Abstract
Recent advances in treatment for adult T-cell leukemia-lymphoma (ATL) are reviewed herein. It is currently possible to select a therapeutic strategy for ATL and predict prognosis by classification of patients by clinical subtypes and clinicopathological factors. Although the overall survival (OS) of patients with ATL has increased marginally because of advances in chemotherapy, further prolongation of survival might be difficult with conventional chemotherapy alone. Promising results have been reported for antiviral therapy using zidovudine and interferon-α, and, indeed, antiviral therapy is currently the standard treatment for patients with ATL in western countries. Remarkably, the 5-year OS rates are 100% for both the smoldering-type and chronic-type ATL. Recently, treatments for ATL have included allogeneic hematopoietic stem cell transplantation and molecular targeted therapies. Furthermore, the anti-CCR4 monoclonal antibody mogamulizumab has been shown to have marked cytotoxic effects on ATL cells, especially in the leukemic type of ATL. In the lymphoma type of ATL, the response rate may be improved by combining mogamulizumab with chemotherapy. It should be recognized that prevention of infection from carriers of human T-cell leukemia virus type-I and transfer of the virus from mother to infant are crucial issues for the eradication of ATL.
Keywords: Adult T-cell leukemia-lymphoma, allogeneic hematopoietic stem cell transplantation, antiviral therapy, chemotherapy, molecular targeted therapy
Adult T-cell leukemia-lymphoma (ATL) is a mature T-cell neoplasm caused by human T-cell leukemia virus type-I (HTLV-1).1 Following the initial report by Uchiyama et al.,2 many key discoveries concerning the mechanism of leukemogenesis of ATL have been made in association with the HTLV-1 tax and HTLV-1 basic leucine zipper factor genes.3,4 Several clinical manifestations of ATL are known and may be classified into four clinical subtypes based on the presence of organ involvement and the results of blood work-up.5 This classification is currently used as the basis for therapeutic strategies.
Therapeutic interventions, including intensive chemotherapy for aggressive ATL, are not associated with satisfactory outcomes, mainly because ATL cells are often resistant to chemotherapeutic agents;6 moreover, patients with ATL frequently suffer from a number of opportunistic infections.5 We reported for the first time that allogeneic hematopoietic stem cell transplantation (allo-HSCT) improved overall survival (OS) in ATL patients.7
In Europe and USA, antiviral therapy has been frequently applied for ATL patients since the therapeutic efficacy of zidovudine (AZT) and interferon-α (IFN) has been demostrated.8,9 More recently, the mechanism of action of AZT combined with IFN (AZT/IFN) has been partially elucidated.10 Antiviral therapy has received greater attention in Europe and USA than in Japan. Finally, new molecular targeted agents are under investigation in patients with ATL.
Herein, we review current treatments for ATL, along with previous and future therapies.
Epidemiology
Approximately 10–20 million people are infected with HTLV-1 worldwide; endemic areas include Central Africa, South America, the Caribbean basin, Iran, south-western Japan and Melanesia.11 In Japan, approximately 1.1 million individuals are infected with HTLV-112 and approximately 1000 HTLV-1 carriers develop ATL each year.13
In late 2000, a decrease in the prevalence of HTLV-1 carriers has been observed in the Kyushu district (south-western island of Japan, an endemic area for ATL); however, the prevalence is increasing in several regions in the non-endemic areas.12 The age-standardized incidence rates of ATL in the Honshu region of Japan and the USA, both of which are considered non-endemic areas, are increasing significantly, although no changes in incidence have been observed in the Kyushu district.14 These results suggest that HTLV-1 is spreading through the migration of carriers from endemic to non-endemic areas. The mortality (per 100 000 person-years) of patients with ATL decreased from 1.86 (95% confidence interval [CI]: 1.84–1.87) to 1.41 (95% CI: 1.40–1.43) in Kyushu during the period of 2000–2009, and from 0.22 (95% CI: 0.22–0.23) to 0.16 (95% CI: 0.16–0.17) in other areas of Japan from 2003–2009, and these trends are statistically significant.13 The number of allo-HSCT performed in Japan has increased since 2000.13 A significant inverse correlation was observed between the decreasing mortality trend and the increasing number of allo-HSCT procedures. The decreasing trend in mortality observed in ATL patients might be associated with allo-HSCT.13
Diagnosis and Clinical Subtype
A diagnosis of ATL is made by anti-HTLV-1 positivity in sera and by confirming the presence of a mature T-cell malignancy. The identification of monoclonal integration of HTLV-1 proviral DNA in tumor cells by Southern blot analysis is required to confirm a diagnosis of ATL.
Adult T-cell leukemia-lymphoma is divided into four clinical subtypes (acute, lymphoma, chronic and smoldering) according to leukemic manifestation in the blood, organ involvement, serum lactate dehydrogenase (LDH) levels and corrected serum calcium levels (Table1).5 Chronic type is divided into two subtypes: the unfavorable chronic type with at least one poor prognostic factor and the favorable chronic type with no poor prognostic factors. Poor prognostic factors include three factors, including serum LDH > upper limit of normal (ULN), serum blood urea nitrogen > ULN and serum albumin < lower limit of normal.15
Table 1.
