Abstract
Background and Purpose
Methcathinone (MCAT) is a potent monoamine releaser and parent compound to emerging drugs of abuse including mephedrone (4-CH3 MCAT), the para-methyl analogue of MCAT. This study examined quantitative structure–activity relationships (QSAR) for MCAT and six para-substituted MCAT analogues on (a) in vitro potency to promote monoamine release via dopamine and serotonin transporters (DAT and SERT, respectively), and (b) in vivo modulation of intracranial self-stimulation (ICSS), a behavioural procedure used to evaluate abuse potential. Neurochemical and behavioural effects were correlated with steric (Es), electronic (σp) and lipophilic (πp) parameters of the para substituents.
Experimental Approach
For neurochemical studies, drug effects on monoamine release through DAT and SERT were evaluated in rat brain synaptosomes. For behavioural studies, drug effects were tested in male Sprague-Dawley rats implanted with electrodes targeting the medial forebrain bundle and trained to lever-press for electrical brain stimulation.
Key Results
MCAT and all six para-substituted analogues increased monoamine release via DAT and SERT and dose- and time-dependently modulated ICSS. In vitro selectivity for DAT versus SERT correlated with in vivo efficacy to produce abuse-related ICSS facilitation. In addition, the Es values of the para substituents correlated with both selectivity for DAT versus SERT and magnitude of ICSS facilitation.
Conclusions and Implications
Selectivity for DAT versus SERT in vitro is a key determinant of abuse-related ICSS facilitation by these MCAT analogues, and steric aspects of the para substituent of the MCAT scaffold (indicated by Es) are key determinants of this selectivity.
Tables of Links
| TARGETS |
|---|
| Transporters |
| DAT, dopamine transporter, SLC6A3 |
| SERT, 5-HT transporter, SLC6A4 |
| LIGANDS | |
|---|---|
| Serotonin (5-HT) | Fenfluramine |
| Amphetamine | Methamphetamine |
| Chloroamphetamine | MPP+, 1-methyl-4-phenylpyridinium |
| Dopamine | Reserpine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Methcathinone (MCAT), the β-keto analogue of methamphetamine, is the parent compound of an emerging class of abused designer drugs (Glennon et al., 1987; Spiller et al., 2011; De Felice et al., 2014). Like its amphetamine analogue, MCAT functions as a monoamine releaser that selectively increases release of dopamine over that of serotonin (5-HT; Glennon et al., 1987; Cozzi et al., 1999; 2013; Baumann et al., 2012). Similarly, in behavioural studies, MCAT produces locomotor activation, stimulus generalization to amphetamine and methamphetamine in assays of drug discrimination, and abuse-related behavioural effects in assays of intracranial self-stimulation (ICSS) and drug self-administration (Kaminski and Griffiths, 1994; Young and Glennon, 1998; Cozzi et al., 2013; Bonano et al., 2014). Because of its amphetamine-like effects and its resultant high abuse potential, MCAT was assigned Schedule I status by the US Drug Enforcement Administration (DEA) in 1993 and Class B status under the UK Misuse of Drugs Act in 1998.
Mephedrone (4-CH3 MCAT) is the para-methyl analogue of MCAT and was recently popularized in Europe and the United States as one of the primary components of bath salts (Spiller et al., 2011; Vardakou et al., 2011; Zuba and Byrska, 2013; Hall et al., 2014). Because of its rapid rise to international notoriety as a designer drug of abuse, as well as its cocaine- and methamphetamine-like discriminative stimulus effects, 4-CH3 MCAT was added to the list of controlled drugs in both the United States and the United Kingdom in 2010–2011 (Gatch et al., 2013). However, despite its structural similarity to MCAT, only differing by substitution of a methyl group for the hydrogen at the para position on the aromatic ring of the MCAT scaffold, 4-CH3 MCAT produces distinct neurochemical and behavioural effects (Bonano et al., 2014; De Felice et al., 2014). For example, in comparison with MCAT, which has high in vitro selectivity to increase dopamine rather than 5-HT release, 4-CH3 MCAT has lower potency to release dopamine, higher potency to release 5-HT, and is less effective than MCAT in producing abuse-related behavioural effects (Baumann et al., 2012; Bonano et al., 2014). Methedrone (4-OCH3 MCAT) and flephedrone (4-F MCAT) are two other para-substituted MCAT analogues that have been identified by US Drug Courts in toxicology screens or in the international drug market (Prosser and Nelson, 2012; Leffler et al., 2014), and 4-F MCAT was recently assigned temporary Schedule I status by the DEA. With these compounds too, the identity of the para substituent appears to influence pharmacological activity. For example, 4-F MCAT has higher potency to release dopamine than 5-HT and produces robust locomotor activation in mice, whereas 4-OCH3 MCAT has higher potency to release 5-HT than dopamine and produces much weaker locomotor stimulation in mice (Marusich et al., 2012; Eshleman et al., 2013; Simmler et al., 2014). Taken together, this evidence suggests that research on structure–activity relationships of para-substituted MCAT analogues might be useful both to predict new drugs of abuse and to identify key structural features that contribute to abuse-related neurochemical and behavioural effects.
