Abstract
Breast and prostate cancers are among the most common of all cancers. They are referred to as hormone-dependent cancers, because estrogen and androgen are involved in their development and growth. The effects of these hormones are mediated by their respective receptors, estrogen receptor (ER) α and androgen receptor. Around 18 years ago, a second ER, ERβ, which has a very similar structure to ERα, was discovered. Its function has been investigated using a variety of methods and biological systems, leading to our present understanding that ERβ can interact with or inhibit ERα and androgen receptor function directly and/or indirectly, suppress cell growth, and influence responsiveness to endocrine therapy. In order to apply the “inhibition of cell growth” function to cancer treatment, several specific ERβ agonists have been synthesized and are being tested for effectiveness in cancer treatment. We need to keep our eyes on ERβ.
Keywords: Breast cancer, estrogen receptor alpha, estrogen receptor beta, hormone therapy, prostate cancer
Etiology of Breast Cancer and Prostate Cancer
Breast cancer is the most threatening neoplastic disease in females; nearly 1.7 million women per year are diagnosed with it, accounting for 25% of all cancers in females worldwide. Prostate cancer is the second most frequently occurring cancer in males, with approximately 1.1 million new diagnoses per year (World Cancer Research Fund International, http://www.wcrf.org). These two leading cancers are so-called “hormone-dependent cancers”, which means that these cancers are initiated and developed by sex steroid hormones, that is, estrogens and androgens.
Androgen and AR, Estrogen and ER
Androgens include adrenal androgens and testosterone. Adrenal androgens are synthesized by the adrenal cortex and testosterone is produced in the testicles of males and the ovaries of females. 5α-Dihydrotestosterone is the most potent ligand of AR and is converted from testosterone in target tissues like prostate; it is processed for excretion by hydroxylation. One hydroxylation product, 3βAdiol, has been reported as the most likely ligand of ERβ in the prostate (Fig.1).1,2 Androgens are precursors of estrogens and therefore very important for ERs. Estrogens are synthesized by aromatization of androgens. The most potent ligand of ER is E2, which is synthesized from either estrone or testosterone (Fig.1).
Fig 1.
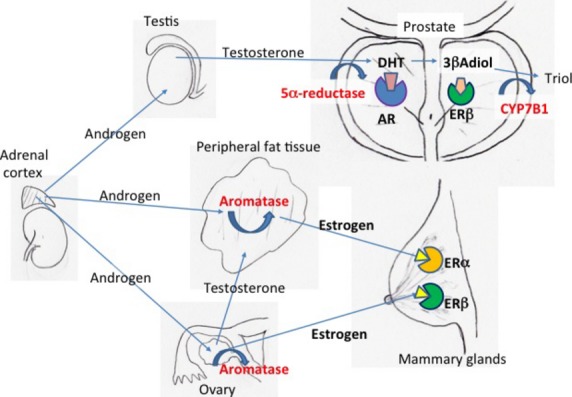
Steroid synthesis in key organs and steroid receptors in prostate and breast. Androgen, precursor estrogen, and testosterone are produced in the adrenal cortex. Androgen is converted into testosterone in testis or ovary and further converted into dihydrotestosterone (DHT) in prostate or into estrogen in ovary and peripheral fat tissue. Estrogen receptor β (ERβ) is present in both prostate and breast, however, its ligand seems to be estrogen in breast and 5α-androstane-3β, 17β-diol (3βAdiol) in prostate. AR, androgen receptor.
Both ER and AR belong to the nuclear receptor superfamily, which is composed of transcriptional factors, regulated by ligand binding. As a result of ligand binding these receptors bind to specific DNA sequences, either ERE3 or androgen response element, in their target genes, and recruit transcription factors, resulting in hormone-stimulated transcription (Fig.2a).
Fig 2.
