibody (red). Merged image shows that 4.1R co-localizes with β-catenin on the bilateral membrane. (B) 4.1R does not co-localize with ZO-1. Frozen sections from wild-type stomach were co-stained with goat anti-4.1R exon13 antibody (green) and rabbit anti ZO-1 antibody (red). Merged image shows distinct localization of 4.1R and ZO-1. (C) Co-immunoprecipation of β-catenin with 4.1R. 4.1R was immunoprecipated from from 4.1R+/+ gastric mucosa lysate using anti-4.1R or pre-immune IgG. 4.1R or β-catenin in the immunoprecipate was detected using anti-4.1R antibody (upper panel) or anti-β-catenin antibody (lower panel) respectively. Note that β-catenin was brought down with 4.1R. (D) Binding of 4.1R to β-catenin. Recombinant His-tagged 4.1R was incubated with GST, GST-tagged β-catenin, GST-tagged α-catenin or GST-tagged cytoplasmic domain of E-cadherin. Binding was assayed by pull down assay, using anti-His antibody for detection. Note that 4.1R only bound to β-catenin. (E) Binding of 4.1R domains to β-catenin. Recombinant His-tagged 4.1R domains (FERM domain; SABD: spectrin-actin binding domain; CTD: C-terminal domain) were incubated with GST-tagged β-catenin. Binding was assayed as above, using anti-His antibody for detection. Note that only FERM bound to β-catenin.
Selective Reduction of β-Catenin in 4.1R-deficient Gastric Epithelial Cells
Having demonstrated the direct and specific interaction between 4.1R and β-catenin, we then examined the effect of lack of 4.1R on junction proteins. Fig 3A shows that while β-catenin was significantly decreased in 4.1R-null gastric epithelia, α-catenin and p120 catenin were unaltered. No decrease was observed in the expression of two tight junction proteins ZO-1 and occludin. Immunohistochemistry also revealed diminished staining of β-catenin in 4.1R null cells (Fig3B). To explore the mechanisms by which lack of 4.1R led to reduction of β-catenin, we compared the transcripts of β-catenin in wild type and 4.1R null gastric mucosa by real time RT-PCR and we found no difference (data not shown). These findings demonstrate that lack of 4.1R selectively affects β-catenin, probably by affecting the stable assembly of β-catenin into adherens junctions.
Fig 3. Selective reduction of β-catenin in 4.1R-/- gastric epithelia.
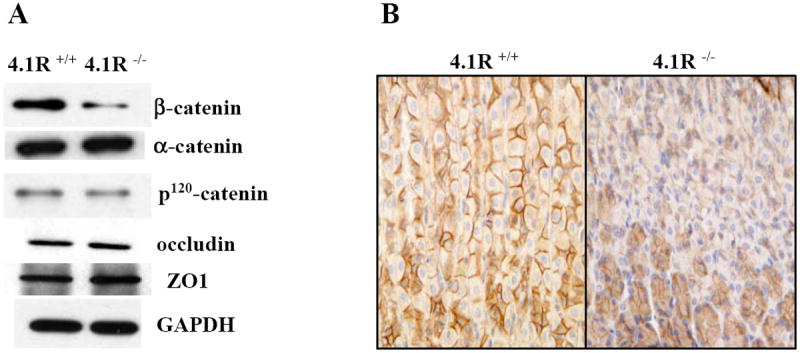
(A) Western blotting analysis. Expression of β-catenin, α-catenin, p120 catenin, ZO-1 and occudin in gastric mucosa was examined by Western blotting analysis. Note the selective reduction of β-catenin in 4.1R-/-gastric mucosa. (B) Immunohistochemistry. β-catenin was stained in paraffin-embedded wild type and 4.1R-/- stomach tissue sections. Note the staining of β-catenin on the bilateral membrane of wild-type gastric epithelial cells (left panel) and the reduction of it in the 4.1R-/- stomach.