Accordingly, the goal of this study was to use quantitative structure–activity relationship (QSAR) analysis (Glennon and Young, 2011) to evaluate molecular determinants of abuse-related neurochemical and behavioural effects of MCAT and six para-substituted MCAT analogues. Figure 1 shows the chemical structure of the MCAT scaffold, which served as the base molecule for all analogues investigated in this study. The para substituent on the benzene ring of the MCAT scaffold, R, was manipulated to include substituents that varied systematically along three physicochemical dimensions [steric (Es), electronic (σp), and lipophilic (πp) ], and Table 1 shows quantitative measures for each substituent on each parameter. Taft's steric parameter Es was one of the first steric parameters employed in QSAR analyses and was derived by measuring rates of hydrolysis of various esters (Taft, 1952; 1953). Hydrolysis rates are dependent upon the contribution of both steric strain and steric hindrance; thus, this integration of steric parameters into a single metric (Es) provides a simple and useful tool for conducting preliminary QSAR analyses. Large Es values indicate low functional steric bulk of the substituent, and increasingly negative Es values indicate progressively greater magnitudes of steric bulk. The lipophilic value πp provides a measure of the degree to which the addition of a given substituent alters partitioning of the compound between aqueous and organic solvents. Thus, πp signifies the relative rate at which a compound penetrates lipid membranes compared with the unsubstituted parent compound (Cammarata and Rogers, 1971). Finally, the electronic value σp provides a measure of the change in free energy that accompanies formation of complexes between ligands (e.g. drugs) and their target proteins (e.g. monoamine transporters) (Yoshida et al., 2012). Substituents with positive σp values are electron-withdrawing, whereas substituents with negative σp values are electron-donating, and the absolute value of σp signifies the degree to which the substituent produces an electronic effect.
Figure 1.

Chemical structure of MCAT scaffold. R = site of para substituent, which was systematically varied to generate MCAT analogues.
Table 1.
Physicochemical parameters, in vitro release activities, and in vivo behavioural ICSS effects of MCAT and its para-substituted analogues
| Drug name | Physicochemical parametera | In vitro release EC50b | Maximal ICSS facilitationd | ||||
|---|---|---|---|---|---|---|---|
| Es | σp | πp | DAT (nM) | SERT (nM) | DAT selectivityc | ||
| MCAT | 1.24 | 0.00 | 0.00 | 12.5 ± 1.1 | 3,860 ± 520 | 309 | 191.9 |
| 4-F MCAT | 0.78 | 0.06 | 0.14 | 83.4 ± 6.0 | 1,290 ± 220 | 15.4 | 156.3 |
| 4-OCH3 MCAT | 0.69 | −0.27 | −0.02 | 506 ± 62 | 120 ± 18 | 0.24 | 110.9 |
| 4-Cl MCAT | 0.27 | 0.23 | 0.71 | 42.2 ± 5.2 | 144 ± 22 | 3.40 | 114.9 |
| 4-Br MCAT | 0.08 | 0.23 | 0.86 | 59.4 ± 5.1 | 60.2 ± 6.7 | 1.01 | 118.0 |
| 4-CH3 MCAT | 0.00 | −0.17 | 0.56 | 49.1 ± 8.3 | 118 ± 26 | 2.41 | 102.5 |
| 4-CF3 MCAT | −1.16 | 0.54 | 0.88 | 2,700 ± 300 | 190 ± 30 | 0.07 | 90.9 |
Physicochemical parameters of para substituent (functional steric bulk, Es; electron-withdrawing capacity, σp; lipophilicity, πp) as reported in Wolff (1980).