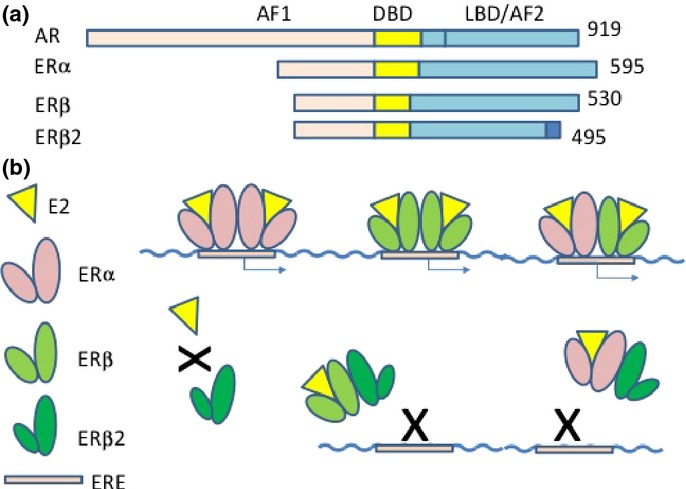
Structure of androgen receptor (AR) and estrogen receptors (ERs), and ER binding to estrogen response element (ERE). (a) Domain structure of AR, ERα, ERβ, and ERβcx. All of them have AF1, DBD, and ligand-binding domain (LBD)/AF2 domains, in order, from the N-terminal. (b) Estogen is a ligand of ERα and ERβ, but not ERβcx. ERα and ERβ could form either a homodimer or heterodimer and bind to ERE. ERβcx could form a heterodimer with ERα or ERβ and inhibit binding to ERE.
Two forms of ER: ERα and ERβ
The first estrogen receptor, ERα, was cloned from the MCF-7 human breast cancer cell line in 1985,4 and the second estrogen receptor, ERβ, was discovered in rat prostate in 1996.5
Estrogen receptor α is expressed mainly in sex organs, that is, breast, uterus, ovary, testis, and epididymis, but also in other organs, for example, liver, kidney, adrenal glands, pituitary gland, and hypothalamus. Expression of ERβ is not predominantly in sex organs except prostate; it is found in skin, bone, brain, lung, urinary bladder, blood vessels, lymphocytes, and fat tissues and appears to be widely distributed throughout the whole body.5
The structure of ERβ is homologous to that of ERα. The DNA-binding domain of ERβ is 96% conserved compared to ERα, and the ligand-binding domain shows 58% conserved residues,5 suggesting that while ERβ could bind the same target genes as ERα, it might have different specific ligands (Fig.2). With E2 bound, helix 12 of the receptor is exposed and binds cofactor proteins, but with other ligands, different cofactors may be recruited, leading to activation of different downstream genes.
Estrogen receptor has been shown to bind to AP1 or specificity protein 16 without dimerization,7 and thus can activate downstream target genes without EREs. E2 activates transcription on AP1 site with ERα; however, it inhibits transcription with ERβ. It suggested that ERα and ERβ have opposite transcriptional effects at AP1 sites when complexed with E2.8,9
Estrogen receptor β variants
Multiple variant forms of ERβ were detected in 1998.10 The most significant form was named ERβ2 (or ERβcx). Estrogen receptor β2 was cloned as full-length cDNA and had identical DNA binding domains, however, its ligand affinity is apparently different. In rodents, an ERβ isoform named ERβins has been described which contains a 54-nt insertion in the ligand-binding domain and shows reduced affinity for estrogens.11,12 ERβ2 and ERβins are different forms but might have the same function in different species.
Both ERα and ERβ1 require ligand binding, and ERβ1 can form homodimers as well as ERα–ERβ1 heterodimers on ERE. However, ERβ2 was found to form heterodimers with ERα or ERβ1 without ligand binding10,13 and inhibit ERE binding of ERβ1 or ERα (Fig.2b). Therefore, ERβ2 is functional modulators of ERα and ERβ1.
Estrogen receptor β has a somewhat lower E2 binding affinity than ERα: the Kd values of E2 calculated from saturation curves are 0.06 nM for ERα and 0.24 nM for ERβ.5 Estrogen receptor β can bind other ligands with rather higher affinity than ERα, for example, 4-hydroxytamoxifen, the phytoestrogen genistein, and, testosterone derivatives, 3βAdiol.
Estrogen Receptor in the Breast
In the mammary glands, both ERα and ERβ are present. Expression of ERα is limited to luminal cells but ERβ is widely distributed, in both basal and luminal epithelial cells, fibroblasts, adipose cells, lymphocytes, and endothelial cells (Fig.3).14–16
Fig 3.
