Abstract
Abundant populations of epithelial progenitor cells maintain the epithelium along the proximal-to-distal axis of the airway. Exposure of lung tissue to ionizing radiation leads to tissue remodeling and potential cancer initiation or progression. However, little is known about the effects of ionizing radiation on airway epithelial progenitor cells. We hypothesized that ionizing radiation exposure will alter the behavior of airway epithelial progenitor cells in a radiation dose- and quality-dependent manner. To address this hypothesis, we cultured primary airway epithelial cells isolated from mice exposed to various doses of 320 kVp X-ray or 600 MeV/nucleon 56Fe ions in a 3D epithelial-fibroblast co-culture system. Colony-forming efficiency of the airway epithelial progenitor cells was assessed at culture day 14. In vivo clonogenic and proliferative potentials of airway epithelial progenitor cells were measured after exposure to ionizing radiation by lineage tracing and IdU incorporation. Exposure to both X-rays and 56Fe resulted in a dose dependent decrease in the ability of epithelial progenitors to form colonies in vitro. In vivo evidence for increased clonogenic expansion of epithelial progenitors was observed after exposure to both X-rays and 56Fe. Interestingly, we found no significant increase in the epithelial proliferative index, indicating that ionizing radiation does not promote increased turnover of the airway epithelium. Therefore, we propose a model in which radiation induces a dose-dependent decrease in the pool of available progenitor cells, leaving fewer progenitors able to maintain the airway long-term. This work provides novel insights into the effects of ionizing radiation exposure on airway epithelial progenitor cell behavior.
Keywords: lung, epithelium, stem cell, radiation quality, 3D co-culture, proliferation
INTRODUCTION
Exposure of lung tissue to Ionizing radiation results in DNA damage and altered cellular signaling, leading to tissue remodeling, fibrosis, and cancer (1–3). Humans are exposed to ionizing radiation (IR) in a variety of situations including cancer treatment, high altitude flight or space travel, and radiological warfare or accidents. However, these different radiation exposures differ in the quality of ionizing radiation delivered. Radiation quality can be characterized by the amount of energy deposited as it traverses a tissue, a property referred to as linear energy transfer (LET). Low-LET radiation, such as X-rays, deposits energy in a diffuse manner and is commonly used in radiation therapy (4). High-LET radiation, such as heavy ions found in cosmic radiation, deposit large, concentrated amounts of energy along the path taken by the high energy ion (4). Studying low- and high-LET radiation is important for optimization of regimens for therapeutic radiation and to better understand occupational exposure effects such as that encountered by astronauts.
Many studies have investigated the effects of ionizing radiation on stem and progenitor cells in a number of organs, including the epidermis, mammary gland, intestine, and hematopoietic system (5–9). A consensus from this work is that tissue stem and progenitor cells have differential radiosensitivity as compared to their differentiated progeny. Despite the wide knowledge base in these systems, less is known of the effects of ionizing radiation on progenitors in the pulmonary system. Club cells, previously known as Clara cells, are non-ciliated secretory progenitors that express Scgb1a1 (also known as CCSP and CC10) (10). Experiments using an inducible Cre recombinase, CreER, driven by the Scgb1a1 promoter to lineage-label Scgb1a1-expressing cells with a fluorescent reporter, have demonstrated that these non-ciliated secretory cells function as self-renewing progenitors that maintain the mouse bronchiolar airway (11). Additionally, Scgb1a1-expressing cells are the primary progenitors responsible for repopulating the airway epithelium following injury (11–14). Not only are these secretory progenitors capable of maintenance and repair in vivo, they can also self-renew to form colonies when placed in a 3D matrix and co-cultured with lung fibroblasts (12, 15).
The goals of the present study were to define the effect of ionizing radiation on maintenance and renewal of the airway epithelium. We used lineage tracing approaches coupled with evaluation of progenitor cell behavior in vitro and in vivo to determine the effects of low- and high-LET radiation on lung epithelial progenitor cells in mice using the bronchiolar epithelium as a model. We found that airway epithelial progenitors isolated from mice exposed to whole-body ionizing radiation lost their ability to form colonies in vitro in a dose-dependent manner. Additionally, we observed highly clonogenic Scgb1a1-expressing progenitor cells in vivo following exposure to either low- or high-LET radiation. However, exposure to radiation did not increase the lung epithelial proliferative index. These data suggest that radiation-resistant progenitor cells clonally expand for normal epithelial maintenance after functional loss of radiation-sensitive progenitors.
MATERIALS AND METHODS
Mice
The Scgb1a1-Cre; Rosa26-mT/mG mice were generated by crossing Scgb1a1-Cre mice with Rosa26-mT/mG (The Jackson Laboratory, Bar Harbor, Maine). The Scgb1a1-CreER; Rosa26R-Confetti mice were established by crossing Scgb1a1-CreER™ mice (kindly provided by Brigid L.M. Hogan, Duke University) with Rosa26R-Confetti mice (The Jackson Laboratory) as previously reported by Chen et al (12). Scgb1a1-CreER mice heterozygous for the Rosa26R-Confetti allele were injected i.p. 3 times every other day with 0.2mg/g body weight tamoxifen in Mazola corn oil to randomly introduce one of four genetic tags into the Scgb1a1-expressing epithelial cells. All mice were maintained in pathogen-free conditions in AAALAC approved animal facility at Duke University. Mice were exposed to a 12-hour light/dark cycle and had free access to food and water. Adult mice between the ages of 2–4 months were sacrificed for experiments according to IACUC approved protocols.