Weakened Attachment of E-cadherin to the Cytoskeleton
β-catenin plays an important role in linking E-cadherin to the actin cytoskeleton [28-30]. We then compared the distribution, expression of E-cadherin, and its linkage to the actin cytoskeleton in wild type and 4.1R-/- gastric epithelia. Immuno-staining and Western blotting revealed no differences in E-cadherin distribution and expression in wild-type and 4.1R-/- gastric epithelia (Figs 4A, 4B and 4C). On the other hand, E-cadherin was more readily extracted by nonionic detergent from 4.1R-/- than from wild-type stomach mucosa (upper panel, Fig 4D). As a control, the lack of 4.1R (and the subsequent reduction of β-catenin) had no effect on the detergent extractability of tight junction protein occludin (lower panel, Fig 4D). It thus appears that the reduced β-catenin in 4.1R-/- epithelial cells specifically leads to weakened attachment of E-cadherin to the cytoskeleton.
Fig 4. Weakened attachment of E-cadherin to cytoskeleton in 4.1R-/- gastric epithelia.
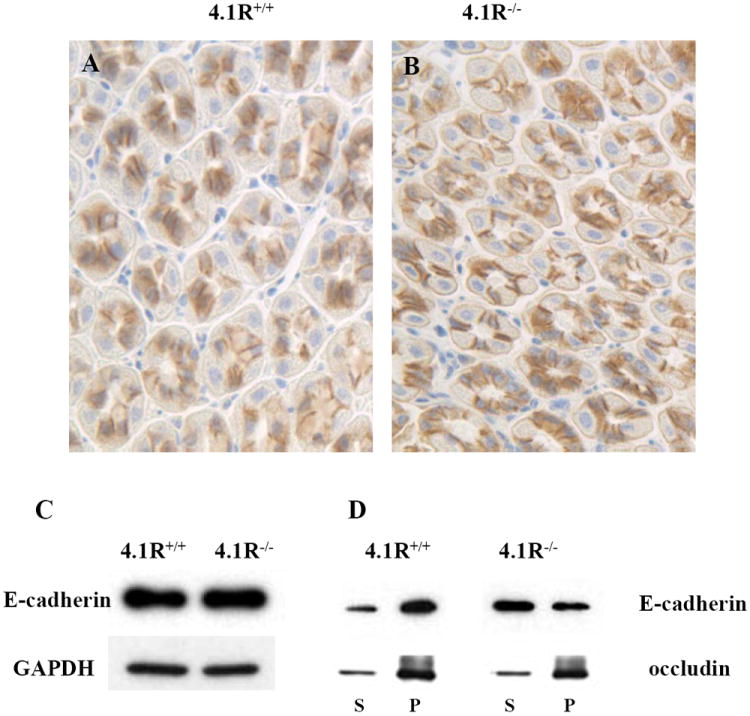
(A and B) Immunostaining of E-cadherin in wild-type and 4.1R-/- gastric epithelia. E-cadherin is located at the bilateral membranes of both wild-type (A) and 4.1R-/-(B) gastric epithelial cells. (C) Western blotting analysis of E-cadherin from gastric mucosa. Total cell lysates from mucosa of wild-type and 4.1R-/- mice were probed with anti-E-cadherin antibody. No difference was observed between wild type and 4.1R-/- mice. GAPDH was used as loading control. (D) Increased detergent extractability of E-cadherin from 4.1R-/- gastric mucosa. Transmembane proteins were extracted from wild type and 4.1R-/- gastric mucosa with buffer containing 0.5% Triton X-100. Western blotting analysis was performed using anti-E-cadherin or anti-occudin antibodies. Note retention of the bulk of E-cadherin in the pellet from wild-type samples, the greater part is recovered in the supernatant from 4.1R-/- samples. No difference was observed in the retention of tight junction protein occudin between wild type and 4.1R-/-.