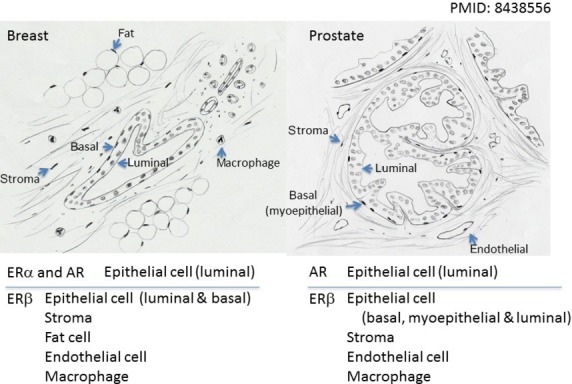
Distribution of estrogen receptor α (ERα), ERβ, and androgen receptor (AR) in breast and prostate. Breast: ERα and AR are located in luminal epithelial cells. ERβ is more widely distributed, in luminal and basal epithelial cells, stroma, fat cell, endothelial cells, and macrophages. Prostate: AR is located in luminal epithelial cells, whereas ERβ is in basal myoepithelial and luminal epithelial cells, stromal cells, endothelial cells, and macrophages.
Estrogen receptor α is the principal receptor for estrogen function in the breast. Estrogen is essential for homeostasis of normal mammary gland, however, activation of ERα results in induction of cell cycling and stimulation of cell growth, and can result in the initiation and development of cancer. Clinically, evaluation of ERα expression in breast cancer specimens is considered essential for the choice of treatment, however, response to hormone therapy cannot be exactly predicted by ERα status.
Function of ERβ in the Breast
Molecular biological studies
In the MCF-7 breast cancer cell line, estradiol increases cell proliferation and causes the cells to form tumors in a mouse xenograft model, however, introducing ERβ into MCF-7 cells causes a reduction of proliferation in vitro and prevents tumor formation in response to estradiol.17 Another cell model with constitutive expression of ERβ resulted in a significant inhibition of cellular growth compared with the mock transfected and prevented establishment and growth of tumors as s.c. xenografts in immunodeficient mice.18 The other xenograft model using the ERα-positive breast cancer cell line T-47D in which ERβ expression was controlled through the Tet-off system also showed that ERβ reduced tumor growth by decreasing expression of vascular endothelial growth factor and platelet-derived growth factor β.19 The mechanism of growth inhibition was reported to be a retardation of transition into the S-phase of the cell cycle,18 and reduced cyclin D1 expression, frequently leading to G1 arrest.20 Microarray analysis in ERβ stably transfected T-47D cells showed that ERβ overexpression specifically downregulated major DNA replication and cell cycle-related genes.21 In MCF-7 cells, ERβ was found to inhibit proliferation by repressing c-myc, cyclin D1, and cyclin A gene transcription, and increasing the expression of p21 and p27, leading to G(2) cell cycle arrest (Fig.4a).17
Fig 4.
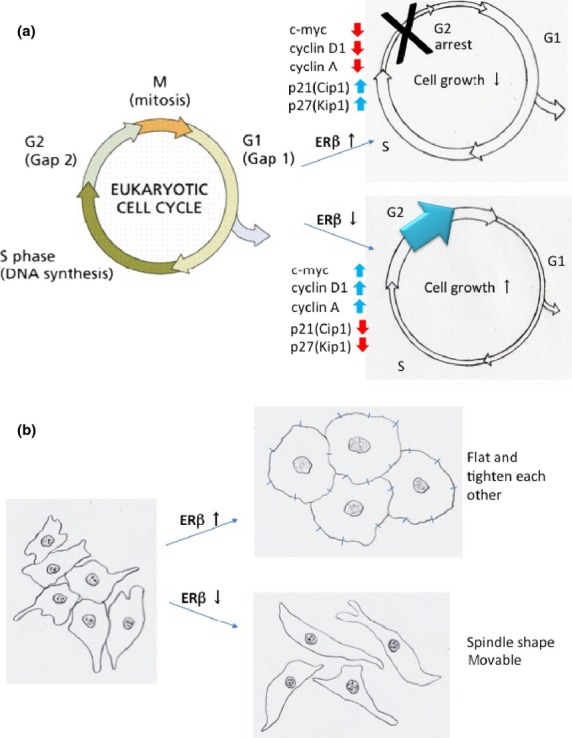
Estrogen receptor β (ERβ) effects on cell cycles and cell shape and adhesion. (a) ERβ effect on cell cycle. ERβ-transfected cells showed G1 arrest whereas ERβ knockout cells showed induction of the G2 population. (b) ERβ effect on cell shape and adhesion. ERβ-transfected cells became flatter and tighter against each other. ERβ knockout cells became spindle-shaped and less tightly associated.
