Abstract
Signal transducer and activator of transcription 3 (STAT3) is constitutively activated in human cancers. Therefore, STAT3 is a therapeutic target of cancer drug discovery. We previously reported that natural products inhibited constitutively activated STAT3 in human prostate tumor cells. We used a dual-luciferase assay to screen 200 natural products isolated from herbal medicines and we identified ginkgetin obtained from the leaves of Ginkgo biloba L. as a STAT3 inhibitor. Ginkgetin inhibited both inducible and constitutively activated STAT3 and blocked the nuclear translocation of p-STAT3 in DU-145 prostate cancer cells. Furthermore, ginkgetin selectively inhibited the growth of prostate tumor cells stimulated with activated STAT3. Ginkgetin induced STAT3 dephosphorylation at Try705 and inhibited its localization to the nucleus, leading to the inhibition of expression of STAT3 target genes such as cell survival-related genes (cyclin D1 and survivin) and anti-apoptotic proteins (Bcl-2 and Bcl-xL). Therefore, ginkgetin inhibited the growth of STAT3-activated tumor cells. We also found that ginkgetin inhibited tumor growth in xenografted nude mice and downregulated p-STAT3Tyr705 and survivin in tumor tissues. This is the first report that ginkgetin exerts antitumor activity by inhibiting STAT3. Therefore, ginkgetin is a good STAT3 inhibitor and may be a useful lead molecule for development of a therapeutic STAT3 inhibitor.
Keywords: Apoptosis, biflavonoid, ginkgetin, prostate cancer, STAT3
Despite the development of new therapies, cancer still remains a deadly disease and leading cause of mortality in the world. The unmet needs of antitumor drug development include finding an agent that is safe, selective, and effective and that also prevents metastasis. Our group is interested in herbal medicines that show low toxicity against human cells. Many compounds originating from traditional medicines have been linked to the prevention and treatment of cancer.1,2 However, the mechanisms by which these agents prevent disease remain largely unknown.
Signal transducer and activator of transcription 3 (STAT3) is a well-known oncogene that plays an important role in tumorigenesis and is a transcription factor considered a potential target for cancer therapy.3 The oncogenic role of STAT3 was established by constitutive activation of this molecule in a wide variety of human tumors including leukemia, lymphomas, and multiple myeloma, as well as head and neck, skin, breast, lung, gastric, colorectal, and prostate cancers.4–8 Activated STAT3 monomers in the cytoplasm form dimers and the STAT dimers then translocate to the nucleus, bind to specific DNA response elements, and modulate the transcription of downstream genes involved in cancer cell survival and proliferation.9–11 Indeed, cancer cells having aberrant STAT3 activity show elevated levels of anti-apoptotic (Mcl-1 and Bcl-xL) and cell cycle regulating proteins (cyclin D1 and c-Myc).12,13
Prostate cancer is the most commonly diagnosed tumor in males in the USA, ultimately affecting 35% of all American men.14 Internationally, prostate cancer is the leading cause of cancer-related deaths.15 In human prostate cancer, the transcription factor STAT3 is constitutively active and has been associated with advanced tumor stages.16,17 Several reports have suggested that activation of STAT3 is associated with promotion of prostate cancer cell proliferation and inhibition of apoptosis.18,19 In addition, progression of prostate cancer to metastatic disease is one of the key issues in clinical management.20
Traditionally, Ginkgo biloba (Ginkgo) has been used in medicine and as a source of food. Extracts from Ginkgo leaves include glycosides and terpenoids, and have been used pharmaceutically.21,22 Here we isolated ginkgetin and its structural isomers, such as isoginkgetin and sciadopitysin, from G. biloba extract, which are biflavonoids. Some flavonoid derivatives have been described to have anti-inflammatory and immune-modulatory activities both in vitro and in vivo.23,24 Furthermore, ginkgetin has been reported to exert anti-inflammatory, antifungal, anti-influenza, and neuroprotective activities.25 However, the underlying antitumor mechanism of ginkgetin remains unclear.
In this study, we determined that ginkgetin exhibits a strong antitumor effect against prostate cancer cells with activated STAT3. We investigated the antitumor effects of ginkgetin on the human prostate cancer cell line DU-145 with activated STAT3, and we determined the biological mechanism underlying ginkgetin-mediated inhibition of the STAT3 activation pathway.