Altered Actin Cytoskeleton Organization in 4.1R-/- Gastric Epithelial Cells
4.1R was first discovered as a red cell cytoskeleton protein and the lack of 4.1R resulted in altered actin cytoskeleton organization in both human and mouse red cells [31, 32]. To determine whether 4.1R also has effect on actin assembly in gastric epithelial cells, we stained the permeabilized cells with rhodamine-phalloidin. Images shown in Fig 5 are taken from the middle part of the gastric glands, where parietal cells predominate. It is important to point out that one of the distinct morphological features of parietal cell is its intracellular secretory canaliculus system, formed by an invagination of the apical cell surface and continuous with the lumen of the gastric gland. This structure is rich in actin and distributed around nucleus. Fig 5A and Fig 5C show that whereas F-actin in the wild-type cells is concentrated around the nucleus, with a sharp, well-defined thread-like pattern, displaying the intracellular secretory canalicular system, in the 4.1R-/- cells it is uniformly distributed throughout the cytoplasm (Fig 5B and Fig 5D). We conclude that as in red cells, 4.1R also plays an important for normal actin cytoskeleton organization in gastric epithelial cells.
Fig 5. Impaired F-actin cytoskeleton organization in 4.1R-/- gastric epithelial cells.
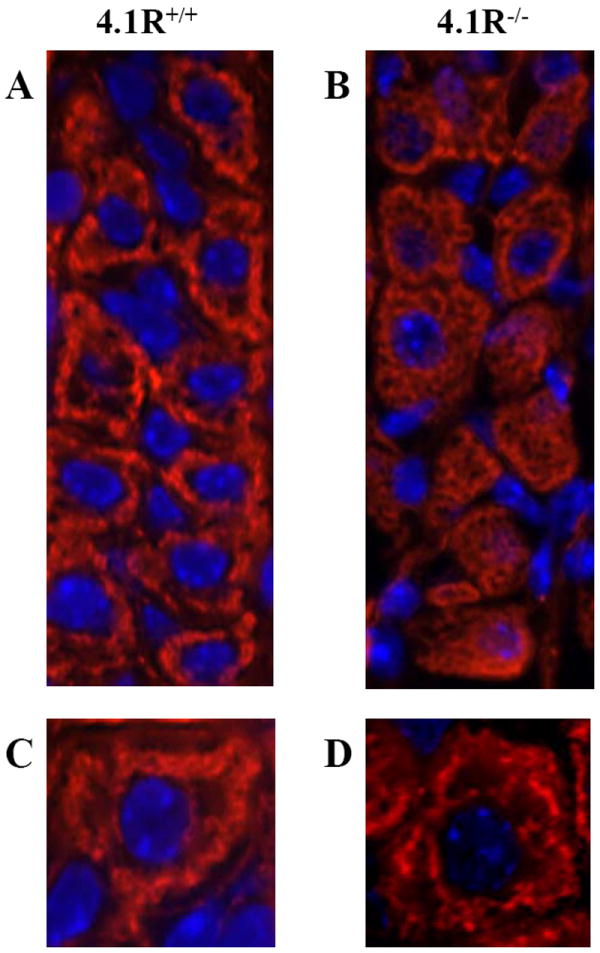
F-actin filaments were stained with rhodamine-phalloidin. In wild-type cells (A and C), actin filaments are distributed in a sharp, well-defined thread-like pattern around nucleus (displaying the intracellular secretory canalicular system), in the 4.1R-/- cells (B and D) it is uniformly distributed throughout the cytoplasm. Blue is DAPI staining for the nucleus. Magnifications: x40 (A, B); x100 (C, D).
Disorganized Glands and Discohensive Cell-cell Contact of 4.1R-/- Gastric Epithelia
To determine the effect of 4.1R deficiency on the overall structure of the stomach epithelia, we examined H&E-stained sections from 22 wild-type (14 male and 8 female) and 22 4.1R-/- (14 male and 8 female) adult mice, aged from 3 to 15 months. Fig 6A shows that wild-type gastric glands were well organized with sharply outlined and closely-packed cells, while those of the 4.1R-/- gastric glands were of irregular appearance, with irregular contours and loose cell-cell contacts (Fig 6B). Fig 6C and 6D are transverse sections of wild type and 4.1R null gastric glands respectively. They revealed that while the wild-type cells are mainly pyramidal or polygonal in outline (Fig 6C), most of those in the 4.1R-/- stomach present a rounded appearance, with short lateral membranes (Fig 6D). Importantly, while cell-cell contacts are tight in wild type gastric epithelia (Fig 6C), they are much less tight in 4.1R-/- mice (Fig 6D). These in vivo findings confirm an important role for 4.1R in the formation of normal cell-cell interactions in gastric epithelial cells.