IdU Drinking Water
5-Iodo-2′-deoxyuridine (IdU; Sigma-Aldrich, St. Louis, MO) was resuspended in sterile drinking water at a concentration of 1 g/L. Fresh IdU drinking water was provided weekly, for 4 weeks, in light protected water bottles.
Radiation Exposure
Mice, eight to ten weeks old, were either exposed to either X-rays or 56Fe radiation. For in vitro experiments using low-LET irradiation, unanesthetized mice were placed in plexiglas restraining tubes, and irradiated with 1, 2, 4, 6, or 8 Gy of 320 kVp X-rays (X-RAD 320 Biological Irradiator, Precision X-ray, Filter#4: 2.5 mm aluminum + 0.1 mm copper, dose rate = 1.95 Gy/min) delivered to the whole body. For in vivo clonal expansion and IdU experiments using low-LET irradiation, unanesthetized mice were placed in plexiglas restraining tubes, and irradiated with 8 Gy of 320 kVp X-rays, delivered only to the thorax by shielding the head and abdomen with lead. For high-LET irradiation, mice were exposed whole body to 0.2, 0.5, 1, and 2.5 Gy of 600 MeV/nucleon 56Fe ions (NASA Space Research Laboratory’s linear accelerator at Brookhaven National Laboratory, dose rate 0.1 Gy/min).
Lung Cell Isolation and Flow Cytometry
Eighteen hours post irradiation, suspensions of primary lung cells were isolated by elastase digestion and subsequently flow sorted for epithelial cells using cell specific surface markers, as previously described (12). Following euthanasia, the chest cavity was opened and the lungs were perfused via the heart with PBS. The trachea was cannulated and lavaged with PBS. The heart and lungs were then removed en block and the lungs instilled with elastase (Worthington Biochemical, Lakewood, NJ) for 10 minutes in a 37°C water bath followed by 3 additional 0.5 mL instillations with a 5 minute incubation period between each instillation. After elastase digestion, the lung lobes were dissected away from the heart and extrapulmonary airways, minced with scissors and further digested by the addition of DNase I (Promega, Madison, WI) for 15 minutes at 37°C. The cell suspension was passed through a 70 μm cell strainer, gently centrifuged (600 × g, 6 min, 4°C), and briefly resuspended in a red blood cell lysis solution (eBioscience Inc., San Diego, CA), then staining buffer (HBSS, 10 mM HEPES, 2% FBS) and centrifuged as above.
Cells were sorted using a FACSVantage cell sorter (BD Biosciences, San Jose, CA). For lung epithelial cells from ubiquitous-RFP (Rosa26-mT/mG) or -GFP mice, cell populations were positively selected for EpCAM (PE-Cy7 anti-EpCAM; eBioscience, San Diego, CA) and negatively selected for 7-aminoactinomycin D (7-AAD, Biolegend) and biotin labeled antibodies to anti-CD34, -CD31, -CD45 (eBioscience) labeled with APC-Cy7 anti-biotin (eBioscience). For GFP labeled Scgb1a1+ cells (Scgb1a1-Cre; Rosa26-mT/mG), GFP positive cells were positively selected for EpCAM and negatively selected for 7-AAD.
Matrigel Cell Culture
Flow sorted epithelial cells were cultured with mouse lung fibroblasts and Matrigel (BD Biosciences) seeded onto Transwell filter inserts (BD Biosciences) as previously described (12). For ubiquitous-RFP + ubiquitous-GFP mixing experiments, red and green epithelial cells were mixed in equal amounts, 2.5×103 each, with 1×105 mouse lung fibroblasts. The epithelial/fibroblast mixture was then added to an equal volume of growth factor reduced Matrigel (BD Biosciences) and seeded to the apical surface of 24-well transwell filter inserts (BD Biosciences) placed in 24-well flat-bottom culture plates. The solution was allowed to polymerize for >30 min at 37°C, then basic medium was added to the basal compartment. Cell cultures were maintained for 14 days at 37°C in a humidified incubator (5% CO2). Colony-forming efficiency was calculated as the percentage of seeded cells that give rise to colonies, visualized on a Zeiss Axiovert40 inverted fluorescent microscope.
Immunofluorescence and Morphometric Analysis
Immunofluorescence imaging was performed on fixed lung tissue embedded in paraffin and processed as previously described (12). The following primary antibodies were used: Rabbit anti-GFP (1:1000, Abcam, Cambridge, MA), Rabbit anti-RFP (1:300, Rockland Immunochemicals, Gilbertsville, PA), Mouse anti-C-terminus β-catenin (1:400, BD Biosciences), Rabbit anti-SCGB1a1 (1:15000, in house), Mouse anti-IdU/BrdU (1:200, BD Biosciences). Primary antibodies were labeled with the appropriate corresponding fluorescently conjugated secondary antibody (Alexa Fluor 488 or Alexa Fluor 594; Invitrogen, Carlsbad, CA). Images were acquired using a Zeiss 780 confocal inverted confocal microscope with Zen Software.