Diagnostic criteria for clinical subtype of adult T-cell leukemia-lymphoma
| Smoldering | Chronic§ | Lymphoma | Acute | |
|---|---|---|---|---|
| Anti-HTLV-1 antibody | + | + | + | + |
| Lymphocyte (×109/L) | <4 | ≥4¶ | <4 | † |
| Abnormal T-lymphocytes | ≥5% | +†† | ≤1% | +†† |
| Flower cells of T-cell marker | Occasionally | Occasionally | No | + |
| LDH | ≤1·5N | ≤2N | † | † |
| Corrected Ca (mmol/L) | <2·74 | <2·74 | † | † |
| Histology-proven lymphadenopathy | No | † | + | † |
| Tumor lesion | ||||
| Skin | ‡ | † | † | † |
| Lung | ‡ | † | † | † |
| Lymph node | No | † | Yes | † |
| Liver | No | † | † | † |
| Spleen | No | † | † | † |
| CNS | No | No | † | † |
| Bone | No | No | † | † |
| Ascites | No | No | † | † |
| Pleural effusion | No | No | † | † |
| GI tract | No | No | † | † |
No essential qualification except terms required for other subtype(s).
No essential qualification if other terms are fulfilled, but histology-proven malignant lesion(s) is required in case abnormal T-lymphocytes are less than 5% in peripheral blood.
Chronic type is divided into two subtypes: the unfavorable chronic type with at least one poor prognostic factor and the favorable chronic type with no poor prognostic factors. Poor prognostic factors include three factors: serum LDH > upper limit of normal (ULN), serum BUN > ULN and serum albumin < lower limit of normal.
Accompanied by T-lymphocytosis (3·5 × 109/L or more).
In case abnormal T-lymphocytes are less than 5% in peripheral blood, histology-proven tumor lesion is required. Ca, calcium; CNS, central nervous system; GI, gastrointestinal; HTLV-1, human T-cell leukemia virus type-I; LDH, lactate dehydrogenase; N, normal upper limit. Source: Shimoyama (1991).
Prognostic Factors and Stratification
The Lymphoma Study Group has identified five prognostic factors: age, total number of involved lesions, serum calcium level, serum LDH level and performance status (PS).16 When ATL is stratified into three different groups (i.e. low risk group and high risk group based on the combination of prognostic factors, and extremely high risk group with high levels of serum calcium), OS is clearly different between the three groups. Nonetheless, these stratifications are not practical clinically as the classification system is rather complicated. In order to provide a more clinically useful system, Shimoyama devised a new clinical classification scheme for the four subtypes mentioned above.5
Several research groups in Japan have reported other factors that may also influence OS in ATL patients. These include deletion of p16, lung resistance-related protein and multi-drug resi-stance associated protein genes, eosinophilia, and expression of CC chemokine receptor 4 (CCR4) and serum interleukin (IL)-5.17
Recently, the Ann Arbor clinical stage, PS, and three continuous variables, age, serum albumin and soluble interleukin-2 receptor, were identified as independent prognostic factors in a multicenter retrospective analysis of 807 patients with newly diagnosed, acute-type and lymphoma-type ATL. Based on these results, Katsuya et al.18 propose a prognostic index for acute-type and lymphoma-type ATL.
Treatment of Adult T-cell Leukemia-lymphoma
The current treatment strategy for patients with ATL is shown in Figure1. Treatment is based on the clinical subtype. Patients with aggressive ATL, such as acute, lymphoma or chronic types, with at least one poor prognostic factor should receive early chemotherapy. In the USA and Europe, antiviral therapy using AZT/IFN is the standard treatment for leukemic-type ATL. In Europe, chemotherapy is the first-line therapy for lymphoma-type ATL, because OS with antiviral therapy alone is very short. 19
Fig 1.