Fig 6. Disorganization of glands and impaired cell-cell adhesion of 4.1R-/- Gastric Epithelia.
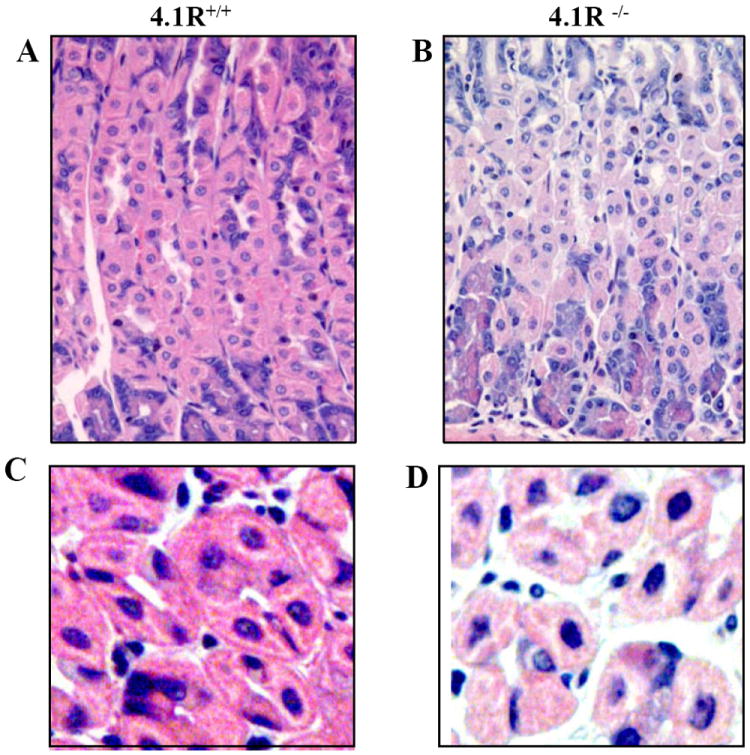
(A and B): the images are of H&E-stained sections of wild-type and 4.1R-/- gastric epithelia under 40x magnification. Note the disorganized glands of 4.1R-/- gastric epithelium. (C and D): enlarged images from A and B reveal tightly packed cells and pyramidal or polygonal parietal cells in the wild-type. Note the irregular outline of gastric glands, rounded cells with short lateral membranes and loose cell-cell contact in 4.1R -/- gastric epithelia.
DISCUSSION
The ubiquitous nature of the 4.1 superfamily proteins has become increasingly clear in recent years, as also have their multifarious functions [12-16]. Yet nearly all such studies have been conducted on cell lines, and the function in situ have been barely touched on. In the present study, we show that 4.1R is a component of adherens junction and it specifically associates with β-catenin in gastric epithelial cells. Using 4.1R knockout mice, we have shown that 4.1R is required for cell-cell adhesion and actin cytocytoskeleton organization. These findings have enabled us to establish the role of 4.1R in linking cadherin/catenin complex to the cytoskeleton through its direct interaction with β-catenin and in regulating the integrity of adherens junction.