Morphometric analysis of confetti-labeled airway epithelial patches and epithelial proliferation was performed using a Zeiss Axiovert40 inverted fluorescent microscope. Sections were selected that contained the greatest representation of the airway epithelial tree and stained on serial sections for IdU/Scgb1A1, beta-catenin/RFP, and GFP-variants (YFP, CFP, GFP)/beta-catenin. All contiguous patches of epithelial cells in a section that share the same lineage tag (cytoplasmic RFP, nuclear GFP, cytoplasmic YPF, and membrane CFP) and share the same lateral membrane (beta-catenin) were counted. For proliferative index, all IdU+CCSP+ were counted and represented over the total number of DAPI+ airway epithelial cells. Patch size and frequency, and proliferative index were plotted using Microsoft Excel and GraphPad Prism.
RESULTS
Low-LET radiation results in cell autonomous and dose-dependent decrease in epithelial colony-forming ability
Previous work by us and others have shown that the murine airway epithelium possess the potential to proliferate and repair after injury as well as the ability to clonally expand and differentiate when cultured in a 3D-in vitro culture system (12, 15). To determine the effects of low-LET radiation exposure on airway epithelial progenitor cells, the colony forming ability of primary mouse lung epithelial cells was assessed in vitro after whole-body exposure of mice to X-rays (Fig. 1A). Epithelial cells were isolated from the lungs of mice that ubiquitously expressed RFP (U-RFP) 18 hours after whole-body exposure to X-rays. Primary epithelial cells were also isolated from the lungs of unirradiated mice ubiquitously expressing GFP (U-GFP) and mixed in equal proportion with the irradiated epithelial cells. These cells were co-cultured in Matrigel along with unirradiated immortalized mouse lung fibroblasts and assessed for colony forming efficiency at culture day 14. X-ray dose-dependent decreases were observed in colony-forming ability of isolated epithelial progenitor cells (Fig. 1B). Colony forming efficiency decreased to 44% and 14% after exposure to 2 Gy and 4 Gy of X-rays, respectively, and was completely lost after exposure to 8 Gy of X-rays. No differences were observed in the colony forming efficiency of un-irradiated U-GFP progenitor cells that were co-cultured with irradiated progenitor cells.
Figure 1. Dose-dependent decrease in lung epithelial colony formation following whole body X-ray irradiation.
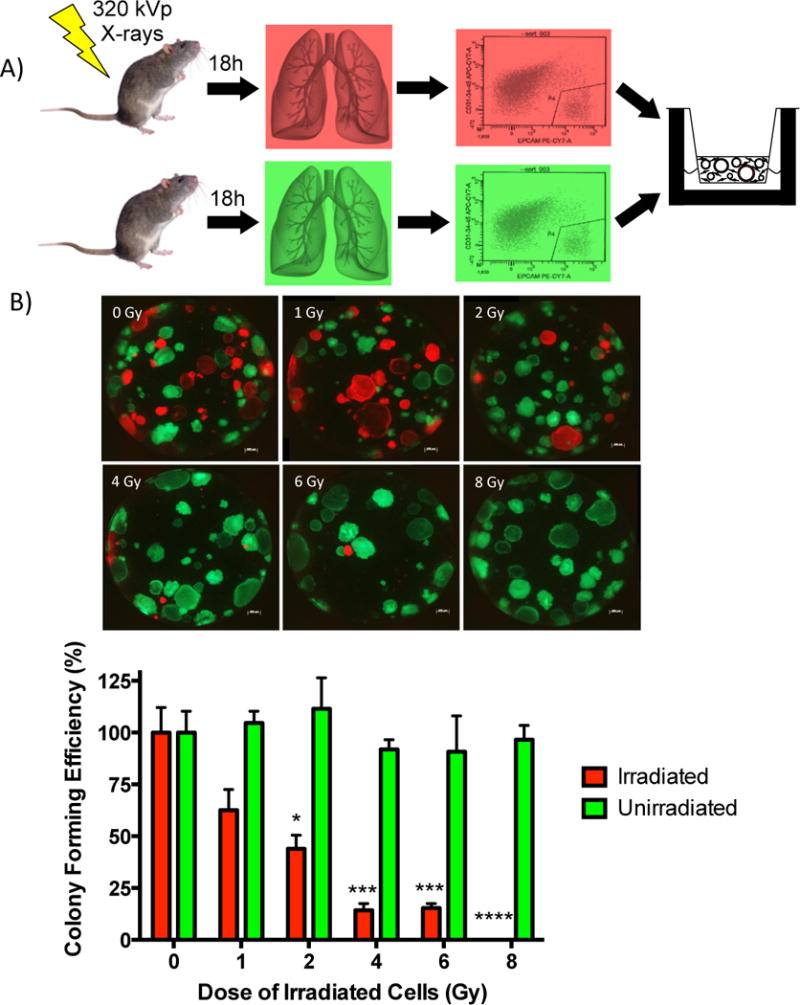
(A) Isolated and fractionated primary epithelial cells from X-ray irradiated U-RFP and unirradiated U-GFP mice were co-cultured with unirradiated mouse lung fibroblasts for 14 days. (B) Colonies were imaged and quantitated and the colony forming efficiency calculated as the percentage (± SEM) of seeded cells that give rise to colonies after 14 days in culture. Graph was plotted as the percent of colony forming efficiency normalized to the control. Significance to unirradiated controls indicated by: * (p ≤ 0.05), *** (p ≤ 0.001), **** (p ≤ 0.0001).