Materials and Methods
Reagents
RPMI-1640, DMEM F-12, Opti-MEM reduced serum medium, FBS, PBS, Trypsin-EDTA, and penicillin–streptomycin solution were purchased from Invitrogen (Grand Island, NY, USA). Antibodies against STAT3, p-STAT3Tyr705, p-STAT3Ser727, poly(ADP-ribose) polymerase (PARP), caspase-3, Bcl-2, and survivin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against cyclin A, cyclin D1, Bcl-xL, Vascular Endothelial Growth Factor (VEGF), and p53 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cell Counting Kit-8 was purchased from Dojindo Molecular Technologies (Rockville, MD, USA). Texas Red (TR)-conjugated dyes against goat anti-rabbit IgG-TR and DAPI were purchased from Santa Cruz Biotechnology. Dual Luciferase Assay kit was purchased from Promega (Madison, WI, USA). Ginkgetin, isoginkgetin, and sciadopitysin were isolated from dried G. biloba leaves.26
Cell culture
Cancer cell lines were obtained from the ATCC (Manassas, VA, USA). Human cancer cell lines HCT-116 (CCL-247, colon carcinoma), DU-145 (HTB-81, prostate carcinoma), LNCap (CRL-1740, prostate carcinoma), and PC-3 (CRL-1435, prostate adenocarcinoma), and as well as human mammary epithelial cell line MCF-10A (CRL-10317, epithelial fibrocystic disease) were maintained in RPMI-1640 (Invitrogen). The culture media were supplemented with 10% heat-inactivated FBS. Cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
Transient transfection and dual-luciferase reporter assay
HCT-116 cells were seeded at a density of 6 × 106 cells in 150-mm2 culture plates. On the following day, cells were transfected with 30 μg 21pSTAT3-TA-Luc vector containing the STAT3 binding element for firefly luciferase activity, and 3 μg pRL-TK vector for Renilla control luciferase activity using X-tremeGene HP DNA Transfection Reagent (Roche Diagnostics, Indianapolis, IN, USA) in Opti-MEM reduced serum medium. After 5 h of transfection, cells were trypsinized and seeded onto black-bottom 96-well plates at a density of 2 × 104 cells per well and then incubated with complete RPMI-1640 medium for 15 h. Cells were treated with either test compounds or 0.1% DMSO for 24 h. After treatment, cells were washed with 150 μL PBS, lysed with 25 μL passive lysis buffer and shaken at 4°C for 15 min. Luciferase activity was evaluated by the Dual Luciferase Reporter Assay kit on a GloMax 96 microplate luminometer (Promega). Relative luciferase activity was calculated according to the following formula: relative luciferase activity (%) = ([normalized luciferase activity of sample treated with a test compound]/[normalized luciferase activity of sample treated with 0.1% DMSO]) × 100.27
Cell proliferation assay
Cells were seeded at a density of 7000 cells per well in 96-well plates with appropriate culture medium containing 10% FBS. On the following day, seeded wells were replenished with fresh complete medium containing either test compounds or 0.1% DMSO. After incubation for 24, 48, or 72 h, 10 μL cell proliferation reagent CCK-8 (Dojindo Molecular Technologies) was added to each well and incubated with the plate for 1–4 h. WST-8 is reduced by dehydrogenases in cells to produce a yellow colored product, formazan, the amount of which was quantitatively measured at 450 nm using an ELISA reader (Bio-Rad Laboratories, Inc. Hercules, CA, USA).
Western blot analysis
The amounts of proteins in lysates were quantitated with the Bio-Rad protein assay dye reagent concentrate. Proteins were resolved on appropriate percentages of acrylamide SDS gels (SDS-PAGE) and transferred to PVDF membranes using transfer buffer (25 mM Tris-Cl [pH 8.3], 1.4% glycine, and 20% methanol). Membranes were blocked with 5% skim milk from Becton Dickinson (Sparks, MD, USA) in TBS-T (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, and 0.05% Tween 20) and probed with primary antibodies for 2 h. Blots were washed with TBS-T and exposed to HRP-conjugated goat-anti-rabbit or anti-mouse IgG for 1 h, then washed with TBS-T, and their chemiluminescence was examined using Luminata Forte Western HRP Substrate (Millipore, Billerica, MA, USA).
Kinase assay
Kinase assay was carried out by Merck Millipore. Protein kinases were tested in a radiometric assay format and the raw data measured by scintillation counting (in cpm).
Confocal laser microscopy
Cells were treated with the test compound or 0.1% DMSO. After the appropriate treatment time, cells were rinsed with PBS buffer and fixed for 10 min at room temperature in 4% paraformaldehyde fixative, rinsed with PBS three times, followed by permeabilization with methanol at −20°C for 10 min. After three rinses, cells were blocked with 1.0% BSA in PBS for 1 h and incubated overnight at 4°C with primary antibodies diluted in PBS containing 1.0% BSA. After washing three times with PBS buffer, cells were incubated with FITC-conjugated IgG. Finally, the cells were washed three times with PBS and treated with 2 μg/mL DAPI in PBS for 2 min to stain chromosomes. Cells were observed using a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Oberkochen, Germany).
Fluorescence-activated cell sorting analysis
Cells were seeded at a density of 1 × 106 cells in 100-mm2 culture plates and cells were treated with test compounds or 0.1% DMSO. After incubation for 24 h, cell culture supernatant and wash solution were centrifuged at 300 g for 5 min at room temperature. Precipitated cells were carefully suspended in 500 μL PBS buffer and fixed with 4 mL ice-cold 70% ethanol. Fixed cells were centrifuged at 300 g for 5 min at room temperature and washed twice with PBS buffer. The collected cells were resuspended in PBS buffer (5 × 105 cells per 500 μL) and treated with 100 μg/mL RNase A and 50 μg/mL propidium iodide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) at 37°C for 45 min. Fixed cells were analyzed on a FACSCalibur flow cytometer until 20 000 cells were counted (Becton Dickinson, San Jose, CA, USA). The distribution of cells across the cycle was analyzed using WinMDI 2.9 (http://facs.scripps.edu/software.html).