The current view about the connection between E-cadherin/catenin complexes and the cytoskeleton is that E-cadherin binds to β-catenin which in turn is linked to the actin skeleton through its interaction with α-catenin. The findings from present study that 4.1R (also an actin-binding protein) specifically binds to β-catenin and that deficiency of 4.1R resulted in significant reduction of β-catenin provide strong evidence that 4.1R is another linker for β-catenin. A working model that accounts for our results in conjunctin with previous findings is shown in Fig 7. In wild type cell (Fig 7A), E-cadherin/catenin complex is firmly attached to the actin cytoskeleton by both α-catenin and 4.1R, which ensures the proper adhesive activity of E-cadherin. In 4.1R null cell (Fig 7B), β-catenin is reduced and the remaining β-catenin is linked the actin cytoskeleton only by α-catenin. This resulted in the weakened attachment of E-cadherin to the skeleton and the impaired cell-cell adhesion since it has been shown that the association of E-cadherin with cytoskeleton strengthens its adhesion activity [33]. In addition, actin is also disorganized.
Fig 7. Working model for role of 4.1R in gastric epithelial cells.
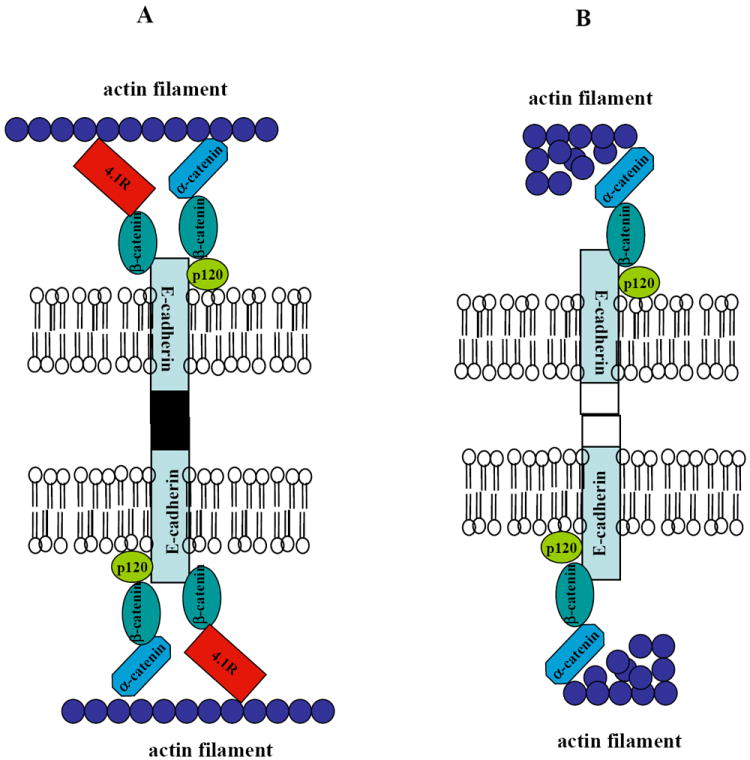
(A) In wild type cell, E-cadherin/catenin complex is well attached to the actin cytoskeleton by both α-catenin and 4.1R. This ensures the adhesive activity of E-cadherin. (B) In 4.1R null cells, β-catenin is reduced and the remaining β-catenin is linked the actin cytoskeleton only by α-catenin. This resulted in weakened attachment of E-cadherin to the skeleton and the impaired cell-cell adhesion. In addition, actin filament is also disorganized.
The concept that α-catenin is a linker for β-catenin is based on the findings that α-catenin binds to both β-catenin [34] and actin filament [35, 36] in vitro. However, it is interesting to note that knockout and/or knockdown of α-catenin has no effect on expression levels of β-catenin and other AJs proteins [37-39]. It is tempting to speculate that 4.1R may be compensating for α-catenin in these occasions.
The conclusion that 4.1R and other members of the protein superfamily act as linker proteins is derived mainly from studies on red cells, in which 4.1R deficiency results in reduction of its membrane attachment proteins, p55 [40] and glycophorin C [41], alterations in the spectrin-actin membrane cytoskeleton [31] and destabilization of the membrane [8, 42]. No similar function for 4.1R in non-erythroid cells has previously been demonstrated. We have shown here that in gastric epithelial cells lack of 4.1R leads to reduced expression of its membrane partner, β-catenin, reduced retention of E-cadherin and altered actin cytoskeletal organization, implying that a bridging function for 4.1R is a general phenomenon in many nonerythroid cells.