We considered the possibility that changes in the colony-forming ability of epithelial cells isolated from irradiated mice may have resulted from either direct or indirect (microenvironmental non-targeted) effects of radiation exposure. To distinguish between these possibilities, epithelial cells were isolated from un-irradiated mice and irradiated to the same doses of X-rays ex-vivo prior to evaluating colony-forming ability in 3D epithelial-fibroblast co-cultures (Fig. 2A). Radiation dose-dependent decreases in epithelial colony forming ability were observed following ex-vivo irradiation that were identical to those observed following whole-body in vivo radiation exposure, for example, colony forming ability was 13% after 4 Gy whole body exposure as well as after 4 Gy ex-vivo exposure (Fig. 2B). Together these data suggest that X-ray exposure induces direct alterations in lung epithelial proliferative capacity, resulting in dose-dependent decrease in colony forming efficiency.
Figure 2. Dose-dependent decrease in lung epithelial colony formation following ex vivo X-ray irradiation.

(A) Isolated and fractionated primary epithelial cells were irradiated with X-rays ex vivo. (B) Colony forming efficiency was calculated as the percentage (± SEM) of seeded cells that give rise to colonies after 14 days in culture. Graph was plotted as the percent of colony forming efficiency normalized to the control. Significance to unirradiated controls indicated by: * (p ≤ 0.05), *** (p ≤ 0.001), **** (p ≤ 0.0001).
Clonal expansion of epithelial progenitor cells in airways of mice following exposure to low-LET radiation
To understand the effect of low-LET radiation exposure on airway epithelial progenitors in vivo, we used a lineage tracing method to assess changes in the clonal behavior of Scgb1a1-expressing progenitor cells of conducting airways. Prior to thoracic X-ray exposure, Scgb1a1-CreER™/Rosa26R-Confetti mice were given tamoxifen to randomly tag airway progenitor cells with one of four lineage reporters. Reporters introduced into Scgb1a1-expressing cells could be distinguished by native fluorescence and/or subcellular distribution and their expression under the control of a ubiquitous promoter allowed visualization of progenitor-progeny relationships as a function of time after exposure to ionizing radiation (Fig. 3A). The frequency of large epithelial patches composed of 3 or more touching cells carrying the same lineage tag significantly increased greater than 2-fold at both 30 and 70 days following low-LET radiation exposure (Fig. 3B). Interestingly, only 6.12% of lineage traced club cells undergo increased patch expansion post-IR, indicating that only a small proportion of progenitors are contributing to epithelial maintenance and regeneration after radiation-induced progenitor cell depletion.
Figure 3. X-ray irradiation induces clonal expansion of club progenitor cells.
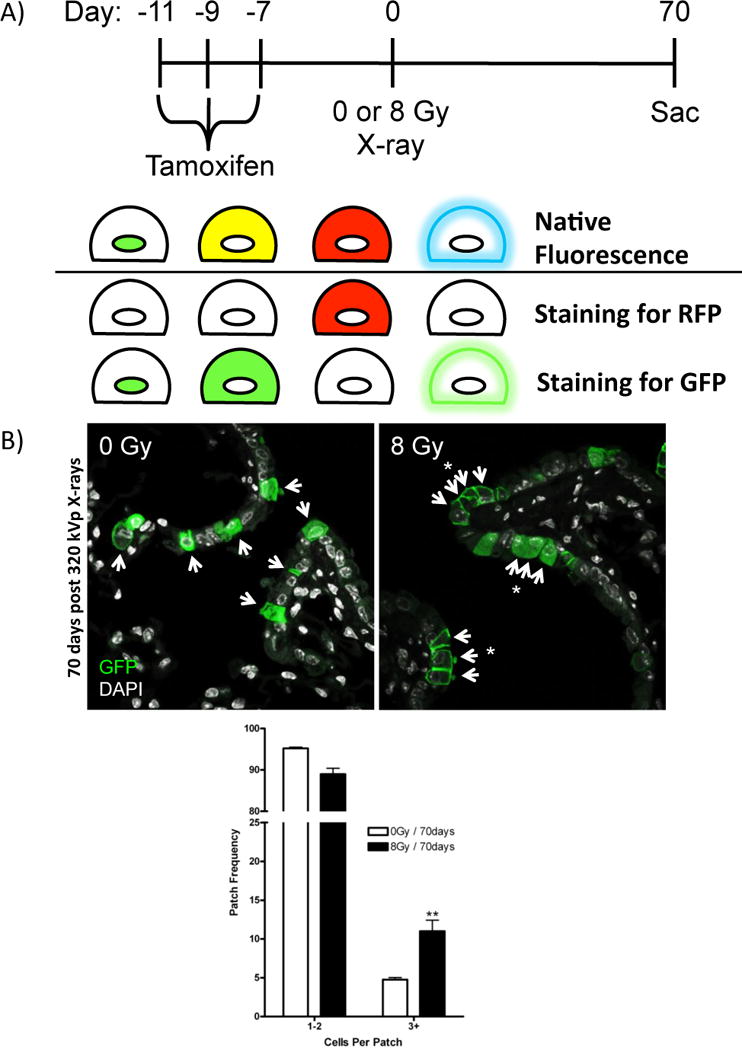
(A) Schematic describing experimental design and immunofluorescence staining to visualize 4 distinct fluorescent protein reporters. Tamoxifen treated Scgb1a1-CreER™; Rosa26R-Confetti mice randomly recombine to induce one of four distinct fluorescent markers that differ in their subcellular distribution (nuclear GFP, cytoplasmic YFP, cytoplasmic RFP, membrane CFP) in Scgb1a1-expressing club cells. RFP lineage tagged airway epithelial cells were assessed by immunofluorescence for RFP; lineage tags of GFP variants (GFP, YFP, CFP) within club progenitor cells were assessed by immunofluorescence of GFP and distinguished by subcellular localization. (B) Patches of lineage tags assessed by immunofluorescence in mice exposed to 0 or 8 Gy X-rays and recovered for 30 or 70 days (RFP not shown, arrows indicate tagged cells, asterisks indicate patches). Quantitation of patches (3+ cells that share the same lineage tag) from unirradiated and X-ray irradiated mice. Data is presented as the relative frequency (± SEM) of patches (3+) per all lineage tagged club cells. Significance to unirradiated controls indicated by: * (p ≤ 0.05), ** (p ≤ 0.01).