In vivo nude mouse tumor xenograft model assay
All animals were maintained in a specific pathogen-free facility under protocols approved by the Institutional Animal Care and Use Committee. Five- to six-week-old female BALB/c nude mice (Orient Bio Inc., Seoul, Korea) were used for animal studies. Tumors were established by s.c. injecting 9 × 106 DU-145 cells/mouse into the right flank of mice on day 0. The compound was dissolved in 0.5% Tween 80 and was given i.p. once daily for 25 days at a concentration of 30 mg/kg. Each group consisted of eight mice. The overall appearance and health of the mice were monitored when the compound was given. Tumor volumes were estimated by the following formula: length (mm) × width (mm) × height (mm)/2. Tumor volumes and weight at the end of treatment were compared using Student's t-test.
Statistical analysis
Data were expressed as the mean ± SD, and the degree of significance was analyzed by Student's t-test. P-values < 0.05 were considered statistically significant.
Results
Ginkgetin inhibits STAT3-dependent luciferase activity in HCT-116 human colorectal cancer cell lines
To find a novel inhibitor of STAT3 from herbal medicines, 200 natural compounds were screened using a STAT3-dependent luciferase assay in HCT-116 colon cancer cells. Ginkgetin was identified as a good inhibitor of STAT3. The structures of ginkgetin and its derivatives are shown in Figure1(a). Inhibition of STAT3 activity by ginkgetin and its derivatives, such as isoginkgetin, sciadopitysin, and tetramethylginkgetin, was evaluated using a dual-luciferase assay in HCT-116 colon cancer cells. STAT3 activity was inhibited by more than 45% with 10 μM ginkgetin (Fig.1b). However, isoginkgetin, sciadopitysin, and tetramethylginkgetin inhibited the activity of STAT3 at 10 μM concentration, 7.3%, 10.8%, and 6.8%, respectively. When DU-145 human prostate cancer cells were treated with ginkgetin and its derivatives at 5 and 10 μM, only ginkgetin downregulated the phosphorylation of STAT3 at Tyr-705 (Fig.1c). These data suggest that ginkgetin is a STAT3 inhibitor.
Fig 1.
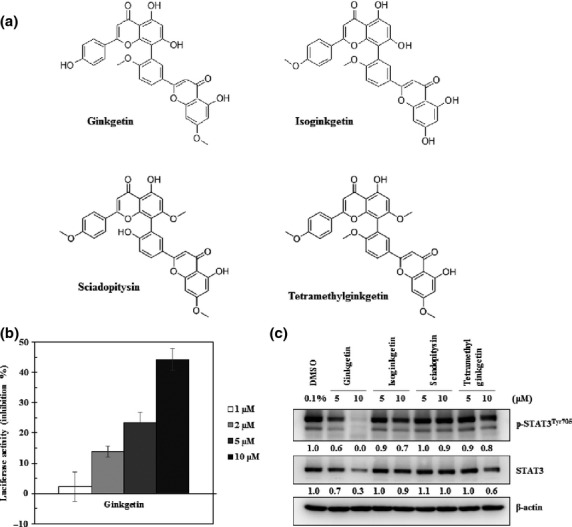
Ginkgetin inhibits signal transducer and activator of transcription 3 (STAT3)-dependent luciferase activity and constitutively active STAT3 in DU-145 cells. (a) Chemical structures of ginkgetin and its derivatives. (b) STAT3 inhibiting activity of ginkgetin was analyzed with a STAT3–luciferase reporter assay using increasing doses of ginkgetin in HCT-116 human colorectal cancer cell lines for 24 h. (c) STAT3 inhibition activities of ginkgetin and its derivatives were compared by immunoblotting after compound treatment with 5 and 10 μM concentration to DU-145 human prostate cancer cells (1 × 106 cells/mL) for 9 h.
Ginkgetin inhibits constitutive and interleukin-6 (IL-6)-induced STAT3 activation and proliferation of DU-145 human prostate cancer cell lines expressing constitutively activated STAT3
Ginkgetin completely inhibited the constitutive activation of STAT3 at 5 μM in DU-145 cells (Fig.2a). Because IL-6 induces STAT3 phosphorylation, we determined whether ginkgetin could inhibit IL-6-induced STAT3 phosphorylation. Enhanced p-STAT3 levels by IL-6 in DU-145 cells were suppressed by ginkgetin (Fig.2a). LNCap cells, which express STAT3 but lack constitutively active STAT3 and are attenuated the proliferation by IL-6 treatment,28 were treated with IL-6 and then examined for phosphorylated STAT3. Interleukin-6-induced phosphorylation of STAT3 in LNCap cells was suppressed by more than 70% after treatment with 5 μM ginkgetin (Fig.2b). However, the proliferation of LNCap cells was only slightly changed by IL-6 treatment and ginkgetin did not affect the growth of the cells in this experimental condition (data not shown).
Fig 2.