EC50 values derived from concentration–effect curves in Figure 2 or published previously for 4-CH3 MCAT (Baumann et al., 2012) and 4-CF3 MCAT (Cozzi et al., 2013).
DAT selectivity calculated as SERT EC50 ÷ DAT EC50.
The effects of each drug were examined on two experimental endpoints: (i) in vivo modulation of ICSS in rats, and (ii) in vitro potency to increase monoamine release through dopamine and 5-HT transporters (DAT and SERT, respectively) in rat brain synaptosomes. ICSS is one type of behavioural procedure that can be used to study abuse potential of monoamine releasers and other classes of drugs (Kornetsky and Esposito, 1979; Wise, 1996; Negus and Miller, 2014). In ICSS, subjects are trained to lever press for pulses of brain stimulation delivered via microelectrodes implanted in reward-related brain regions, such as the medial forebrain bundle. Many drugs of abuse increase (‘facilitate’) low ICSS rates maintained by low frequencies or intensities of brain stimulation; thus, ICSS facilitation is often interpreted as an abuse-related drug effect. ICSS results show substantial congruence with other preclinical measures of abuse potential, such as drug self-administration and conditioned place preference (Vlachou and Markou, 2011; Negus and Miller, 2014), and ICSS also has the ability to discriminate between abuse-related and abuse-limiting drug effects in a single procedure. In vitro measures of drug-induced monoamine release were determined as a correlate to behavioural data because previous results with a different set of monoamine releasers indicated a correlation between in vitro selectivity for DAT versus SERT and in vivo efficacy to produce an abuse-related facilitation of ICSS in rats (Bauer et al., 2013). QSAR analysis was accomplished by correlating measures for each physicochemical parameter with data from neurochemical and behavioural studies. We predicted that in vitro selectivity for DAT over SERT would correlate with maximal ICSS facilitation, and that at least one of the physicochemical parameters would correlate with both DAT selectivity and maximal ICSS facilitation. Our results supported this hypothesis and suggested that the ‘steric bulk’ of the para substituent, as quantified by Taft's steric parameter (Es), was a key determinant of abuse-related neurochemical and behavioural effects of these para-substituted MCAT analogues.
Methods
In vitro release assays
Animals
All animal care was carried out in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and experimental procedures were carried out in accordance with the Institutional Animal Care and Use Committee and the National Institutes of Health guidelines on care and use of animal subjects in research (National Research Council, 2011). Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 15 rats were used in the in vitro experiments described here and another 33 rats were used in the ICSS experiments described in the succeeding text. Male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 250–350 g were housed three per cage with free access to food and water and maintained on a 12 h light/dark cycle with lights on from 07:00 to 19:00 h.
Procedure
Rats were killed by CO2 narcosis and brains were processed to yield synaptosomes as previously described (Rothman et al., 2003). For release assays, 9 nM [3H]1-methyl-4-phenylpyridinium ( [3H]MPP+) was used as the radiolabelled substrate for DAT, whereas 5 nM [3H]5-HT was used as a substrate for SERT. All buffers used in the release assay methods contained 1 μM reserpine to block vesicular uptake of substrates. The selectivity of release assays was optimized for a single transporter by including unlabelled blockers to prevent the uptake of [3H]MPP+ or [3H]5-HT by competing transporters. Synaptosomes were preloaded with radiolabelled substrate in Krebs-phosphate buffer for 1 h (steady state). Release assays were initiated by adding 850 μL of preloaded synaptosomes to 150 μL of test drug. Release was terminated by vacuum filtration, and retained radioactivity was quantified by liquid scintillation counting. Effects of test drug concentrations were expressed as percentage of maximum release, with maximum release (i.e. 100% Emax) was defined as the release produced by 10 nM tyramine for DAT and NET assay conditions, and 100 nM tyramine for SERT assay conditions. These doses of tyramine evoke the efflux of all ‘releasable’ tritium from synaptosomes.
Earlier studies have used these procedures to evaluate MCAT, 4-CH3 MCAT, and 4-CF3 MCAT. For this study, MCAT effects were redetermined, and effects of the additional analogues 4-F MCAT, 4-OCH3 MCAT, 4-Cl MCAT and 4-Br MCAT were also determined. Correlational analysis described later used data from the present study and data for 4-CH3 MCAT and 4-CF3 MCAT from earlier studies (Baumann et al., 2012; Cozzi et al., 2013).