We next sought to determine whether clonal expansion of epithelial progenitor cells resulted from increased radiation-induced turnover of the epithelium. We measured epithelial cell proliferative responses to X-ray exposure as a surrogate to follow epithelial turnover. Immunofluorescent detection of nuclear 5-iodo-2-deoxyuridine (IdU) was used to determine the cumulative labeling index after 7 or 30 days of continuous IdU labeling in either control mice or mice exposed to 8 Gy X-rays (Fig. 4A). Cumulative IdU labeling indices were not different between control and X-ray exposed mice at either time point evaluated (Fig. 4B). IdU labeling indices were 9.7% and 8.3% at the 7 day time point and 21.2% and 21.7% at the 30 day time point, for control and X-ray exposed mice, respectively. However, contiguous patches of IdU-labeled epithelial cells were more frequently observed in X-ray exposed mice and were correlated with the clonogenic patches (data not shown). These data suggest that X-ray exposure does not increase the rate of epithelial turnover. Together with lineage tracing data, our results suggest that radiation reduces the pool of epithelial progenitor cells available for proliferation, leading to increased cycling of radiation-resistant progenitors to maintain the epithelium.
Figure 4. Proliferative kinetics of airway epithelium following X-ray irradiation.

(A) Proliferative index of the airway epithelium post-IR was assessed at 7 and 30 days using continuous IdU labeling. (B) Proliferative kinetics data are expressed as the percent (± SEM) IdU+ airway epithelial cells per total DAPI+ airway epithelium. No significant changes were found.
Epithelial colony-forming ability and expansion of epithelial progenitor cells in airways of mice following exposure to high-LET radiation
High-charge and energy (HZE) nuclei, such as 56Fe, are a high-LET and potentially highly damaging component of space radiation. Since evolutionary pressure may have led to protection of progenitor cell pools against terrestrial sources of low-LET radiation, no such selective pressure exists to develop defenses against high-LET radiation. To determine the effects of high-LET radiation on airway epithelial progenitor cells, we used whole body exposure to 600 MeV/nucleon 56Fe ionizing radiation in conjunction with lineage tracing and either in vitro or in vivo analysis of progenitor cell behavior. Scgb1a1-expressing cells were lineage tagged, isolated following whole body exposure to high-LET radiation, placed in co-culture with unirradiated mouse lung fibroblasts, and assessed for colony forming ability at 14 days (Fig. 5A). No significant decrease in colony forming ability occurred following exposure to low doses of high-LET radiation; however, after exposure to 2.5 Gy 56Fe, we observed a significant decrease of 79% in the ability of Scgb1a1 lineage-labeled progenitor cells to form colonies (Fig. 5B). Compared to results from X-ray exposure (Fig. 1B), we show that, when exposed to the same dose, the airway progenitor cells exposed to high-LET radiation had a lower colony forming efficiency, with a relative biological effectiveness of approximately 2.
Figure 5. Dose dependent changes in lung epithelial colony formation following 600 MeV/nucleon 56Fe irradiation.