Members of 4.1 family are expressed in practically all types of epithelial cells, but in few cases have their functions in the cell been identified. In the context of present study, it should be noted that unlike 4.1R, 4.1B, 4.1G and 4.1N are not localized to the bilateral membrane of stomach epithelial cells (our unpublished data). Besides 4.1R and 4.1B KO mice [43], we have recently generated 4.1G and 4.1N KO mice. We expect future studies on epithelial cells of these different knockout mice will enable us to gain more explicit insights into the functions of these proteins in epithelia biology.
Acknowledgments
This work was supported in part by NIH grants DK26263, DK32094 and HL31579.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conboy J, Kan YW, Shohet SB, Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986;83:9512–6. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parra M, Gascard P, Walensky LD, Gimm JA, Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA, Snyder SH, Mohandas N, Conboy JG. Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. J Biol Chem. 2000;275:3247–55. doi: 10.1074/jbc.275.5.3247. [DOI] [PubMed] [Google Scholar]
- 3.Parra M, Gascard P, Walensky LD, Snyder SH, Mohandas N, Conboy JG. Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein 4.1 (EPB41) gene family. Genomics. 1998;49:298–306. doi: 10.1006/geno.1998.5265. [DOI] [PubMed] [Google Scholar]
- 4.Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci. 1999;19:6457–67. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould KL, Bretscher A, Esch FS, Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. Embo J. 1989;8:4133–42. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilgenbus KK, Milatovich A, Francke U, Furthmayr H. Molecular cloning, cDNA sequence, and chromosomal assignment of the human radixin gene and two dispersed pseudogenes. Genomics. 1993;16:199–206. doi: 10.1006/geno.1993.1159. [DOI] [PubMed] [Google Scholar]
- 7.Lankes WT, Furthmayr H. Moesin: a member of the protein 4.1-talin-ezrin family of proteins. Proc Natl Acad Sci U S A. 1991;88:8297–301. doi: 10.1073/pnas.88.19.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takakuwa Y, Tchernia G, Rossi M, Benabadji M, Mohandas N. Restoration of normal membrane stability to unstable protein 4.1-deficient erythrocyte membranes by incorporation of purified protein 4.1. J Clin Invest. 1986;78:80–5. doi: 10.1172/JCI112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi ZT, Afzal V, Coller B, Patel D, Chasis JA, Parra M, Lee G, Paszty C, Stevens M, Walensky L, Peters LL, Mohandas N, Rubin E, Conboy JG. Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. J Clin Invest. 1999;103:331–40. doi: 10.1172/JCI3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marfatia SM, Lue RA, Branton D, Chishti AH. In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. J Biol Chem. 1994;269:8631–4. [PubMed] [Google Scholar]
- 11.Pasternack GR, Anderson RA, Leto TL, Marchesi VT. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985;260:3676–83. [PubMed] [Google Scholar]
- 12.Krauss SW, Larabell CA, Lockett S, Gascard P, Penman S, Mohandas N, Chasis JA. Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division. J Cell Biol. 1997;137:275–89. doi: 10.1083/jcb.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattagajasingh SN, Huang SC, Hartenstein JS, Snyder M, Marchesi VT, Benz EJ. A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein. J Cell Biol. 1999;145:29–43. doi: 10.1083/jcb.145.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Ferreiro CM, Luque CM, Correas I. 4.1R proteins associate with interphase microtubules in human T cells: a 4.1R constitutive region is involved in tubulin binding. J Biol Chem. 2001;276:44785–91. doi: 10.1074/jbc.M107369200. [DOI] [PubMed] [Google Scholar]
- 15.Krauss SW, Heald R, Lee G, Nunomura W, Gimm JA, Mohandas N, Chasis JA. Two distinct domains of protein 4.1 critical for assembly of functional nuclei in vitro. J Biol Chem. 2002;277:44339–46. doi: 10.1074/jbc.M204135200. [DOI] [PubMed] [Google Scholar]