Data analysis
Statistical analyses were carried out using GraphPad Prism (v. 6.0; GraphPad Scientific, San Diego, CA, USA). EC50 values for stimulation of release were calculated based on non-linear regression analysis. Selectivity for DAT over SERT was also calculated as SERT EC50 ÷ DAT EC50, such that larger ratios indicate greater selectivity for DAT.
ICSS
Preparation of animals
Male Sprague-Dawley rats (Harlan, Frederick, MD, USA) weighing at least 300 g at the time of surgery were individually housed and maintained on a 12 h light/dark cycle with lights on from 06:00 to 18:00 h. Rats had free access to food and water except during testing.
Rats were anaesthetized with isoflurane (3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) until unresponsive to toe pinch, prior to implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode, which was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, was stereotaxically implanted into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull). Three screws were placed in the skull, and the uninsulated anode was wrapped around one of the skull screws to serve as the ground. Dental acrylic secured the skull screws and electrode. Ketoprofen (5 mg kg−1) was used for post-operative analgesia immediately and 24 h after surgery. Animals were allowed at least 7 recovery days prior to commencing ICSS training. Experiments were conducted in sound-attenuating boxes that contained modular acrylic and metal test chambers equipped with a response lever, three stimulation lights positioned above the lever, a 2 W house light, and an ICSS stimulator (Med Associates, St. Albans, VT, USA). Electrodes were connected to the stimulator via a swivel commutator (Model SL2C, Plastics One, Roanoke, VA, USA). Operation of equipment and data collection were controlled by Med-PC IV computer software (Med Associates).
Training
Rats were trained and tested under a fixed-ratio 1 (FR 1) schedule of brain stimulation using a behavioural procedure identical to that previously described (Bonano et al., 2014). During behavioural sessions, each lever press resulted in the delivery of a 0.5 s train of square wave cathodal pulses (0.1 ms pulse duration). Brain stimulation was accompanied by illumination of the stimulus lights over the lever, and additional responding within the 0.5 s stimulation period did not earn additional stimulation. Stimulation intensity was set at 150 μA and stimulation frequency was held constant at 126 Hz during initial 60 min training sessions. Stimulation intensity was then individually adjusted for each rat to the lowest value that sustained a high rate of reinforcement (>30 stimulations per min). This intensity (100–290 μA across rats) was then held constant for the remainder of the study, and frequency manipulations were introduced. Sessions involving frequency manipulations consisted of three sequential 10 min components. During each component, a descending series of 10 frequencies (2.2–1.75 log Hz in 0.05 log increments) was presented, with each frequency available for a 1 min trial. Each frequency trial began with a 10 s time out, during which responding had no scheduled consequences, and five non-contingent ‘priming’ stimulations were delivered at the frequency of stimulation that would be available during that trial. This time out was then followed by a 50 s response period, during which responding produced electrical stimulation under a FR 1 schedule as described earlier. Training continued until rats reliably responded for only the first three to six frequency trials of each component over a period of at least 3 consecutive training days.
Testing
The MCAT analogues tested in this study were 4-F MCAT, 4-OCH3 MCAT, 4-Cl MCAT, 4-Br MCAT and 4-CF3 MCAT. Two additional compounds, MCAT and 4-CH3 MCAT, were studied previously (Bonano et al., 2014), and data from those studies were included in correlational analyses described later. Each drug was studied in dose–effect and time–course procedures. In dose–effect studies, a 1.0–1.5 log unit range of doses was tested for each drug with the goal of testing a dose range from a low dose that produced little or no effect to a high dose that produced maximal facilitation of ICSS. For these studies, test sessions consisted of three sequential ‘baseline’ components followed first by a 30 min time out period and then by three sequential ‘test’ components. A single dose of test drug was administered i.p. at the beginning of the time out period. In time–course studies, the highest dose of each compound was tested. Test sessions consisted of three consecutive baseline components followed by immediate drug injection and then by pairs of consecutive test components beginning 10, 30, 100 and 300 min after drug injection. Test sessions were completed on Tuesdays and Fridays, and three-component training sessions were conducted on all other weekdays. The order of testing with vehicle and drug doses was varied across subjects using a Latin-square design, and experiments with any single compound were completed prior to beginning tests with another compound. Furthermore, tests with different drugs in a single rat were separated by at least 1 week, and a saline vehicle test session was conducted during this period to ensure that injections or drug exposures did not alter individual ICSS baselines.