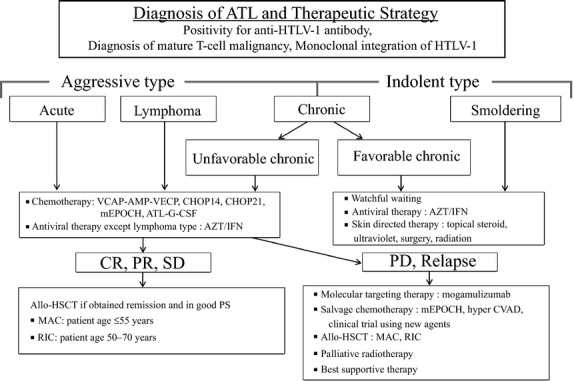
Treatment algorithm for adult T-cell leukemia-lymphoma (ATL) patients. ATL diagnosis is based on anti-HTLV-1 antibody positivity in the serum, the presence of mature T-cell malignancy, and the Southern blot detection of monoclonal integration of HTLV-1 proviral DNA in the tumor cells. ATL treatment is usually determined according to the clinical subtypes and prognostic factors. The presence of an aggressive-type ATL (acute, lymphoma and chronic types with poor prognostic factors) or indolent-type ATL (chronic and smoldering types without poor prognostic factors) is critical when making treatment decisions. Patients with an aggressive-type (acute, lymphoma and unfavorable chronic type) generally receive immediate combination chemotherapy or antiviral therapy with zidovudine and interferon-α (AZT/IFN), except for those with the lymphoma type. The international consensus meeting primarily recommends the VCAP-AMP-VECP regimen. Other therapeutic regimens include CHOP14, CHOP21, mEPOCH and ATL-G-CSF. The patients undergo further treatment with allogeneic hematopoietic stem cell transplantation, which is particularly effective in young patients with good performance statuses, and those who have achieved remission before transplantation. In Japan, patients with an indolent-type ATL without any skin lesions are usually followed up under a watchful waiting policy until the disease transforms to an aggressive type. Antiviral therapy is frequently performed for favorable chronic and smoldering ATL patients in non-Japanese nations, and skin directed therapy is applied for smoldering ATL with skin manifestations. allo-HSCT, allogeneic hematopoietic stem cell transplantation; ATL-G-CSF, combination chemotherapy consisting of vincristine, vindesine, doxorubicin, mitoxantrone, cyclophosphamide, etoposide, ranimustine, and prednisone with granulocyte-colony stimulating factor support; AZT/IFN, zidovudine and interferon-α; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP14 is performed every 2 weeks and CHOP21 is performed every 3 weeks); CR, complete remission; hyper CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MAC, myeloablative conditioning; mEPOCH, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) with modifications; PD, progressive disease; PR, partial remission; PS, performance status; RIC, reduced intensity conditioning; SD, stable disease; VCAP-AMP-VECP, vincristine, cyclophosphamide, doxorubicin and prednisone (VCAP)-doxorubicin, ranimustine and prednisone (AMP)-vindesine, etoposide, carboplatin and prednisone (VECP).
Chemotherapy
Several chemotherapy combinations have been investigated for ATL patients in Japan, although the median OS range was only 6–8.5 months.20 The Japan Clinical Oncology Group-Lymphoma Study Group (JCOG-LSG) has conducted a number of clinical trials for ATL patients in Japan, with complete response (CR) rates of 17–43% and median OS of 5–13 months in prospective multicenter studies.21 The JCOG-LSG conducted a randomized clinical trial in patients with aggressive ATL in which a VCAP-AMP-VECP regimen (Fig.2) was compared to a biweekly doxorubicin, cyclophosphamide, vincristine and prednisone (CHOP14) regimen.22 The VCAP-AMP-VECP regimen reduced one course of VCAP-AMP-VECP from the original LSG15 regimen and added cytarabine to the intrathecal administration of methotrexate and prednisone as a prophylaxis against central nervous system (CNS) relapse. The CR rate and median OS of the VCAP-AMP-VECP regimen and CHOP14 regimen were 40% (95% CI: 27.6–54.2) versus 25% (95% CI: 14.5–37.3), and 13 versus 11 months, respectively. The CR rate of the VCAP-AMP-VECP regimen was significantly higher than that of CHOP14. In terms of the OS, there was no significant difference in the two groups (hazard ratio [HR] = 0.751, 95% CI: 0.50–1.13).22 The VCAP-AMP-VECP regimen is considered a standard chemotherapeutic regimen for aggressive ATL in Japan.
Fig 2.
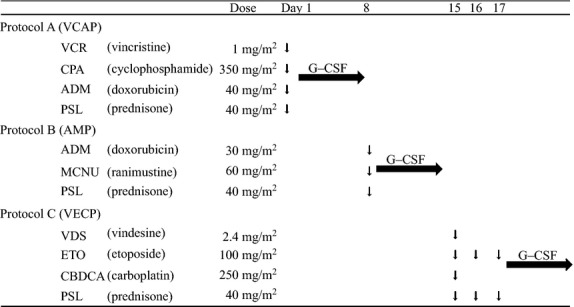
The VCAP-AMP-VECP regimen. A, B and C are repeated every 28 days for 6 cycles. Cytarabine (40 mg), methotrexate (15 mg) and prednisone (10 mg) are administered intrathecally before cycles 2, 4 and 6. VCAP-AMP-VECP, vincristine, cyclophosphamide, doxorubicin and prednisone (VCAP)-doxorubicin, ranimustine and prednisone (AMP)-vindesine, etoposide, carboplatin and prednisone (VECP); G-CSF, granulocyte-colony stimulating factor.
Stem Cell Transplantation
In general, autologous HSCT has not been successful because of ATL relapses or infectious complications.23 We and other Japanese researchers have reported that allo-HSCT could improve the outcome of ATL,7 mainly using conventional myeloablative regimens (MAC); however, high transplant-related mortality poses a challenge (Table2).
Table 2.