Animal studies
A study using rat mammary glands revealed that ERβ was expressed in 60–70% of cells at all stages of development, however, 90% of ERβ-expressing cells did not express proliferation markers.22 The generation of BERKO mice has revealed that mammary gland development, for example, ductal elongation and lobular formation, is the same in BERKO and wild-type mice.14 However, BERKO shows reduced levels of adhesion molecules, that is, E-cadherin, connexin 32, occludin, and integrin α2 (Fig.4b), and increased cell proliferation, indicating a role for ERβ in the final terminal differentiation of the mammary gland.23
Clinical studies
Many studies have examined the association of ERβ mRNA or protein expression with clinical parameters of breast cancer. Although this is still a matter of controversy, most reports have shown that higher expression of ERβ1 is significantly correlated with good prognosis, and expression tends to decrease during progression.24–27 Estrogen receptor β agonist was reported to prevent ductal carcinoma in situ from invasive change.27 A multivariate analysis of 442 invasive breast cancers with adjuvant tamoxifen revealed that ERβ1 status emerged as an independent predictor of recurrence and mortality, especially in a population with triple negative cancers.26 In several studies on its interaction with tamoxifen, higher ERβ expression was an independent predictor of better response.26,28,29 Overexpression of ERβ1 was also associated with increased sensitivity to 4-hydroxytamoxifen.30 A study of ERα-positive breast cancer patients who had been treated with neoadjuvant hormone therapy found that ERβ mRNA was neither independently predictive of response to preoperative toremifene nor improved predictions based on ERα mRNA levels, which are positively correlated with response.31
Estrogen Receptor β2
From a clinical point of view, it would be interesting if ERβ2 could affect the sensitivity to hormone treatment of ERα-positive breast cancer. There have been several reports about the relationship between ERβ2 and tamoxifen treatment but the results are conflicting. For example, ERβ2 protein expression was reported to be a good predictive marker for endocrine therapy,32 and ERβ2 mRNA to be independently predictive of tamoxifen treatment outcome in ERα-positive tumors,33 but a more detailed study suggested that expression of ERβ2 in primary lesions correlated with a poor response to tamoxifen, especially in cancers with a low Allred score for progesterone receptor.34 In summary, ERβ1 or ERβ2 appear to interfere with ERα function and downregulate ERα downstream genes. Therefore, the presence of ERβ1 or ERβ2 could affect the sensitivity to drugs that directly bind ERα, that is, tamoxifen or raloxifene.
Estrogen Receptors in the Prostate
As a male sex-accessory tissue, the prostate has been regarded as androgen-regulated, with AR considered as the hormone receptor responsible for the regulation of prostatic growth. Testosterone is produced in and secreted by the testis and is converted into DHT in androgen-responsive tissue such as the prostate and it elicits cell growth and proliferation as well as cancer development. Therefore, a traditional therapy for prostate cancer has been androgen ablation, by castration, administration of estrogenic agents like diethylstilbestrol to suppress androgen production indirectly by the hypothalamo-pituitary-gonadal axis, use of gonadotropin-releasing hormone agonists to downregulate the pituitary or use of AR antagonists.35–37
Estrogen receptor α exists in stroma and is very important for prostate development,38 and it appears in ductal epithelial cells in very short time when it is necessary for ductal branching.39 However, it is rarely present in the adult prostate, in which ERβ is the most abundant ER. The latter is strongly expressed in the basal and secretory compartments of benign prostate epithelium as well as in the stroma and the infiltrating immune cells.40–42
Association of ERα and ERβ in human prostate cancer
Estrogen receptor β exists in some prostate cancer cell lines, but not ERα.43 Using an adenoviral delivery system to induce ERβ expression in prostatic cancer cells, it was shown that ERβ has antiproliferative, anti-invasive, and pro-apoptotic effects.44 Another study, using xenografts of ERβ-expressing LNCap prostate cancer cells, showed that E2 treatment inhibited tumor establishment and growth.45 Experiments using stably transfected ERβ1 or ERβ2 in prostate cancer cell lines revealed that ERβ1 inhibits proliferation, whereas ERβ2 increases proliferation, therefore, ERβ2 is oncogenic but ERβ1 has tumor-suppressing effects.46
Intensive investigation of the expression of ERs, especially ERβ and its variants, in human prostate and prostatic diseases, such as hyperplasia and prostate cancer, has shown that ERβ expression declines during hyperplastic changes40,47,48 and ERβ1 is lost during cancer progression, whereas its splice variant, ERβ2, is expressed in advanced prostate cancer.46,49 An association between a single nucleotide polymorphism located in the promoter region of the ERβ gene and risk of developing prostate cancer was shown in a large population-based case–control study.50
This evidence highly suggested antiproliferative effects of ERβ. It has been suggested that the two ERs play opposing roles in prostate cancer: that ERα is oncogenic and promotes cell proliferation and survival, whereas ERβ is predominantly protective, being anticarcinogenic and pro-apoptotic.51–55 Expression of ERα has also been observed in prostate cancer, however, it was located in stromal cells. Therefore, a model in knockout mice was investigated to clarify the function of ERs.