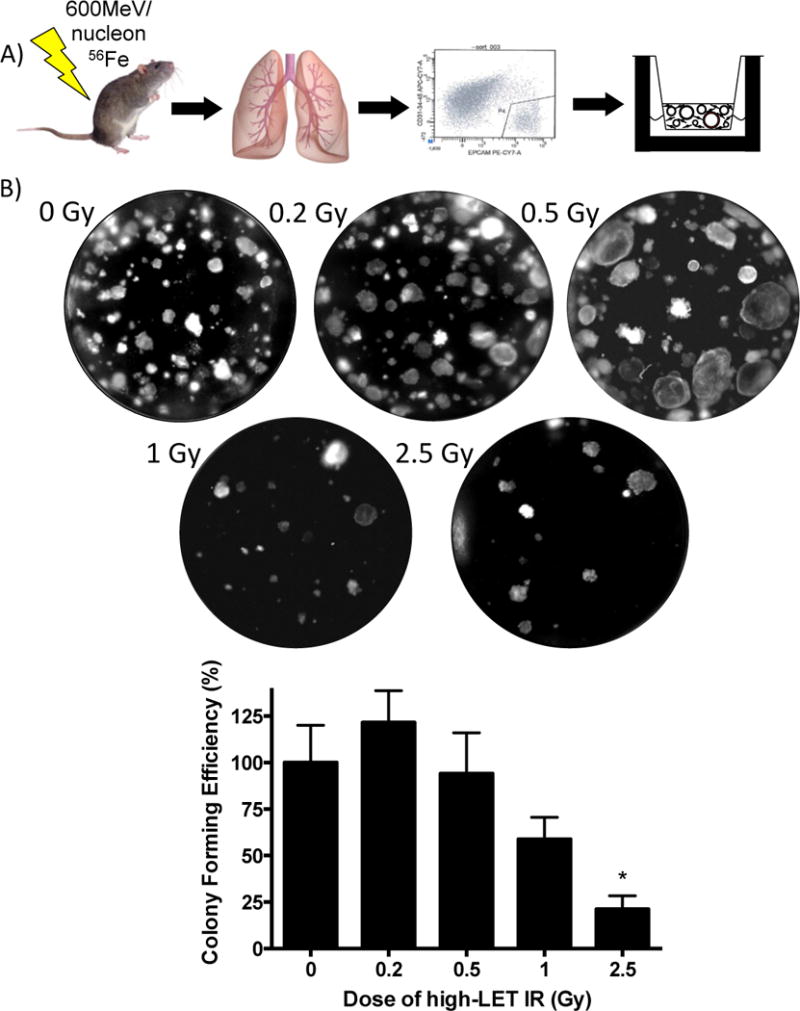
(A) Primary GFP lineage tagged club epithelial cells were isolated from unirradiated mice or mice exposed to 600 MeV/nucleon 56Fe (0, 0.2, 0.5, 1.0, and 2.5 Gy) mice (Scgb1a1-Cre; Rosa26-mT/mG), fractionated, and co-cultured with mouse lung fibroblasts for 14 days. (B) Colonies were quantitated and the colony forming efficiency calculated as the percentage (± SEM) of seeded cells that give rise to colonies after 14 days in culture. Graph was plotted as the percent of colony forming efficiency normalized to the control. Significance to unirradiated controls indicated by: * (p ≤ 0.05).
We next evaluated the effect of high-LET exposure on airway epithelial progenitor cells in vivo. Scgb1a1-CreER™, Rosa26R-Confetti mice were given tamoxifen to lineage label airway progenitor cells and exposed to 0 or 2.5 Gy 56Fe (Fig. 6A). No significant differences in lineage patch size distribution were observed between control mice and mice exposed to whole-body 0.5 Gy 56Fe (data not shown). However, after whole body irradiation with 2.5 Gy 56Fe, we observed a significant increase in the abundance of large lineage-labeled patches indicative of increased clonal expansion of airway progenitor cells (Fig. 6B). Taken together, airway epithelial cells showed greater sensitivity when exposed to high-LET radiation, resulting in decreased colony forming ability. However, after in vivo high-LET radiation exposure, the remaining pool of proliferation-competent epithelial progenitors was able to divide and maintain the epithelium.
Figure 6. 56Fe irradiation induces clonal expansion of club progenitor cells.
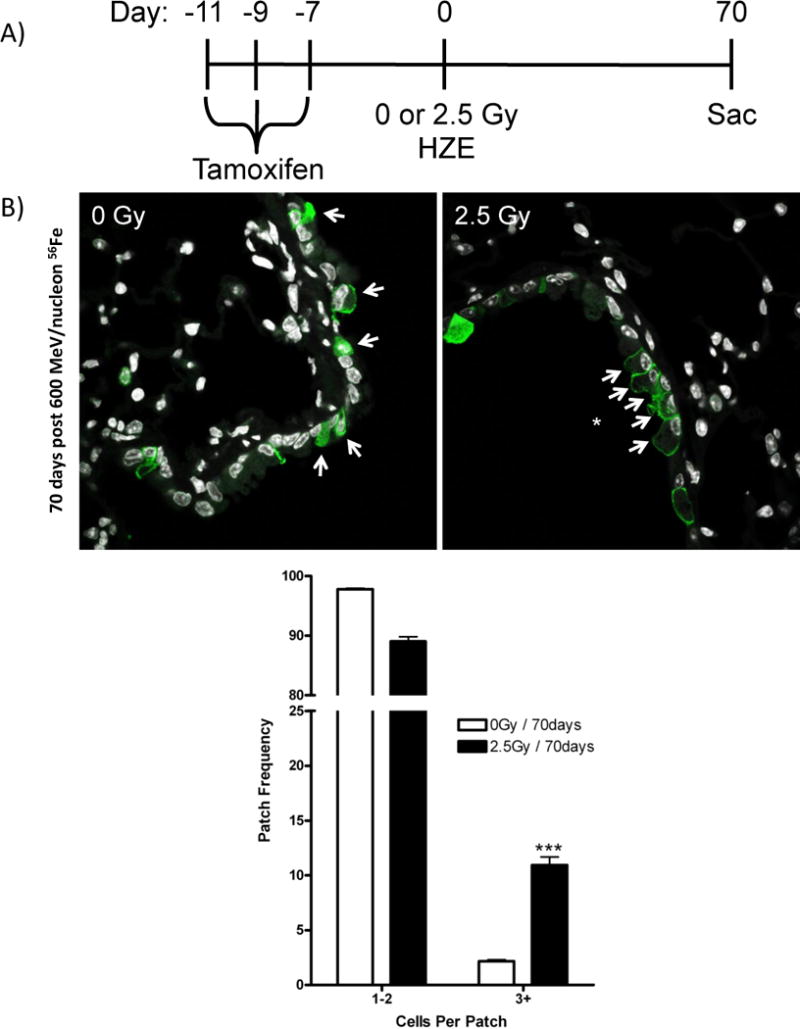
(A) Patches of GFP lineage tags (cytoplasmic RFP, nuclear GFP, cytoplasmic YFP, membrane CFP) assessed by immunofluorescence of GFP in formalin-fixed lung tissue from tamoxifen treated Scgb1a1-CreER™; Rosa26R-Confetti mice exposed to 2.5 Gy 600 MeV/nucleon 56Fe and recovered for 70 days. (B) Quantitation of lung epithelial patches (1–2 or 3+ cells that share the same lineage tag) from unirradiated and 56Fe irradiated mice. Arrows indicate tagged cells and asterisks indicate patches. Data is presented as the relative frequency (± SEM) of patches (1–2 vs 3+) per all lineage tagged airway epithelial cells. Significance to unirradiated controls indicated by: *** (p ≤ 0.001).