Data analysis
ICSS data were analysed as described previously (Bauer et al., 2013; Negus and Miller, 2014). The primary dependent variable in this ICSS procedure was the reinforcement rate in stimulations per minute during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percentage of maximum control rate (%MCR), with the MCR defined as the mean of the maximal rates observed during the second and third baseline components for any given session in any given rat. Thus, %MCR values for each trial were calculated as %MCR = (reinforcement rate during a frequency trial ÷ maximum control rate) × 100. For each experimental manipulation, data from all test components for each dose or time point were averaged first within each rat and then across rats to yield mean test curves for each manipulation. Results from test sessions were compared by repeated measures two-way anova, with ICSS frequency as one factor and dose or time as the second factor. A significant anova was followed by the Holm–Sidak post hoc test with the criterion for significance set at P < 0.05.
As a summary measure of drug effects on ICSS, the total number of stimulations delivered per component across all 10 frequency trials was also calculated for each dose and time point. Test data were normalized to individual baseline data using the equation % baseline total stimulations per component = (mean total stimulations per test component ÷ mean total stimulations per baseline component) × 100. Data were then averaged across rats in each experimental condition.
Correlational analysis
Correlations were evaluated between in vitro and in vivo drug effects using linear regression and a Pearson correlation test as described previously for a different set of monoamine releasers (Bauer et al., 2013). Specifically, in vitro selectivity to increase DAT- versus SERT-mediated release was compared with maximal in vivo facilitation of ICSS (defined as the maximum increase in percentage baseline total stimulations per component produced by any dose of a given drug). Correlational analysis was also used to assess QSAR between physicochemical features and the in vitro and in vivo effects of each drug (Glennon and Young, 2011). Specifically, steric (Es), electronic (σp) and lipophilic (πp) constants of para substituents on the MCAT scaffold (Wolff, 1980) were compared with in vitro potency for DAT- and SERT-mediated release, in vitro DAT versus SERT selectivity, and maximal in vivo facilitation of ICSS. The measure used to represent steric bulk of para substituents in this study was Taft's steric parameter, Es, which was developed to reflect the steric influence of substituents on the rate of hydrolysis; thus, Taft's steric constant represents a functional measure of steric bulk. Correlations and statistical analyses were carried out using Prism 6.0 (GraphPad Scientific, San Diego, CA, USA), and correlations were considered statistically significant if P < 0.05.
Materials
This study examined effects of MCAT and six analogues with different substitutions at the para, or 4- position on the phenyl ring. For MCAT, the para substituent is hydrogen, and for the purposes of this study, the MCAT analogues are designated using the nomenclature ‘4-R MCAT,’ with ‘R’ being the substitution for hydrogen at the para position. In some cases, these compounds also have other generic names or other chemical names based on the abbreviation ‘MAP’ (for methylaminopropiophenone, the chemical name for MCAT), and all compounds were synthesized as their racemic HCl salts using previously published procedures. The alternative names and published syntheses for the compounds studied are as follows: MCAT (Findlay et al., 1981), 4-F MCAT (flephedrone; Archer, 2009), 4-OCH3 (methedrone; Lespagnol and Hallot, 1954), 4-Cl MCAT (Trepanier and Sprancmanis, 1964), 4-Br MCAT (4-BMAP; Foley and Cozzi, 2003), 4-CH3 MCAT (mephedrone; McDermott et al., 2011) and 4-CF3 MCAT (4-TFMAP; Cozzi et al., 2013). All compounds were dissolved in sterile saline for i.p. injection. [3H]5-HT (specific activity = 30 Ci mmol−1) was purchased from Perkin Elmer (Shelton, CT, USA). [3H]MPP+ (specific activity = 85 Ci mmol−1) was purchased from American Radiolabeled Chemicals (St. Louis, MO, USA). All other chemicals and reagents were acquired from Sigma-Aldrich (St. Louis, MO, USA).