Animal models
BERKO mice show high proliferation and high apoptosis in the epithelial cells of the ventral prostate56,57 and increased prostatic hyperplasia,56,58 indicating that ERβ1 is important for maintaining a normal prostate and for suppressing tumor growth. Activation of ERα in BERKO mice leads to aberrant proliferation, inflammation, and the development of premalignant lesions; in contrast, activation of ERβ in ERKO mice is critical in prostatic stromal–epithelial cell signaling and mediating antiproliferative effects that balance the proliferative action of androgens on the epithelium.59 In another experiment, diethylstilbestrol treatment in ERKO or BERKO mice induced prostatic squamous metaplasia in WT and BERKO mice, but not in ERKO mice.60 Administration of testosterone and estrogen together to BERKO or ERKO mice showed that WT and BERKO mice developed prostatic intraepithelial neoplasia, whereas ERKO mice did not.
Aromatase knockout mice, which lack synthesis of estrogen, is a good model to evaluate the effect of endogenous estrogen. ArKO mice had reduced incidences of hyperplastic lesions.61 A selective ERβ agonist induces apoptosis in ArKO mouse prostate.62 All of these investigations showed that BERKO, which is lacking ERβ, developed induced proliferation and hyperplastic lesion in its prostate, whereas ERKO, which is lacking ERα, did not, indicating that estrogenic action to stimulate prostatic disease is ERα responsive and, in contrast, ERβ is a guard to protect prostatic disease.
Studies of CYP7B1 and 3βAdiol
In the prostate, the most abundant estrogen is not E2 but 3βAdiol.2 The enzyme CYP7B1 is responsible for the hydroxylation of 3βAdiol to Triol for excretion from the body.
Mice in which CYP7B1 has been knocked out have increased concentrations of 3βAdiol in tissues where there is DHT, like prostate. Decreased proliferation of ventral prostate epithelial cells has been observed in CYP7B1 knockout mice, which is attributed to activation of ERβ by 3βAdiol.2 Treatment of the prostate cancer cell line PC3 with 3βAdiol induces a broad range of antitumor changes, including decreased proliferation, increased cell adhesion, and reduced cell migration and invasive capabilities in vitro.63 Estrogen receptor β-mediated inhibition of cell migration by 3βAdiol was also observed in the prostate cancer cell line DU145.64
Conclusions and Future Perspectives
It has been widely reported that incidence of both breast and prostate cancer are higher in Western populations than Asian. One possible explanation for this phenomenon is diet. Soybean products contain phytoestrogens, which are very good activating ligands of ERβ. One such compound, genistein, was found to reduce the potential for neoplastic transformation in breast and prostate tissues;65,66 similar results were obtained with other isoflavones.67 In addition, several ERβ-selective agonists have been synthesized68 and used to examine the effect of ERβ stimulation. Treatment of the prostate cancer cell line DU145 with one such agonist, diarylpropionitrile, decreased cell proliferation.69 Diarylpropionitrile also prevented the development of prostatic hyperplasia and inflammation in testosterone-treated luteinizing hormone receptor knockout mice, which were lacking postnatal androgen production.70 Another ERβ selective agonist, 8β-VE2, was shown to reverse the hyperplasia observed in the prostates of ArKO mice and induced cell death in benign prostate hypertrophy and prostate cancer.71 Another novel selective ERβ agonist, SERBA-1, was also reported to show beneficial effects in a benign prostate hypertrophy model.68 New therapies targeting ERβ seem promising.