Summary of published reports on allogeneic hematopoietic stem cell transplantation in ATL
| Reference | Patient Number | Median age (range) | Sex M/F | Subtype | Donor | Donor HTLV-1 Ab | Stem cell source | Disease Status at SCT | Conditioning regimen | Cause of death | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Utsunomiya (BMT, 2001) | 10 | 45 (33–51) | 7/3 | Acute: 8 | MSD: 9 | Neg: 7 | BM: 8 | CR: 4 | MAC: 10 | TRM: 4 | Median leukemia-free survival 17.5+ M (range 3.7–34.4+) | |
| Lymphoma: 1 | MUD: 1 | Posi: 3 | PB: 1 | PR: 5 | ||||||||
| Other: 1 | BM + PB: 1 | NR: 1 | ||||||||||
| Kami (BJH, 2003) | 11 | 47 (15–59) | 7/4 | Acute: 5 | MSD: 9 | Neg: 9 | BM: 7 | CR: 6 | MAC: 9 | TRM: 7 | 1Y-OS | |
| Lymphoma: 4 | PMRD: 1 | Posi: 2 | PB: 3 | PR: 1 | RIC: 2 | 54.5 ± 30.0% | ||||||
| Other: 2 | MUD: 1 | BM + PB: 1 | PD: 4 | |||||||||
| Fukushima (Leukemia, 2005) | 40 | 44 (28–53) | 22/18 | Acute: 30 | MSD: 27 | Neg: 27 | BM: 21 | CR: 15 | MAC: | TRM: 16 | 3Y-0S | |
| Lymphoma: 10 | PMRD: 5 | Posi: 9 | PB + 19 | PR: 13 | most cases | Unk: 1 | 45.3% | |||||
| NUD: 8 | NE: 4 | NC: 3 | ATL: 4 | |||||||||
| PD: 9 | ||||||||||||
| Kato (BBMT, 2007) | 33 | 49 (24–59) | 18/15 | Acute: 20 | MUD: 33 | Neg: 33 | BM: 33 | CR + PR: 15 | MAC: 27 | TRM: 9 | 1Y-OS | |
| Lymphoma: 7 | NR: 14 | RIC: 6 | ATL: 2 | 49.5% | ||||||||
| NE: 6 | NE: 4 | NE: 3 | ||||||||||
| Shiratori (BBMT, 2008) | 15 | 57 (41–66) | 3/12 | Acute: 6 | MSD: 10 | Neg: 13 | BM: 8 | CR: 9 | MAC: 5 | TRM: 2 | 3Y-OS | |
| Lymphoma: 8 | MRD: 5 | Posi: 2 | PB: 4 | PR: 5 | RIC: 10 | ATL: 2 | 73.3% | |||||
| Other: 1 | BM + PB: 3 | PD: 1 | ||||||||||
| Nakase (BMT, 2006) | 8 | 49 (45–59) | 2/6 | Acute: 5 | MUD: 3 | Neg: 8 | BM: 8 | CR: 6 | MAC: 5 | TRM: 2 | Median OS 20M | |
| Lymphoma: 3 | PMUD: 5 | Non-CR: 2 | RIC: 3 | ATL: 1 | (range 0-43) | |||||||
| Nakamura (IJH, 2012) | 10 | 51 (31–64) | 6/4 | Acute: 9 | PMUD: 10 | Neg: 10 | UCB | CR: 2 | MAC: 6 | ATL: 4 | 2Y-OS: 40% | |
| Lymphoma: 1 | PR: 4 | RIC: 4 | Sepsis: 1 | (95% CI 67-12) | ||||||||
| SD: 1 | GVHD+ATL: 1 | |||||||||||
| PD: 3 | ||||||||||||
| Fukushima (IJH, 2013) | 27 | 52 (41–63) | 18/9 | Acute: 17 | MUD: 1 | Neg: 27 | UCB | CR: 5 | MAC: 9 | TRM: 10 | 3Y-OS: 27.4% | |
| Lymphoma: 10 | PMUD: 26 | PR: 11 | RIC: 18 | ATL: 9 | ||||||||
| RIF: 5 | ||||||||||||
| REL: 6 | ||||||||||||
| Bazarbachi (BMT, 2014) | 17 | 47 (21–58) | 9/8 | Acute: 5 | MSD: 6 | ND | ND | CR: 9 | MAC: 4 | ATL: 8 | 3Y-OS: 34.3% | |
| Lymphoma: 10 | MUD: 7 | PR: 4 | RIC: 13 | GVHD: 2 | ||||||||
| Chro/Smold: 2 | UnK: 1 | PD: 4 | Sepsis: 1 | |||||||||
| PMRD: 3 |
ATL, adult T-cell leukemia-lymphoma. BBMT, Biology of Blood and Marrow Transplantation; BJH, British Journal of Haematology; BMT, bone marrow transplantation; Chro/Smold, chronic/smoldering; CR, complete remission; GVHD, graft-versus-host disease; IJH, International Journal of Hematology; M, month; MAC, myeloablative conditioning; MSD, HLA-matched sibling donor; MUD, HLA-matched unrelated donor; NC, no change; ND, not described; NE, not evaluable; Neg, negative; NR, no response; OS, overall survival; PD, progressive disease; Posi, positive; PMRD, HLA partially matched related donor; PMUD, HLA partially matched unrelated donor; PR, partial remission; RIC, reduced intensity conditioning; SCT, stem cell transplantation; SD, stable disease; TRM, transplant-related mortality; UCB, unrelated cord blood; UnK, unknown.