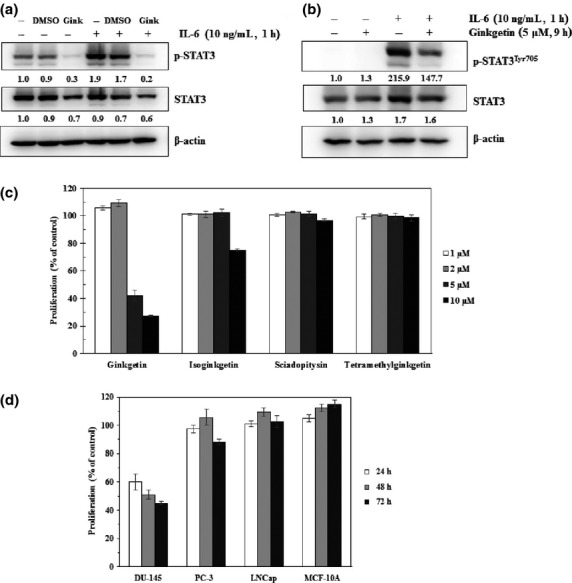
Ginkgetin (Gink) inhibits constitutive and interleukin-6 (IL-6)-induced signal transducer and activator of transcription 3 (STAT3) activation and proliferation of human prostate cancer cell lines that contain activated STAT3. (a) Treatment with 5 μM ginkgetin for 9 h inhibits not only constitutive p-STAT3Tyr705 but also activation of STAT3 by IL-6 treatment (10 ng/mL, 1 h) in DU-145 cells. (b) Ginkgetin reduces activated p-STAT3Tyr705 level by IL-6 treatment (10 ng/mL, 1 h) in LNCap cell lines. (c) Proliferation of DU-145 human prostate cancer cells was measured with the CCK-8 proliferation assay kit at 48 h after treatment with ginkgetin and its derivatives. (d) Ginkgetin (5 μM) was applied to various human prostate cancer cell lines and a normal epithelial cell line (MCF-10A) for increasing periods of time. Cells were plated in triplicate and treated with the indicated concentrations of biflavonoids for 24, 48, and 72 h.
Next we examined whether ginkgetin modulates the proliferation of DU-145 cells. Results in Figure2(c) show that ginkgetin suppressed the proliferation of DU-145 cells in a dose-dependent manner with a GI50 value of 5 μM, whereas the other biflavonoids such as isoginkgetin, sciadopitysin, and tetramethylginkgetin did not inhibit growth of the cells at the same concentration. It was reported that the proliferation of DU-145 cells is inhibited by STAT3 siRNA and LNCap prostate tumor cells has non-activated STAT3.27 To examine the capacity of ginkgetin to inhibit growth in STAT3-dependent, STAT3-independent, and normal cells, the same proliferation assay was carried out in prostate tumor cells DU-145, LNCap, PC-3, and normal MCF-10A cells. As shown Figure2(d), ginkgetin selectively inhibited the growth of DU-145 cells but not normal cells. These data suggest that ginkgetin primarily suppresses the proliferation of STAT3-dependent cancer cells.
Ginkgetin inhibits STAT3 tyrosine phosphorylation leading to blocking of translocation and downregulation of STAT3 target genes
Ginkgetin inhibited STAT3 phosphorylation at tyrosine-705 in a time-dependent manner, with maximum inhibition occurring after 9 h of treatment with 5 μM (Fig.3a,b). The total amounts of STAT3 and p-STAT3 were decreased after 24 h of treatment. Under these conditions, the phosphorylation of other STAT family proteins, such as STAT1 and STAT5, was not inhibited by ginkgetin (Fig.3a).
Fig 3.
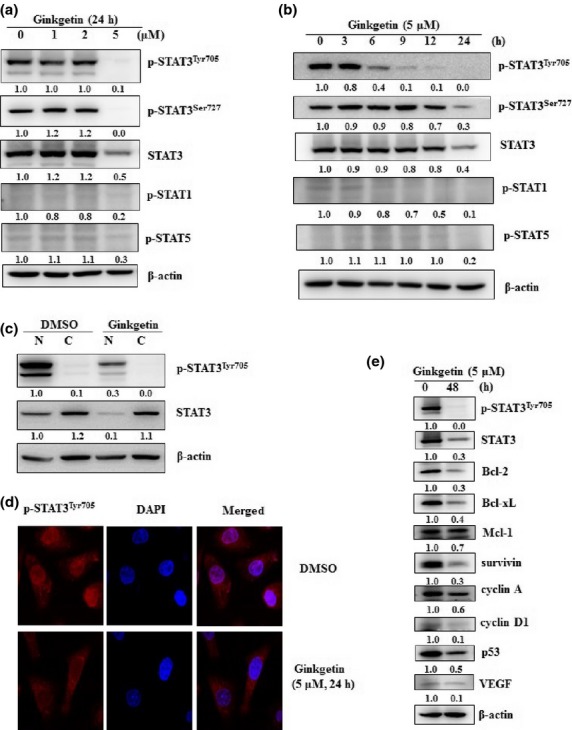
Ginkgetin downregulates signal transducer and activator of transcription 3 (STAT3) phosphorylation and leads to a block of nuclear translocation in DU-145 cells. (a, b) Ginkgetin was used to treat DU-145 human prostate cancer cells at increasing doses for 24 h (a) and at 5 μM for increasing times (b). Immunoblotting was then used to quantify the expression levels of phosphorylated (p-)STAT3 and STAT3 protein. (c) After treatment with 5 μM ginkgetin for 9 h, the cytoplasm (C) and nuclei (N) of DU-145 cells were fractioned. Immunoblotting was then carried out to detect p-STAT3 and STAT3 in both fractions. (d) Confocal laser scanning microscopy was used to confirm that ginkgetin downregulated the expression of p-STAT3 in the nuclei of DU-145 human prostate cancer cells. The cells were fixed and permeabilized. Phosphorylated STAT3 (red) was immunostained with rabbit anti-STAT3 followed by FITC-conjugated secondary antibodies and the nucleus (blue) was stained with DAPI. The third panels show the merged images of the first and second panels. (e) Protein levels of STAT3 target genes were confirmed by immunoblotting after ginkgetin treatment (5 μM for 48 h). The anti-apoptotic proteins Bcl-2 and Bcl-xL were downregulated by ginkgetin treatment. VEGF, vascular endothelial growth factor.