Results
In vitro monoamine release mediated by DAT and SERT
All seven compounds considered in this study produced concentration-dependent increases in DAT-mediated [3H]MPP+ release and SERT-mediated [3H]5-HT release from rat brain synaptosomes. Data for 4-CH3 MCAT and 4-CF3 MCAT have been published previously (Baumann et al., 2012; Cozzi et al., 2013), and Figure 2 shows data for the other five compounds. Table 1 shows EC50 values for each compound to increase DAT- and SERT-mediated monoamine release. Selectivity for inhibition of DAT over SERT is also reported for each compound. This selectivity varied across a range of more than 4000-fold, with MCAT functioning as the most DAT-selective compound and 4-CF3 MCAT functioning as the most SERT-selective compound.
Figure 2.
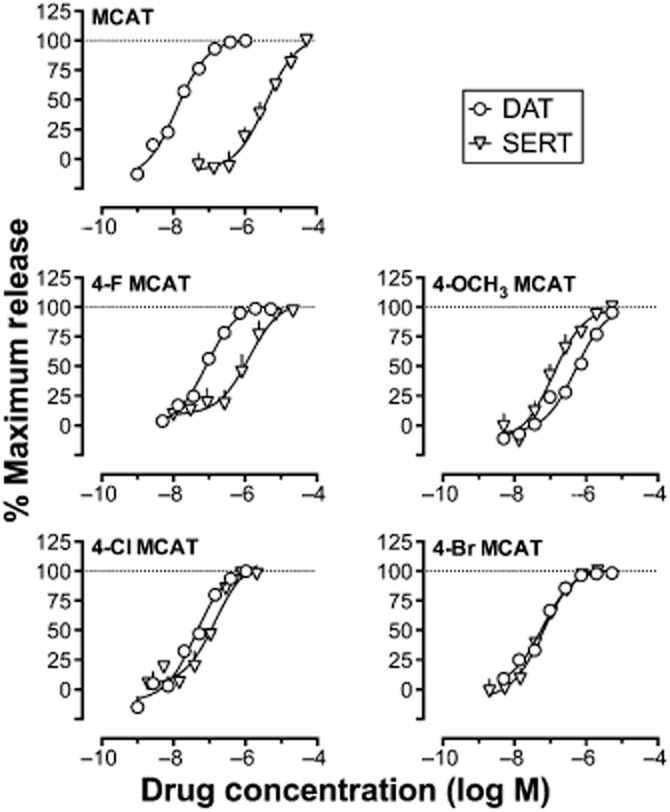
Effects of test drugs on DAT- and SERT-mediated monoamine release in rat brain synaptosomes. Abscissae: log concentration of drug (molar). Ordinates: percentage of maximum release. All points show mean ± SD for n = 3 separate experiments. EC50 values shown in Table 1 were derived from these concentration–effect curves.
ICSS
Electrical brain stimulation maintained a frequency-dependent increase in ICSS rates under baseline conditions. Across the 33 rats used in these studies, the average ± SEM baseline MCR was 61 ± 2 stimulations per trial, and the mean ± SEM number of total baseline stimulations across all frequencies was 291 ± 14 stimulations per component.
All seven compounds considered in this study produced dose-dependent changes in ICSS. Data for MCAT and 4-CH3 MCAT have been reported previously (Bonano et al., 2014). Figure 3 shows dose–effect data for the other five compounds. Two-way anova indicated significant main effects of frequency and dose and significant frequency × dose interactions for all drugs. Only the interaction effects are reported below for each drug. 4-F MCAT [F(36,144) = 10.54, P < 0.0002] produced dose-dependent facilitation of low ICSS rates maintained by low brain stimulation frequencies with no evidence at these doses and pretreatment times of depression of high ICSS rates maintained by high brain stimulation frequencies. 4-OCH3 MCAT [F(36,180) = 5.55, P < 0.0001], 4-Cl MCAT [F(36,180) = 7.31, P < 0.0001], and 4-Br MCAT [F(36,180) = 5.30, P < 0.0001] also produced facilitation of low ICSS rates maintained by low stimulation frequencies; however, higher doses of these compounds also significantly decreased high ICSS rates. Lastly, 4-CF3 MCAT [F(27,135) = 1.69, P = 0.027] produced exclusive depression of high ICSS rates maintained by high brain stimulation frequencies.
Figure 3.