During endocrine therapy, many cancers acquire resistance against these therapies, for example, anti-estrogen, tamoxifen, or aromatase inhibitor resistance in breast cancer and anti-androgen or flutamide in prostate cancer. Estrogen receptor β-targeting therapy can be different from ERα or AR, as ERβ needs to be stimulated whereas ERα or AR need to be inhibited. However, acquisition of resistance is the main problem during hormone treatment of hormone-dependent cancer. Mechanisms of acquisition are under investigation by gene expression analysis and some clues to close this phenomenon were revealed. One is activation of cell growth signaling other than the estrogen–ER or androgen–AR pathways, for example, the PI3K/Akt/mTOR pathways.72,73 Therefore, targeting inhibition of PI3K/Akt/mTOR pathways is applied to patients with hormone receptor-positive advanced breast cancer74,75 and showed prolongation of progression-free survival.76 Estrogen receptor β is the frontier of nuclear receptor transcriptional factor, which has high potential to be a target of cancer treatment. To succeed in applying ERβ-targeting therapy to breast and prostate cancer, there must be more knowledge of ERα or AR. Further investigation is still awaited.
Disclosure Statement
The authors have no conflict of interest.
Glossary
Abbreviations
- 3βAdiol
5α-androstane-3β, 17β-diol
- AP1
Activator protein 1
- AR
Androgen receptor
- ArKO
Aromatase knockout
- BERKO
ERβ knockout
- CYP7B1
5α-androstane-3β, 17β-diol hydroxylase
- DHT
Dihydrotestosterone
- E2
17β-estradiol
- ER
Estrogen receptor
- ERE
Estrogen response element
- ERKO
ERα knockout
References
- Oliveira AG, Coelho PH, Guedes FD, Mahecha GA, Hess RA, Oliveira CA. 5alpha-Androstane-3beta,17beta-diol (3beta-diol), an estrogenic metabolite of 5alpha-dihydrotestosterone, is a potent modulator of estrogen receptor ERbeta expression in the ventral prostrate of adult rats. Steroids. 2007;72:914–22. doi: 10.1016/j.steroids.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589–94. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman JM, Allan GF, Tsai SY, Tsai MJ, O'Malley BW. Transcriptional activation by the estrogen receptor requires a conformational change in the ligand binding domain. Mol Endocrinol. 1993;7:1266–74. doi: 10.1210/mend.7.10.8264659. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–93. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W, Saville B, Hoivik D, Safe S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol. 1997;11:1569–80. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G, Bergers G, Picard D, Busslinger M. Hormone-dependent transcriptional regulation and cellular transformation by Fos-steroid receptor fusion proteins. Proc Natl Acad Sci U S A. 1991;88:5114–8. doi: 10.1073/pnas.88.12.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Valentine C, et al. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–85. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Inoue S, Watanabe T, et al. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998;26:3505–12. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Fuller PJ. Identification of a splice variant of the rat estrogen receptor beta gene. Mol Cell Endocrinol. 1997;132:195–9. doi: 10.1016/s0303-7207(97)00133-0. [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998;139:1082–92. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003;22:5011–20. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Warner M, Gustafsson JA. Estrogen receptors ER alpha and ER beta in proliferation in the rodent mammary gland. Proc Natl Acad Sci U S A. 2004;101:3739–46. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri C, Saji S, Sakaguchi H, et al. The expression of oestrogen receptor (ER)-beta and its variants, but not ERalpha, in adult human mammary fibroblasts. J Mol Endocrinol. 2004;33:35–50. doi: 10.1677/jme.0.0330035. [DOI] [PubMed] [Google Scholar]
- Speirs V, Skliris GP, Burdall SE, Carder PJ. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol. 2002;55:371–4. doi: 10.1136/jcp.55.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–8. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- Behrens D, Gill JH, Fichtner I. Loss of tumourigenicity of stably ERbeta-transfected MCF-7 breast cancer cells. Mol Cell Endocrinol. 2007;274:19–29. doi: 10.1016/j.mce.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson JA. Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006;66:11207–13. doi: 10.1158/0008-5472.CAN-06-0017. [DOI] [PubMed] [Google Scholar]
- Helguero LA, Lindberg K, Gardmo C, Schwend T, Gustafsson JA, Haldosen LA. Different roles of estrogen receptors alpha and beta in the regulation of E-cadherin protein levels in a mouse mammary epithelial cell line. Cancer Res. 2008;68:8695–704. doi: 10.1158/0008-5472.CAN-08-0788. [DOI] [PubMed] [Google Scholar]