For STAT3 to regulate the expression of its target genes, it needs to translocate from the cytosol to the nucleus, a process that requires STAT3 tyrosine phosphorylation. To confirm the localization of ginkgetin-inhibited STAT3 in tumor cells, DU-145 cells were fractionated using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Rockford, IL, USA) after 9 h of treatment with 5 μM ginkgetin (Fig.3c). We found that, in ginkgetin-treated cells, the levels of both p-STAT3 and STAT3 were decreased in the nuclear fraction. To visualize ginkgetin-mediated alteration of STAT3 localization in DU-145 cells, the cells were then fixed, stained, and observed under the microscope with goat anti-rabbit IgG-TR (TR-conjugated) to detect p-STAT3 and DAPI to detect the nucleus. From these experiments, we confirmed that the level of p-STAT3 in the nucleus was decreased following treatment (Fig.3d). Therefore, the expression of cell survival-related genes such as cyclin D1 and survivin was decreased and the anti-apoptotic proteins Bcl-2 and Bcl-xL were downregulated by ginkgetin treatment (Fig.3e). These data suggest that ginkgetin induced STAT3 dephosphorylation at Tyr705 and inhibited its localization to the nucleus, leading to the inhibition of expression of STAT3 target genes and, therefore, the growth of STAT3-activated tumor cells.
Ginkgetin selectively suppresses STAT3 Tyr705 phosphorylation but not through inhibition of upstream tyrosine kinases and tyrosine phosphatases
The JAK and Src family proteins are the most well-known upstream kinases that phosphorylate STAT proteins on their tyrosine residues, and STAT3 has been reported to be activated by soluble tyrosine kinases (TYK) of the JAK family. Therefore, an in vitro kinase assay was carried out for c-Src, epidermal growth factor receptor (EGFR), JAK2, platelet-derived growth factor (PDGF), TYK2, and mammalian target of rapamycin (mTOR) with 5 μM ginkgetin. Results showed that ginkgetin did not inhibit known STAT3-related kinase activity such as EGFR, mTOR, PDFG, Src, and TYK2 (Table1). However, ginkgetin partially inhibited JAK2 and cSRC activity (Table1).
Table 1.
In vitro kinase assay of known signal transducer and activator of transcription 3-related activity (5 μM ginkgetin)
| Inhibition, % | |
|---|---|
| EGFR | 3 |
| JAK2 | 27 |
| cSRC | 43 |
| mTOR | 0 |
| PDGFRβ | 2 |
| Src (T341M) | 5 |
| TYK2 | −7 |
EGFR, epidermal growth factor receptor; mTOR, mammalian target of rapomycin; PDGFRβ, platelet-derived growth factor receptor β; TYK2, tyrosine kinase 2.
Protein tyrosine phosphatases (PTPase) are also involved in the negative regulation of JAK/STAT signaling. Because p-STAT3Tyr705 is dephosphorylated by soluble tyrosine phosphatases, various STAT3 phosphatases were analyzed.29 Next, we investigated whether siRNA-mediated suppression of STAT3-related tyrosine phosphatases, including SHP1, SHP2, and MEG2, would abrogate the inhibitory effect of ginkgetin on STAT3 activation. These knockdown experiments did not reveal any specific PTPases involved in the rescue of STAT3 dephosphorylation following treatment with ginkgetin in DU-145 cells (data not shown). Therefore, further studies are required to elucidate the precise mechanism of STAT3 dephosphorylation stimulated by ginkgetin treatment in DU-145 cells.
Ginkgetin causes accumulation of cells in the G0/G1 phase of the cell cycle and induces apoptosis
Because a decline in levels of cyclin D1 was observed in ginkgetin-treated cells, we sought to determine the effect of ginkgetin on cell cycle phase distribution. Ginkgetin induced accumulation of a cell population at the G0/G1 phase in a time-dependent manner with maximum inhibition occurring after 9 h (55.5% of G0/G1 phase) of treatment with 5 μM (Fig.4a). We also investigated whether ginkgetin-induced suppression of constitutively active STAT3 in DU-145 cells leads to apoptosis. Cells were treated with varying doses of ginkgetin for different times, and then examined for caspase-3 activation by Western blot. We found a time- and dose-dependent cleavage of caspase-3 by ginkgetin and led to the cleavage of a 116 kDa PARP protein into an 87-kDa fragment (Fig.4b,c). These results clearly suggest that ginkgetin inhibits the growth of DU-145 cells by inducing apoptosis. These data are consistent with ginkgetin-induced downregulated expression of anti-apoptotic genes such as Bcl-2 and Bcl-xL (Fig.3e).