DISCUSSION
In the present study we used an in vitro epithelial-stromal co-culture assay in combination with in vivo lineage tracing to better understand the effects of radiation on progenitor cells that maintain the airway epithelium. Whole body exposure to low-LET radiation resulted in a dose-dependent decrease in in vitro colony forming efficiency of airway progenitors removed lung tissue 18 hours after radiation injury. Following ex vivo exposure of isolated lung epithelial progenitor cells to low-LET IR, a similar dose-dependent decrease in in vitro colony forming efficiency was observed. These data suggest that radiation-induced decreases in epithelial in vitro colony forming ability reflect the direct effects of radiation on progenitors without a significant contribution made by either systemic or local non-target effects. In accordance with the results using low-LET radiation, exposure to high-LET radiation resulted in a significant decrease in colony forming ability after 2.5 Gy 56Fe compared to unexposed controls, without significant effects at 0.5 Gy. When comparing results from low- and high-LET exposure we calculate a relative biological effectiveness of approximately 2 for 56Fe particles compared to X-rays. In contrast to the radiation-induced decrease in colony forming ability in vitro, a significant increase in clonal expansion of progenitor cells was observed in vivo following exposure to either low- or high-LET radiation. Taken together, these findings suggest that radiation exposure results in a dose-dependent reduction of proliferation-competent progenitor cells leading to clonal expansion of remaining progenitors in vivo.
We have previously shown that ablation of mouse airway progenitor cells through parenteral exposure to naphthalene is repaired through expansion of chemically resistant progenitors that localize to neuroepithelial bodies (NEB) and bronchoalveolar duct junctions (BADJ) of terminal bronchioles (13, 14). Chemical resistance of NEB-associated progenitors results from the differentiation-modulating properties of the local microenvironment (13). However, it is not clear what mechanisms lead to preservation of progenitor cells that survive after exposure to radiation. Epithelial progenitor cells undergoing clonal expansion following exposure to radiation were not associated with NEB and BADJ microenvironments that harbor naphthalene-resistant airway progenitors. Furthermore, whereas resistance of progenitor cells to naphthalene-induced airway injury has been associated with reduced expression of genes involved in bioactivation of toxicants (16), it is not clear whether progenitor cells that retain proliferative capacity following radiation necessarily represent a resistant population versus progenitors that survive the damaging effects of radiation by chance. Further studies are needed to explore mechanisms by which epithelial progenitor cells of airways retain their proliferative capacity following exposure to radiation.
A surprising finding from our study was that the doses of radiation exposure that were sufficient to decrease lung progenitor in vitro colony formation was not sufficient to increase the normally slow rate of epithelial turnover in airways, as indicated by cumulative IdU labeling. Nevertheless, this radiation exposure was sufficient to increase progenitor cell clonal expansion in vivo. Epithelial cell damage and lung injury resulting from exposure to inhaled or systemically delivered toxicants typically results in the activation of repair responses including proliferation of surviving epithelial progenitor cells. This is evident in models of progenitor cell depletion such as naphthalene-induced lung injury, which results in the clonal expansion of naphthalene-resistant progenitors to replenish depleted epithelium (13, 14). However, whole-body exposure of mice to doses of X-rays up to 8 Gy and 56Fe radiation up to 2.5 Gy resulted in no apparent loss of epithelial integrity. Exposure to these doses of radiation appeared to cause some epithelial progenitors to lose their ability to proliferate because of the decreased colony-forming ability in vitro. This interpretation is also consistent with the observation of clonal expansion of some epithelial progenitor cells into patches in vivo in the absence of an associated increase in the overall epithelial proliferative index. Taken together, our results suggest that, within the dose range we studied, radiation to the lung inhibits proliferation of some epithelial progenitor cells, which places an additional proliferative demand on the pool of progenitors that remain competent for proliferation after radiation for epithelial maintenance (Fig. 7). Together our data suggest that airway progenitor cells are functionally compromised following radiation exposure up to 8 Gy of X-rays or 2.5 Gy of 56Fe, but are retained within the epithelium rather than being eliminated by cell death pathways. Other changes to the function of radiation-damaged epithelial cells, such as acquiring a senescent phenotype, remain to be determined. Our data contrast those observed in frequently cycling progenitors of rapidly replacing epithelia, such as in the gut (17), and suggest that radiation-induced epithelial damage has the potential to accumulate in lung tissue. These findings have potentially important implications for lung homeostasis and repair among individuals receiving long-term low dose radiation or higher dose radiotherapy. By impairing the ability of some progenitor cells to divide with the resulting clonal expansion of remaining progenitors, radiation has the potential to impair normal repair and promote aberrant tissue remodeling.