- Lin CY, Strom A, Li Kong S, et al. Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 2007;9:R25. doi: 10.1186/bcr1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci U S A. 2000;97:337–42. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster C, Makela S, Warri A, et al. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci U S A. 2002;99:15578–83. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–41. [PubMed] [Google Scholar]
- Omoto Y, Kobayashi S, Inoue S, et al. Evaluation of oestrogen receptor beta wild-type and variant protein expression, and relationship with clinicopathological factors in breast cancers. Eur J Cancer. 2002;38:380–6. doi: 10.1016/s0959-8049(01)00383-5. [DOI] [PubMed] [Google Scholar]
- Honma N, Horii R, Iwase T, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–34. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- Huang B, Omoto Y, Iwase H, et al. Differential expression of estrogen receptor alpha, beta1, and beta2 in lobular and ductal breast cancer. Proc Natl Acad Sci U S A. 2014;111:1933–8. doi: 10.1073/pnas.1323719111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA. Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res. 2004;10:7490–9. doi: 10.1158/1078-0432.CCR-04-1114. [DOI] [PubMed] [Google Scholar]
- Iwase H, Zhang Z, Omoto Y, et al. Clinical significance of the expression of estrogen receptors alpha and beta for endocrine therapy of breast cancer. Cancer Chemother Pharmacol. 2003;52(Suppl 1):S34–8. doi: 10.1007/s00280-003-0592-1. [DOI] [PubMed] [Google Scholar]
- Murphy LC, Peng B, Lewis A, et al. Inducible upregulation of oestrogen receptor-beta1 affects oestrogen and tamoxifen responsiveness in MCF7 human breast cancer cells. J Mol Endocrinol. 2005;34:553–66. doi: 10.1677/jme.1.01688. [DOI] [PubMed] [Google Scholar]
- Cappelletti V, Celio L, Bajetta E, et al. Prospective evaluation of estrogen receptor-beta in predicting response to neoadjuvant antiestrogen therapy in elderly breast cancer patients. Endocr Relat Cancer. 2004;11:761–70. doi: 10.1677/erc.1.00822. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, Green AR, Karthik S, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–35. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- Vinayagam R, Sibson DR, Holcombe C, Aachi V, Davies MP. Association of oestrogen receptor beta 2 (ER beta 2/ER beta cx) with outcome of adjuvant endocrine treatment for primary breast cancer–a retrospective study. BMC Cancer. 2007;7:131. doi: 10.1186/1471-2407-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji S, Omoto Y, Shimizu C, et al. Expression of estrogen receptor (ER) (beta)cx protein in ER(alpha)-positive breast cancer: specific correlation with progesterone receptor. Cancer Res. 2002;62:4849–53. [PubMed] [Google Scholar]
- Huggins C. Endocrine control of prostatic cancer. Science. 1943;97:541–4. doi: 10.1126/science.97.2529.541. [DOI] [PubMed] [Google Scholar]
- Horning ES. The effects of castration and stilboestrol on prostatic tumours in mice. Br J Cancer. 1949;3:211–30. doi: 10.1038/bjc.1949.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RW, Emery DS. Management of early prostatic carcinoma. Calif Med. 1959;91:57–61. [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–97. [PubMed] [Google Scholar]
- Omoto Y. Estrogen receptor-alpha signaling in growth of the ventral prostate: comparison of neonatal growth and postcastration regrowth. Endocrinology. 2008;149:4421–7. doi: 10.1210/en.2007-1413. [DOI] [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Lee CS, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5. [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–9. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Omoto Y, Imamov O, Warner M, Gustafsson JA. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. Proc Natl Acad Sci U S A. 2005;102:1484–9. doi: 10.1073/pnas.0409168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–82. [PubMed] [Google Scholar]
- Cheng J, Lee EJ, Madison LD, Lazennec G. Expression of estrogen receptor beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004;566:169–72. doi: 10.1016/j.febslet.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Corey E, Quinn JE, Emond MJ, Buhler KR, Brown LG, Vessella RL. Inhibition of androgen-independent growth of prostate cancer xenografts by 17beta-estradiol. Clin Cancer Res. 2002;8:1003–7. [PubMed] [Google Scholar]
- Dey P, Jonsson P, Hartman J, Williams C, Strom A, Gustafsson JA. Estrogen receptors beta1 and beta2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Mol Endocrinol. 2012;26:1991–2003. doi: 10.1210/me.2012.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali D, Staibano S, Prezioso D, et al. Estrogen receptor beta expression in human prostate tissue. Mol Cell Endocrinol. 2001;178:47–50. doi: 10.1016/s0303-7207(01)00418-x. [DOI] [PubMed] [Google Scholar]