Fig 4.
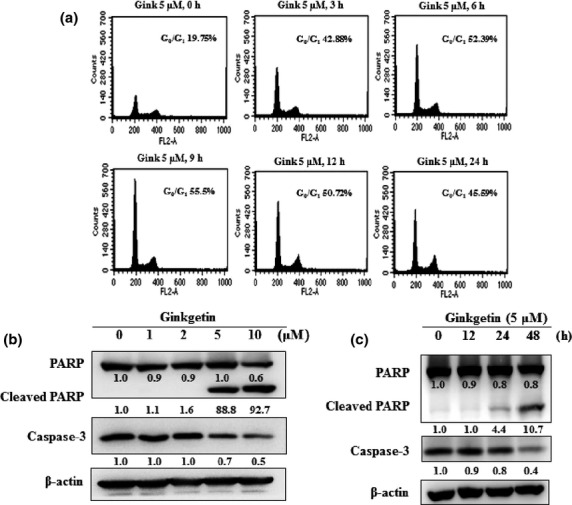
Ginkgetin (Gink) induces G0/G1 arrest and apoptosis in DU-145 prostate cancer cells. (a) Cell cycle distribution was analyzed with a FACSCalibur flow cytometer. Over an increasing time period, DU-145 cells were arrested at G0/G1 phase. The ratios of cells in each phase were analyzed with a WinMDI 2.9 analyzer. (b, c) DU-145 cells were treated with the indicated concentrations of ginkgetin for 24 h and for increasing times at 5 μM up to 48 h. Whole-cell extracts were prepared and immunoblotted with antibodies for caspase-3 and poly(ADP-ribose) polymerase (PARP). The β-actin antibody was used to verify equal protein loading.
Ginkgetin inhibits tumor growth in a mouse xenograft model of DU-145 cells
To explore the antitumor efficacy of ginkgetin in vivo, DU-145 cells were s.c. implanted into the right flank of nude mice on day 0 and ginkgetin feeding was started on day 1. We began i.p. injections of the compound (30 mg/kg) or vehicle five times per week for 23 days (eight mice per group). On day 23, the mice were killed and tumor volume and weight were measured. Treatment with ginkgetin reduced tumor volume by 65.6% and tumor weight by 67.4% in the mice xenografted with DU-145 cells relative to their vehicle-treated counterparts (Fig.5a,b). To measure the level of p-STAT3 in tumor tissues, the tumor tissues of eight mice treated with vehicle and eight mice treated with ginkgetin were lysed and analyzed by Western blot. As shown in Figure5(c), the level of p-STAT3 in ginkgetin-treated mice decreased in comparison with that of the control group. We also analyzed survivin, which is a target protein of STAT3. The expression of survivin in ginkgetin-treated tumor tissues was fully suppressed (Fig.5d). Therefore, ginkgetin reduces tumor volume and tumor weight in the nude mouse xenograft by inhibiting p-STAT3.
Fig 5.
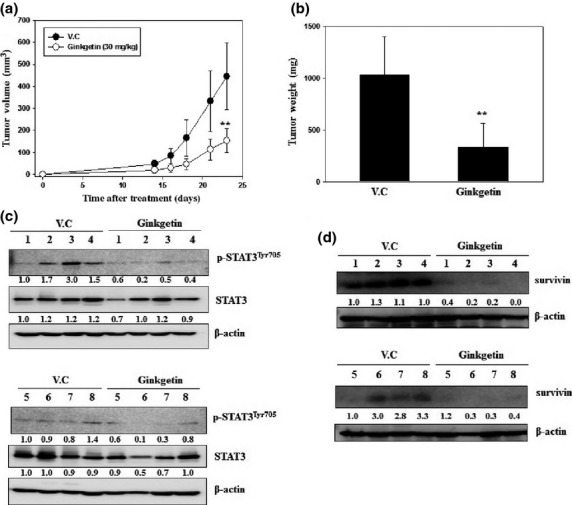
Ginkgetin reduces tumor volume and tumor weight in a nude mouse xenograft model with DU-145 human prostate cancer cells. (a) Tumor volumes from DU-145 cells were estimated after 23 days by the formula length (mm) × width (mm) × height (mm)/2 in DU-145 cells (**P < 0.01, tumor volume [65.6% suppression]). (b) Tumor weight from in DU-145 cells after 23 days (**P < 0.01, tumor weight [67.4% suppression]). (c,d) Collected tumor tissues from each group were sliced and homogenized in RIPA lysis buffer. The lysed tissues were rotated at 4°C for 30 min and were centrifuged at 4°C, 10,000 g for 30 min. The lysates were quantitated with Bio-Rad protein assay dye reagent and 100 μg protein was prepared for Western blot loading samples. Expression of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) (c) in ginkgetin-treated xenograft mouse tissue was compared with that in vehicle-treated tissue. Expression of survivin (d), a transcription factor and STAT3 target protein, in ginkgetin-treated xenograft mouse was compared with that in vehicle-treated tissue. V.C, (Vehicle).