Figure 7. Model for changes in airway epithelial progenitor cell behavior post-IR.
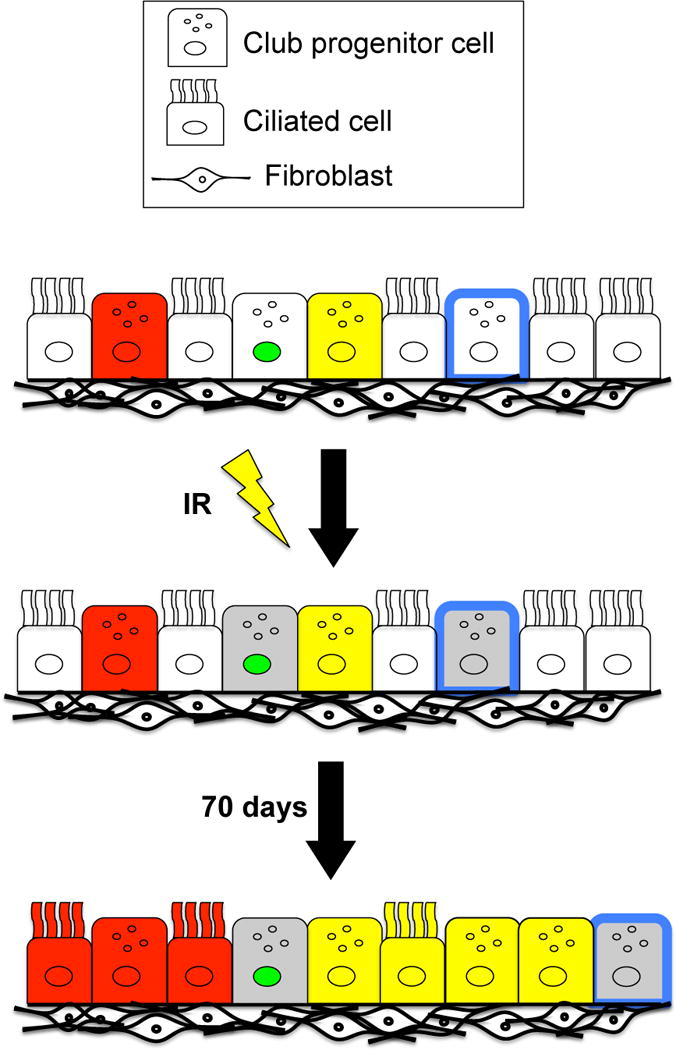
Following exposure to ionizing radiation, a dose-dependent decrease in the pool of available progenitor cells occurs (indicated by grey club cells). Therefore, as the lung epithelium undergoes normal turnover, fewer progenitors have the capacity to divide, so that the progenitors that retain proliferate capacity must divide more frequently to maintain the airway epithelium, giving rise to patches. This proliferative response following exposure to radiation could contribute to carcinogenesis.
Acknowledgments
We are grateful to Karen Terry for important technical contributions, Lixia Luo for assistance with mouse colony management and Mark Onaitis for critical discussion. We thank members of the Duke NSCOR as well as the staff at the NASA Space Research Laboratory (NSRL) linear accelerator for assistance exposing mice to radiation at Brookhaven National Laboratory. This work was supported by NASA NSCOR grant NNX11AC60G (BRS and DGK) and CIRM grant LA1_C12-06915 (BRS).
References
- 1.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment – tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–75. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 2.Bekker-Jensen S, Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair. 2010;9:1219–28. doi: 10.1016/j.dnarep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Abratt RP, Morgan GW, Silvestri G, Willcox P. Pulmonary complications of radiation therapy. Clin Chest Med. 2004;25:167–77. doi: 10.1016/S0272-5231(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 4.Okayasu R. Repair of DNA damage induced by accelerated heavy ions-a mini review. Int J Cancer. 2012;130:991–1000. doi: 10.1002/ijc.26445. [DOI] [PubMed] [Google Scholar]
- 5.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Graczet AD, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1111–32. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–76. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–85. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci. 2007;104:618–23. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotiropoulou PA, Candi A, Mascré G, Clercq SD, Youssef KK, Lapouge G, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–82. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- 10.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest. 2012;122:2724–30. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee J, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–60. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–81. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 14.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci. 2009;106:9286–91. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44:794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–78. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsch DG, Santiago PM, di Tomasso E, Sullivan JM, Hou W, Dayton T, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–6. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]