Therefore, allo-HSCT with reduced intensity conditioning regimens (RIC) was prospectively evaluated. Okamura et al.24 report the safety and feasibility of allo-HSCT with RIC using peripheral blood stem cells from an HLA-matched sibling donor in older patients with ATL who achieved remission after chemotherapy. A total of 29 patients were registered, and the 5-year OS rate was 34% (95% CI: 18–51), indicating the potential curability of the disease.25 Unrelated bone marrow (uBM) and cord blood transplantation with RIC were also prospectively evaluated as alternative strategies to allo-HSCT; follow up is currently under way.
By 2012, more than 1000 ATL patients had received various types of allo-HSCT. Currently, approximately 120 ATL patients undergo allo-HSCT each year in Japan.26 Based on the incidence rate,27 approximately 10% of ATL patients receive allo-HSCT each year. Several related aspects have been reported in a nationwide retrospective study. Based on the stem cell sources, the 3-year OS rate was highest for patients with related HLA-matched donors (41%, 95% CI: 33–49), followed by those with uBM (39%, 95% CI: 29–49).28 In terms of the effect of acute graft-versus-host-disease (GVHD) on OS, grade I/II acute GVHD was significantly associated with a longer OS.29 Regarding the effect of the conditioning regimen intensity on OS, although no significant difference was observed in the OS between MAC and RIC, a trend for superior OS was observed with RIC in older patients.30 Bazarbachi et al.31 report the results from the European Group for Blood and Marrow Transplantation's Lymphoma Working Party, and allo-HSCT might salvage ATL patients in non-Japanese patients.
Immunotherapy
Anti-tumor immune system activity has also been recognized in ATL patients who have received allo-HSCT.29 Cytotoxic T-cells that targeted the HTLV-1 specific tax protein were detected in patients who were in remission after allo-HSCT.32
The discontinuation of immunosuppressive agents or donor lymphocyte infusions was effective in some ATL patients who relapsed after allo-HSCT; many of them developed GVHD subsequently.33,34 The graft versus (Gv)-ATL effect, in particular the graft versus-tax (Gv-tax) effect after allo-HSCT, has been reported in ATL patients.32 Therefore, immunotherapy targeting the tax protein may be effective in patients whose tumor cells express the tax protein. Indeed, a vaccine targeting tax was shown to induce anti-tumor activity in a mouse model.35 Based on these findings, the anti-ATL vaccine, where the tax peptide is pulsed into autologous dendritic cells, was administered to three previously treated ATL patients as a clinical trial; the treated patients exhibited clinical effects without any serious adverse events except for a slight fever and transient skin reaction.36 These results suggest that further improvements in immunotherapy are warranted.
Antiviral Therapy
Antiviral therapy using AZT/IFN was initially described by Gill et al.8 and Hermine et al.9 Gill et al. report an overall response rate (ORR) of 58% for 19 ATL patients, including 7 previously treated patients. Although a high ORR was achieved, the median OS of only 4.8 months in 12 of the previously untreated patients was considered unsatisfactory.8 Subsequently, several follow-up studies of antiviral therapy using AZT/IFN have been conducted for ATL patients in Europe; however, the median OS could only be prolonged by 6–18 months.37
Bazarbachi et al. conducted a meta-analysis of antiviral therapy for ATL patients; they report that the median OS achieved with antiviral therapy was superior to that achieved with combination chemotherapy for ATL patients, especially for the leukemic subtypes, such as the smoldering, chronic and acute types of ATL.19 Remarkably, a 5-year OS rate of 100% was achieved in patients with chronic and smoldering types of ATL with this antiviral therapy. It was concluded that antiviral therapy using AZT/IFN was the gold standard for the leukemic subtypes of ATL, although patients with the lymphoma type showed less benefit from antiviral therapy than from combination chemotherapy.19,38 Takasaki et al.39 report that the prognosis of indolent-type ATL in Japan is worse than that reported previously.5 Bazarbachi et al.19 report excellent results with antiviral therapy; therefore, it is important to verify the efficacy of antiviral therapy for Japanese ATL patients. Because the national health insurance system in Japan has not yet approved the use of these two drugs in the treatment of ATL patients, a randomized phase III clinical trial was recently initiated by the JCOG-LSG for treating indolent-type ATL with antiviral therapy consisting of AZT and IFN versus watchful waiting. This clinical trial will provide conclusive information regarding the optimal standard treatment for indolent-type ATL.
Molecular Targeted Therapy
Anti-CCR4 antibody therapy
The overexpression of CCR4 has been reported in tumor cells of various lymphoid neoplasms. The ratio of expression varies among different disease entities and is higher in mature T-cell and NK-cell neoplasms. Approximately 90% of ATL cases are CCR4-positive.40 CCR4 expression has also been shown to affect the prognosis of ATL patients; multivariate analysis revealed that CCR4 positivity was a significant unfavorable prognostic factor.40
Mogamulizumab (KW-0761) is a first-in-class defucosylated humanized anti-CCR4 monoclonal antibody that has been generated by protein engineering;41 mogamulizumab shows highly potent ADCC activity because of its high affinity of binding to effector cells, including NK cells.