- Leav I, Lau KM, Adams JY, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Takahashi S, Urano T, et al. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun. 2001;289:692–9. doi: 10.1006/bbrc.2001.6038. [DOI] [PubMed] [Google Scholar]
- Thellenberg-Karlsson C, Lindstrom S, Malmer B, et al. Estrogen receptor beta polymorphism is associated with prostate cancer risk. Clin Cancer Res. 2006;12:1936–41. doi: 10.1158/1078-0432.CCR-05-0269. [DOI] [PubMed] [Google Scholar]
- Attia DM, Ederveen AG. Opposing roles of ERalpha and ERbeta in the genesis and progression of adenocarcinoma in the rat ventral prostate. Prostate. 2012;72:1013–22. doi: 10.1002/pros.21507. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol. 2009;55:533–42. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Celhay O, Yacoub M, Irani J, Dore B, Cussenot O, Fromont G. Expression of estrogen related proteins in hormone refractory prostate cancer: association with tumor progression. J Urol. 2010;184:2172–8. doi: 10.1016/j.juro.2010.06.089. [DOI] [PubMed] [Google Scholar]
- Chang WY, Prins GS. Estrogen receptor-beta: implications for the prostate gland. Prostate. 1999;40:115–24. doi: 10.1002/(sici)1097-0045(19990701)40:2<115::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Muthusamy S, Andersson S, Kim HJ, et al. Estrogen receptor beta and 17beta-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer. Proc Natl Acad Sci U S A. 2011;108:20090–4. doi: 10.1073/pnas.1117772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Makela S, Andersson LC, et al. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98:6330–5. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamov O, Morani A, Shim GJ, et al. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci U S A. 2004;101:9375–80. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P, Leav I, Pursell B, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155:174–86. doi: 10.1111/j.1749-6632.2009.04360.x. [DOI] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432–42. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512–20. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- McPherson SJ, Hussain S, Balanathan P, et al. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc Natl Acad Sci U S A. 2010;107:3123–8. doi: 10.1073/pnas.0905524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondi D, Piccolella M, Biserni A, et al. Estrogen receptor beta and the progression of prostate cancer: role of 5alpha-androstane-3beta,17beta-diol. Endocr Relat Cancer. 2010;17:731–42. doi: 10.1677/ERC-10-0032. [DOI] [PubMed] [Google Scholar]
- Guerini V, Sau D, Scaccianoce E, et al. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits prostate cancer cell migration through activation of the estrogen receptor beta subtype. Cancer Res. 2005;65:5445–53. doi: 10.1158/0008-5472.CAN-04-1941. [DOI] [PubMed] [Google Scholar]
- Fritz WA, Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Mol Cell Endocrinol. 2002;186:89–99. doi: 10.1016/s0303-7207(01)00663-3. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–8S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- Slater M, Brown D, Husband A. In the prostatic epithelium, dietary isoflavones from red clover significantly increase estrogen receptor beta and E-cadherin expression but decrease transforming growth factor beta1. Prostate Cancer Prostatic Dis. 2002;5:16–21. doi: 10.1038/sj.pcan.4500546. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discovery. 2011;10:778–92. doi: 10.1038/nrd3551. [DOI] [PubMed] [Google Scholar]
- Pravettoni A, Mornati O, Martini PG, et al. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol Cell Endocrinol. 2007;263:46–54. doi: 10.1016/j.mce.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Savolainen S, Pakarainen T, Huhtaniemi I, Poutanen M, Makela S. Delay of postnatal maturation sensitizes the mouse prostate to testosterone-induced pronounced hyperplasia: protective role of estrogen receptor-beta. Am J Pathol. 2007;171:1013–22. doi: 10.2353/ajpath.2007.060979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P, Strom A, Gustafsson JA. Estrogen receptor beta upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene. 2014;33:4213–25. doi: 10.1038/onc.2013.384. [DOI] [PubMed] [Google Scholar]
- Toren P, Zoubeidi A. Targeting the PI3K/Akt pathway in prostate cancer: Challenges and opportunities (Review) Int J Oncol. 2014;45:1793–801. doi: 10.3892/ijo.2014.2601. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Han SX, Bai E, et al. Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncol Rep. 2013;29:1563–9. doi: 10.3892/or.2013.2245. [DOI] [PubMed] [Google Scholar]
- Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40:862–71. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Jerusalem G, Rorive A, Collignon J. Use of mTOR inhibitors in the treatment of breast cancer: an evaluation of factors that influence patient outcomes. Breast Cancer. 2014;6:43–57. doi: 10.2147/BCTT.S38679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]