- 16.Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ., Jr Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J Biol Chem. 2000;275:30573–85. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- 17.Ramez M, Blot-Chabaud M, Cluzeaud F, Chanan S, Patterson M, Walensky LD, Marfatia S, Baines AJ, Chasis JA, Conboy JG, Mohandas N, Gascard P. Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int. 2003;63:1321–37. doi: 10.1046/j.1523-1755.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 18.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 19.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–87. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeichi M. a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–52. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 21.Patel SD, Chen CP, Bahna F, Honig B, Shapiro L. Cadherin-mediated cell-cell adhesion: sticking together as a family. Curr Opin Struct Biol. 2003;13:690–8. doi: 10.1016/j.sbi.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–44. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 23.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashiro S, Abe H, Mabuchi I. IQGAP2 is required for the cadherin-mediated cell-to-cell adhesion in Xenopus laevis embryos. Dev Biol. 2007;308:485–93. doi: 10.1016/j.ydbio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 26.An XL, Takakuwa Y, Manno S, Han BG, Gascard P, Mohandas N. Structural and functional characterization of protein 4.1R-phosphatidylserine interaction: potential role in 4.1R sorting within cells. J Biol Chem. 2001;276:35778–85. doi: 10.1074/jbc.M101364200. [DOI] [PubMed] [Google Scholar]
- 27.Gimm JA, An X, Nunomura W, Mohandas N. Functional characterization of spectrin-actin-binding domains in 4.1 family of proteins. Biochemistry. 2002;41:7275–82. doi: 10.1021/bi0256330. [DOI] [PubMed] [Google Scholar]
- 28.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–63. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 29.Aberle H, Schwartz H, Hoschuetzky H, Kemler R. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J Biol Chem. 1996;271:1520–6. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- 30.Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–25. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yawata A, Kanzaki A, Gilsanz F, Delaunay J, Yawata Y. A markedly disrupted skeletal network with abnormally distributed intramembrane particles in complete protein 4.1-deficient red blood cells (allele 4.1 Madrid): implications regarding a critical role of protein 4.1 in maintenance of the integrity of the red blood cell membrane. Blood. 1997;90:2471–81. [PubMed] [Google Scholar]
- 32.Salomao M, Zhang X, Yang Y, Lee S, Hartwig JH, Chasis JA, Mohandas N, An X. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci U S A. 2008;105:8026–31. doi: 10.1073/pnas.0803225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13:600–3. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 34.Pokutta S, Weis WI. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol Cell. 2000;5:533–43. doi: 10.1016/s1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- 35.Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem. 2002;277:18868–74. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–7. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, Butz S, Kemler R, Gruss P. An alpha-E-catenin gene trap mutation defines its function in preimplantation development. Proc Natl Acad Sci U S A. 1997;94:901–6. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 39.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–17. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 40.Alloisio N, Dalla Venezia N, Rana AP, Andrabi K, Texier P, Gilsanz F, Cartron JP, Delaunay J, Chishti AH. Evidence that red blood cell protein p55 may participate in the skeleton-membrane linkage that involves protein 4.1 and glycophorin C. Blood. 1993;82:1323–1327. [PubMed] [Google Scholar]
- 41.Reid ME, Takakuwa Y, Conboy J, Tchernia G, Mohandas N. Glycophorin C content of human erythrocyte membrane is regulated by protein 4.1. Blood. 1990;75:2229–34. [PubMed] [Google Scholar]
- 42.Tchernia G, Mohandas N, Shohet S. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J clin invest. 1981;68:454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi C, McCarty JH, Troutman SA, Eckman MS, Bronson RT, Kissil JL. Loss of the putative tumor suppressor band 4.1B/Dal1 gene is dispensable for normal development and does not predispose to cancer. Mol Cell Biol. 2005;25:10052–9. doi: 10.1128/MCB.25.22.10052-10059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