Based on the phase I study, a phase II study for CCR4-positive relapsed ATL was conducted in Japan wherein 1.0 mg/kg of mogamulizumab was intravenously administered once a week for 8 weeks; of the 26 patients evaluable for efficacy assessment, the ORR was 50% (95% CI: 30–70), and response rates according to disease lesions were 100% for blood, 63% for skin, and 25% for nodal and extranodal lesions. The median progression-free survival and OS were 5.2 and 13.7 months, respectively.42 Subsequently, a randomized clinical trial was conducted for evaluating VCAP-AMP-VECP treatment with or without mogamulizumab in newly diagnosed CCR4-positive aggressive ATL patients in Japan. Combination therapy with VCAP-AMP-VECP plus mogamulizumab demonstrated a CR rate of 52% (95% CI: 33–71), which was 19% higher than treatment with VCAP-AMP-VECP alone (33%, 95% CI: 16–55).43
Although mogamulizumab was very effective for relapsed ATL, adverse drug reactions, including infusion reaction (89%) and skin rash (63%), were frequently observed in the phase II study. Severe skin rash was observed occasionally, and one patient developed Stevens-Johnson syndrome during the phase II study.42
Molecular targeted therapy with small molecules
Recently, molecular targeted therapy with small molecules, such as tyrosine kinase inhibitors, angiogenesis inhibitors and proteasome inhibitors, has been applied for various malignancies. The proteasome inhibitor bortezomib has been reported to suppress the growth of ATL cell lines and freshly isolated ATL cells;44 a trial for relapsed or refractory ATL patients is currently under way in Japan to investigate the clinical efficacy of bortezomib.
Supportive Therapy
Hypercalcemia associated with disease progression and opportunistic infections caused by immunodeficiency are problematic events in ATL patients.5 Patients with hypercalcemia need immediate treatment with hydration, antidiuretics, calcitonin, steroid hormones and bisphosphonates. Furthermore, urgent chemotherapy using anti-cancer agents for ATL is needed in severe cases of hypercalcemia.
As CNS relapse is known to occur frequently in ATL patients, the intrathecal administration of the anti-cancer agents methotrexate, cytarabine and prednisone is required for prophylaxis.22
Opportunistic infections from bacteria, fungi, virus, protozoans and parasites are frequently observed in ATL patients, and appropriate treatment is needed.5 In Japan, prophylactic treatment includes the use of fluconazole for Candida, itraconazole for Aspergillus and trimethoprim-sulfamethoxazole for Pneumocystis jirovecii.
Recent Findings of Genomic Heterogeneity of Adult T-cell Leukemia-lymphoma Cells
The initial pathogenic event for ATL is HTLV-1 integration; however, additional genetic alterations in ATL have also been implicated in its pathogenesis.45 Umino et al.46 recently reported the clonal heterogeneity of ATL tumor cells involving different genomic alterations; they demonstrated that these cells originated from a common cell. It was suggested that approximately 70% of ATL cases undergo clonal evolution, and that genetic instability may attribute to the accumulation of genomic alterations. The existence of multiple clones with genomic instability is one factor that renders ATL cells resistant to conventional chemotherapy. Even if a proportion of cells are killed by chemotherapy, there is always the possibility that a new resistant clone will emerge. Therefore, it is feasible to use allo-HSCT that can cure ATL patients by eliminating the HTLV-1-integrated recipient ATL clones through immune reaction, and by replacing the hematopoietic system with the donor type. Whole genome sequencing revealed that carriers have 103 to 104 orders of distinct clones with different HTLV-1 integration sites, and that most clones harbored one copy of HTLV-1.47 This indicates that HTLV-1 carriers potentially have 103 to 104 malignant clones. If the number of pre-malignant cells increases, there is a greater possibility that malignant transformation can occur. Therefore, it is important to reduce the number of pre-malignant cells in carriers of HTLV-1 in order to prevent the development of ATL.
Prevention of Adult T-cell Leukemia-lymphoma Development
An ongoing nationwide prospective investigation (Joint Study on Predisposing Factors of ATL Development) was initiated in 2001 to identify HTLV-1 carriers with the highest risk of developing ATL. Four risk factors have been associated with ATL development in HTLV-1 carriers, including age ≥40 years, high HTLV-1 proviral loads in peripheral blood mononuclear cells, family history of ATL, and any clinical signs or symptoms.48 Although it is obviously very important to prevent the development of ATL in HTLV-1 carriers with any of these risk factors, there are currently no available means towards this end.
The prevention of HTLV-1 infection is also of utmost importance because ATL is caused by HTLV-1 infection. HTLV-1 infection is thought to be transmitted by breastfeeding from the mother to infant, sexual intercourse or blood transfusion. The incidence of ATL development in HTLV-1 carriers differs according to the route of infection.49 A nationwide prospective study is currently under way in Japan using three different nursing methods: cessation of breastfeeding, short nursing periods and ordinary nursing.
Future Directions
Histone deacetylase (HDAC) inhibitors, such as vorinostat (suberoylanilide hygroxamic acid: SAHA), panobinostat (LBH-589) and MS-275, have been recognized for their abilities to inhibit HTLV-1-infected cell lines and freshly isolated ATL cells.50 Clinical use of these HDAC inhibitors for the treatment of ATL patients is expected.