These results indicate that the inhibition of tumor growth by ginkgetin occurred both in vivo and in vitro through modulation of the STAT3 pathway, suggesting that ginkgetin could be used for cancer therapy.
Discussion
Ginkgetin was isolated from leaf extracts of G. biloba, which is used globally as a herbal medicine.30 Ginkgetin has various biological functions including anti-inflammatory, antifungal, anti-influenza, osteoblast differentiation stimulating, neuroprotective, and antitumor activities.31 The antitumor activities of ginkgetin have been reported by various groups, including our laboratory.32 However, the mechanisms underlying its antitumor activities are not fully understood. In this study, we elucidated the mechanism behind the selective toxicity of ginkgetin against STAT3-activated tumor cells. Ginkgetin selectively suppressed the growth of prostate cancer cells with activated STAT3. However, ginkgetin did not inhibit the growth of normal cells (MCF-10A) or of prostate cancer cells without STAT3 activation, such as PC-3 and LNCap prostate tumor cells (Fig.2d). Additionally, we confirmed that ginkgetin at an effective dose against tumor cells showed little or no additional toxicity toward healthy tissues in an in vivo model. Our results revealed that ginkgetin is the only biflavonoid investigated that has antitumor activity against STAT3-activated tumor cells. Therefore, designing a specific STAT3 inhibitor based on biflavonoid or flavonoid moieties will prove highly useful.
Our mechanistic study indicates that ginkgetin inhibited the activation of STAT3 signaling by interfering with STAT3 phosphorylation and reducing STAT3 nuclear localization (Fig.3a–d). The JAK–STAT pathway serves as an intracellular mediator of IL-6 signaling and is evolutionarily conserved from flies to mammals. Cytokine binding to the IL-6r–Gp130 receptor complex leads to JAK activation, STAT phosphorylation on tyrosine residues, STAT dimerization, nuclear translocation, and target gene activation. We found that ginkgetin suppressed both constitutive and IL-6-induced STAT3 activation (Fig.2a,b). Ginkgetin specifically inhibits STAT3 activation, as it has no effect on STAT1 or STAT5 phosphorylation (Fig.3a). It was reported that blocking STAT3 in cancer cells upregulates expression of p53, leading to p53-mediated tumor cell apoptosis. Although ginkgetin inhibited STAT3 activity,33 it also downregulated p53 expression (Fig.3e). We do not yet know why treatment with ginkgetin for 48 h decreased the protein expression of p53. It is possible that apoptosis occurred at that time, thus downregulating many proteins including p53 and STAT3.
The inhibition of constitutive and IL-6-induced STAT3 activation by ginkgetin suppressed STAT3 transcription activity and downregulated STAT3-targeted genes. These genes were involved in proliferation (cyclin D1), anti-apoptosis (Bcl-2 and Bcl-xL), and survival (survivin). Constitutively active STAT3 can contribute to oncogenesis by protecting cancer cells from apoptosis; this implies that suppression of STAT3 activation by ginkgetin may facilitate apoptosis. Furthermore, constitutively active STAT3 has been implicated in the induction of resistance to apoptosis, possibly through the expression of Bcl-2, Bcl-xL, and cyclin D1.34 The downregulation of cyclin D1 expression by ginkgetin correlated with suppression of proliferation and accumulation of cells in G0/G1 (Fig.4a), which is consistent with a requirement for cyclin D1. Expression of Bcl-xL is regulated by STAT3, which has an anti-apoptotic function in cancer cells.35 The downregulation of Bcl-2 and Bcl-xL expression may contribute to the ability of ginkgetin to induce apoptosis in DU-145 cells. We further observed that ginkgetin downregulated the protein expression of survivin protein in these cells. Furthermore, ginkgetin suppressed DU-145 tumor growth and STAT3 activity in our mouse xenograft model, and it completely inhibited the expression of survivin in the tumor tissues grown from DU-145 cells in an animal model (Fig.5). This result reveals that p-STAT3 is a biomarker for ginkgetin for further studies, including clinical testing, and indicates the pharmacodynamics of ginkgetin.
Many STAT3 inhibitors have been reported, most of which inhibit STAT3 activation through STAT3 SH2 domain-mediated ligand binding.36 It has also been reported that certain natural products inhibited STAT3 activation through the upregulation of phosphatases.37 However, ginkgetin inhibits STAT3 activation by a different mode of action. Therefore, these results provide new insight into the inhibition of STAT3 activation. We consider this a strength of ginkgetin.
To our knowledge, this is the first systematic study showing the selective induction of apoptosis in prostate cancer cells versus normal cells using a biflavonoid STAT3 inhibitor. Most importantly, we show that ginkgetin is highly effective in prostate cancer cells with activated STAT3. These results provide new insight into the action of ginkgetin, which potently inhibits the STAT3 signaling pathway. Our findings suggest that ginkgetin has a potential role in the prevention and treatment of prostate cancer.