Furthermore, various combinations of treatment, including chemotherapy, allo-HSCT, immunotherapy and molecular targeted therapies may help to cure a higher proportion of ATL patients in the future.
Conclusions
Although new therapeutic options are gradually improving the curability of ATL, treatment remains challenging for ATL patients. Nevertheless, to increase the ATL cure rate, rigorous investigation is necessary for optimizing therapeutic combinations, prevention of ATL development in HTLV-1 carriers, and reduction in the number of HTLV-1 carriers.
Acknowledgments
The authors are very grateful for the helpful suggestions made by Professor Keitaro Matsuo at the Kyushu University School of Medicine. We thank Ms Anri Takenaka for her excellent secretarial assistance. This work was supported in part by Grants-in-Aid from the Ministry of Health, Labor, and Welfare of Japan (AU, MS) and the Japan Society for the Promotion of Science (MS, #24390249). This work was further supported by a Grant-in-Aid from the Ministry of Health, Labor, and Welfare of Japan (AU, IC, #H22-Ganrinsho-Ippan-028).
Disclosure Statement
Dr Utsunomiya has received honoraria from Kyowa Hakko Kirin. The other authors have no competing interests to declare.
References
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92. [PubMed] [Google Scholar]
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Ann Rev Immunol. 2001;19:475–96. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- Zhao T, Matsuoka M. HBZ and its roles in HTLV-1 oncogenesis. Front Microbilol. 2012;3:247. doi: 10.3389/fmicb.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87) Br J Haematol. 1991;79:428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- Ohno N, Tani A, Uozumi K, et al. Expression of functional lung resistance-related protein predicts poor outcome in adult T-cell leukemia. Blood. 2001;98:1160–5. doi: 10.1182/blood.v98.4.1160. [DOI] [PubMed] [Google Scholar]
- Utsunomiya A, Miyazaki Y, Takatsuka Y, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:15–20. doi: 10.1038/sj.bmt.1702731. [DOI] [PubMed] [Google Scholar]
- Gill PS, Harrington W, Jr, Kaplan MH, et al. Treatment of adult T-cell leukemia lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995;332:1744–8. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- Hermine O, Bouscary D, Gessain A, et al. Treatment of adult T-cell leukemia lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332:1749–51. doi: 10.1056/NEJM199506293322604. [DOI] [PubMed] [Google Scholar]
- Kinpara S, Kijiyama M, Takamori A, et al. Interferon-α (IFN-α) suppresses HTLV-1 gene expression and cell cycling, while IFN-α combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology. 2013;10:52. doi: 10.1186/1742-4690-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–68. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J Med Virol. 2012;84:327–35. doi: 10.1002/jmv.23181. [DOI] [PubMed] [Google Scholar]
- Chihara D, Ito H, Matsuda T, et al. Association between decreasing trend in the mortality of adult T-cell leukemia/lymphoma and allogeneic hematopoietic stem cell transplants in Japan: analysis of Japanese vital statistics and Japan Society for Hematopoietic Cell Transplantation (JSHCT) Blood Cancer J. 2013;3:e159. doi: 10.1038/bcj.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara D, Ito H, Katanoda K, et al. Increase in incidence of adult T-cell leukemia/lymphoma in non-endemic areas of Japan and the United States. Cancer Sci. 2012;103:1857–60. doi: 10.1111/j.1349-7006.2012.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama M. Chemotherapy of ATL. In: Takatsuki K, editor. Adult T-Cell Leukemia. Oxford: Oxford University Press; 1994. pp. 221–37. [Google Scholar]
- Lymphoma Study Group (1984–1987) Major prognostic factors of patients with adult T-cell leukemia lymphoma: a cooperative study. Leuk Res. 1991;15:81–90. doi: 10.1016/0145-2126(91)90087-a. [DOI] [PubMed] [Google Scholar]
- Tsukasaki K, Tobinai K. Clinical trials and treatment of ATL. Leuk Res Treatment. 2012;2012:101754. doi: 10.1155/2012/101754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuya H, Yamanaka T, Ishitsuka K, et al. Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J Clin Oncol. 2012;30:1635–40. doi: 10.1200/JCO.2011.38.2101. [DOI] [PubMed] [Google Scholar]
- Bazarbachi A, Plumelle Y, Ramos JC, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177–83. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- Uozumi K. Treatment of adult T-cell leukemia. J Clin Exp Hematop. 2010;50:9–25. doi: 10.3960/jslrt.50.9. [DOI] [PubMed] [Google Scholar]
- Tsukasaki K, Tobinai K, Hotta T, Shimoyama M. Lymphoma Study Group of JCOG. Jpn J Clin Oncol. 2012;42:85–95. doi: 10.1093/jjco/hyr168. [DOI] [PubMed] [Google Scholar]
- Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458–64. doi: 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- Tsukasaki K, Maeda T, Arimura K, et al. Poor outcome of autologous stem cell transplantation for adult T cell leukemia/lymphoma: a case report and review of the literature. Bone Marrow Transplant. 1999;23:87–9. doi: 10.1038/sj.bmt.1701533. [DOI] [PubMed] [Google Scholar]
- Okamura J, Utsunomiya A, Tanosaki R, et al. Allogeneic stem-cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T-cell leukemia/lymphoma. Blood. 2005;105:4143–5. doi: 10.1182/blood-2004-11-4193. [DOI] [PubMed] [Google Scholar]
- Choi I, Tanosaki R, Uike N, et al. Long-term outcomes after hematopoietic SCT for adult T-cell leukemia/lymphoma: results of prospective trials. Bone Marrow Transplant. 2011;46:116–8. doi: 10.1038/bmt.2010.92. [DOI] [PubMed] [Google Scholar]
- Hematopoietic Cell Transplantation in Japan. Annual Report of Nationwide Survey 2013. The Japanese Data Center for Hematopoietic Cell Transplantation/The Japan Society for Hematopoietic Cell Transplantation 2014, 15 March 2014.
- Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536–45. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2011;116:1369–76. doi: 10.1182/blood-2009-10-247510. [DOI] [PubMed] [Google Scholar]
- Kanda J, Hishizawa M, Utsunomiya A, et al. Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: a retrospective cohort study. Blood. 2012;119:2141–8. doi: 10.1182/blood-2011-07-368233. [DOI] [PubMed] [Google Scholar]
- Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood. 2012;120:1734–41. doi: 10.1182/blood-2012-03-414490. [DOI] [PubMed] [Google Scholar]
- Bazarbachi A, Cwynarski K, Boumendil A, et al. Outcome of patients with HTLV-1-associated adult T-cell leukemia/lymphoma after SCT: a retrospective study by the EBMT LWP. Bone Marrow Transplant. 2014;49:1266–8. doi: 10.1038/bmt.2014.143. [DOI] [PubMed] [Google Scholar]
- Harashima N, Kurihara K, Utsunomiya A, et al. Graft-versus-tax response in adult T-cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res. 2004;64:391–9. doi: 10.1158/0008-5472.can-03-1452. [DOI] [PubMed] [Google Scholar]
- Yonekura K, Utsunomiya A, Takatsuka Y, et al. Graft-versus-adult T-cell leukemia/lymphoma effect following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:1029–35. doi: 10.1038/bmt.2008.39. [DOI] [PubMed] [Google Scholar]
- Itonaga H, Tsushima H, Taguchi J, et al. Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: the Nagasaki Transplant Group experience. Blood. 2013;121:219–25. doi: 10.1182/blood-2012-07-444372. [DOI] [PubMed] [Google Scholar]
- Kannagi M, Harashima N, Kurihara K, et al. Tumor immunity against adult T-cell leukemia. Cancer Sci. 2005;96:249–55. doi: 10.1111/j.1349-7006.2005.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suehiro Y, Hasegawa A, Iino T, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T-cell leukaemia/lymphoma in a pilot study. Br J Haematol. 2015 doi: 10.1111/bjh.13302. ; doi: 10.1111/bjh.13302. [DOI] [PubMed] [Google Scholar]
- Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15:e517–26. doi: 10.1016/S1470-2045(14)70202-5. [DOI] [PubMed] [Google Scholar]
- Bazarbachi A, Suarez F, Fields P, Hermine O. How I treat adult T-cell leukemia/lymphoma. Blood. 2011;118:1736–45. doi: 10.1182/blood-2011-03-345702. [DOI] [PubMed] [Google Scholar]
- Takasaki Y, Iwanaga M, Imaizumi Y, et al. Long- term study of indolent adult T-cell leukemia lymphoma. Blood. 2010;115:4337–43. doi: 10.1182/blood-2009-09-242347. [DOI] [PubMed] [Google Scholar]
- Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–34. [PubMed] [Google Scholar]
- Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16:1520–31. doi: 10.1158/1078-0432.CCR-09-2697. [DOI] [PubMed] [Google Scholar]
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761 for relapsed adult T-cell leukemia lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837–42. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- Ishida T, Jo T, Takemoto S, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive ATL: a randomized phase II study. Br J Haematol. 2015 doi: 10.1111/bjh.13338. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou Y, Nosaka K, Koya Y, Yasunaga JI, Toyokuni S, Matsuoka M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia. 2004;18:1357–63. doi: 10.1038/sj.leu.2403400. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191–5. doi: 10.1111/j.1349-7006.1989.tb02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umino A, Nakagawa M, Utsunomiya A, et al. Clonal evolution of adult T-cell leukemia/lymphoma takes place in the lymph nodes. Blood. 2011;117:5473–8. doi: 10.1182/blood-2010-12-327791. [DOI] [PubMed] [Google Scholar]
- Bangham CR, Cook LB, Melamed A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin Cancer Biol. 2014;26:89–98. doi: 10.1016/j.semcancer.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M, Watanabe T, Utsunomiya A, et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood. 2010;116:1211–9. doi: 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- Murphy EL, Hanchard B, Figueroa JP, et al. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250–3. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, et al. Histone deacetylase inhibitors induce growth arrest and apoptosis of HTLV-1-infected T-cells via blockade of signaling by nuclear factor κB. Leuk Res. 2008;32:287–96. doi: 10.1016/j.leukres.2007.05.026. [DOI] [PubMed] [Google Scholar]