Acknowledgments
This work was supported by the KRIBB Research Initiative Program, the Bio-Synergy Research Project (2012M3A9C404877), and the Foreign Plant Extract Library Program (2011-00497), funded by the Ministry of Science, Information & Communication Technology and Future Planning (ICT), Korea.
Disclosure Statement
The authors have no conflict of interest.
References
- Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–78. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–50. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CL, Jove R, Burakoff SJ. Constitutive activation of the Janus kinase-STAT pathway in T lymphoma overexpressing the Lck protein tyrosine kinase. J Immunol. 1997;159:5206–10. [PubMed] [Google Scholar]
- Sartor CI, Dziubinski ML, Yu CL, Jove R, Ethier SP. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–87. [PubMed] [Google Scholar]
- Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–55. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulghani J, Gu L, Dagvadorj A, et al. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–28. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394:145–51. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Drewry JA, Shahani VM, Page BD, Gunning PT. Molecular disruption of oncogenic signal transducer and activator of transcription 3 (STAT3) protein. Biochem Cell Biol. 2009;87:825–33. doi: 10.1139/o09-044. [DOI] [PubMed] [Google Scholar]
- Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–7. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. 1. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Mora LB, Buettner R, Seigne J, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- Horinaga M, Okita H, Nakashima J, Kanao K, Sakamoto M, Murai M. Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer. Urology. 2005;66:671–5. doi: 10.1016/j.urology.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003;9:370–6. [PubMed] [Google Scholar]
- Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther. 2004;3:11–20. [PubMed] [Google Scholar]
- Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, Patel HR. The metastatic cascade in prostate cancer. Surg Oncol. 2006;15:117–28. doi: 10.1016/j.suronc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Fuchs PA, Katayama N, Noda K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca(2+)-loaded brain neurons. Brain Res. 1994;635:125–9. doi: 10.1016/0006-8993(94)91431-1. [DOI] [PubMed] [Google Scholar]
- Su Y, Sun CM, Chuang HH, Chang PT. Studies on the cytotoxic mechanisms of ginkgetin in a human ovarian adenocarcinoma cell line. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:82–90. doi: 10.1007/s002100000240. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Choi JH, Son KH, Chang HW, Kang SS, Kim HP. Suppression of mouse lymphocyte proliferation in vitro by naturally-occurring biflavonoids. Life Sci. 1995;57:551–8. doi: 10.1016/0024-3205(95)00305-p. [DOI] [PubMed] [Google Scholar]
- Kim HK, Son KH, Chang HW, Kang SS, Kim HP. Inhibition of rat adjuvant-induced arthritis by ginkgetin, a biflavone from Ginkgo biloba leaves. Planta Med. 1999;65:465–7. doi: 10.1055/s-2006-960815. [DOI] [PubMed] [Google Scholar]
- Kwak WJ, Han CK, Son KH, et al. Effects of Ginkgetin from Ginkgo biloba Leaves on cyclooxygenases and in vivo skin inflammation. Planta Med. 2002;68:316–21. doi: 10.1055/s-2002-26742. [DOI] [PubMed] [Google Scholar]
- Kang SS, Kim JS, Kwak WJ, Kim KH. Flavonoids from the leaves of Ginkgo biloba, Korea. J Pharmacogn. 1990;21:111–20. [Google Scholar]
- Shin DS, Kim HN, Shin KD, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69:193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- Tsui KH, Lin YF, Chen YH, Chang PL, Juang HH. Mechanisms by which interleukin-6 regulates prostate-specific antigen gene expression in prostate LNCaP carcinoma cells. J Androl. 2011;32:383–93. doi: 10.2164/jandrol.109.009878. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Tremblay ML, DiGiovanni J. Protein tyrosine phosphatases, TC-PTP, SHP1, and SHP2, cooperate in rapid dephosphorylation of Stat3 in keratinocytes following UVB irradiation. PLoS One. 2010;5:e10290. doi: 10.1371/journal.pone.0010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lim MH, Chun IK, Won YH. Effects of flavonoids of Ginkgo biloba on proliferation of human skin fibroblast. Skin Pharmacol. 1997;10:200–5. doi: 10.1159/000211505. [DOI] [PubMed] [Google Scholar]
- Lim H, Son KH, Chang HW, Kang SS, Kim HP. Effects of anti-inflammatory biflavonoid, ginkgetin, on chronic skin inflammation. Biol Pharm Bull. 2006;29:1046–9. doi: 10.1248/bpb.29.1046. [DOI] [PubMed] [Google Scholar]
- You OH, Kim SH, Kim B, et al. Ginkgetin induces apoptosis via activation of caspase and inhibition of survival genes in PC-3 prostate cancer cells. Bioorg Med Chem Lett. 2013;23:2692–5. doi: 10.1016/j.bmcl.2013.02.080. [DOI] [PubMed] [Google Scholar]
- Niu G, Wright KL, Ma Y, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25:7432–40. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Zushi S, Shinomura Y, Kiyohara T, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–30. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Page B, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors. Expert Opin Ther Pat. 2011;21:66–83. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
- Costantino L, Barlocco D. STAT 3 as a target for cancer drug discovery. Curr Med Chem. 2008;15:334–43. doi: 10.2174/092986708783955464. [DOI] [PubMed] [Google Scholar]