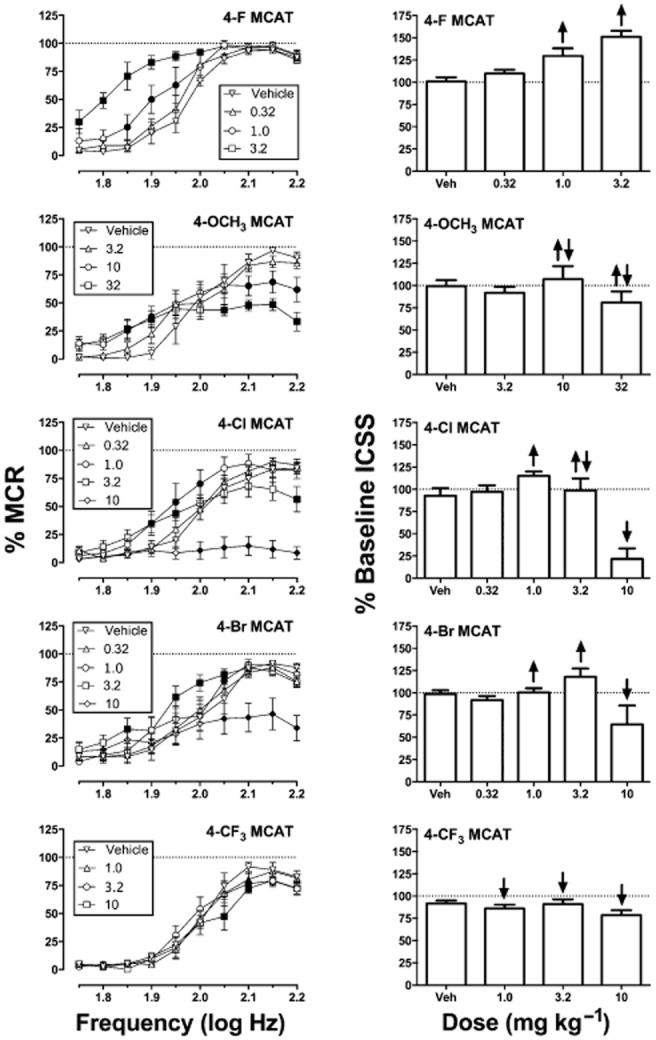
Effects of test drugs on ICSS in rats. Left panels show drug effects on full ICSS frequency–rate curves. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percentage of maximum control reinforcement rate (% MCR). Drug doses are indicated in units of mg kg−1. Filled symbols represent frequencies at which reinforcement rates were statistically different from vehicle rates as determined by two-way anova followed by Holm-Sidak post hoc test, P < 0.05. Right panels show summary ICSS data for drug effects across all frequencies. Abscissae: drug dose in mg kg−1. Ordinates: percentage of baseline number of stimulations per component delivered across all brain stimulation frequencies. Upward/downward arrows indicate significant drug-induced increases/decreases in ICSS, relative to vehicle, for at least one brain stimulation frequency as determined by analysis of full frequency–rate curves. All data show mean ± SEM for five rats (4-F MCAT) or six rats (all other drugs). Maximal ICSS facilitation values shown in Table 1 were taken from the right panels for each drug.
Figure 4 shows the time course of effects produced by the highest tested dose of each compound. Two-way anova indicated significant main effects of frequency and time and significant frequency × time interactions for all drugs, and only the interaction effects are reported later for each drug. 4-F MCAT {3.2 mg·kg−1; [F(36,144) = 5.28, P < 0.0001] } produced facilitation of ICSS within 10 min after administration, and significant ICSS rate-increasing effects were no longer apparent by 300 min. 4-OCH3 MCAT {32 mg kg−1; [F(36,144) = 8.34, P < 0.0001] } produced only depression of ICSS after 10 min, but both rate-increasing and rate-decreasing effects were apparent after 30 and 100 min. 4-Cl MCAT {10 mg kg−1; [F(36,180) = 7.10, P < 0.0001] } and 4-Br MCAT {10 mg kg−1; [F(45,225) = 4.25, P < 0.0001] } produced rate-decreasing effects that peaked after 10 min and were still significant after 300 min. Rate-increasing effects were apparent only 300 min after administration of this high dose of 4-Cl MCAT and at no time after 4-Br MCAT. 4-CF3 MCAT {10 mg kg−1; [F(36,180) = 1.96, P = 0.002] } produced exclusive depression of ICSS from 10 to 100 min, and these effects were no longer apparent after 300 min.
Figure 4.
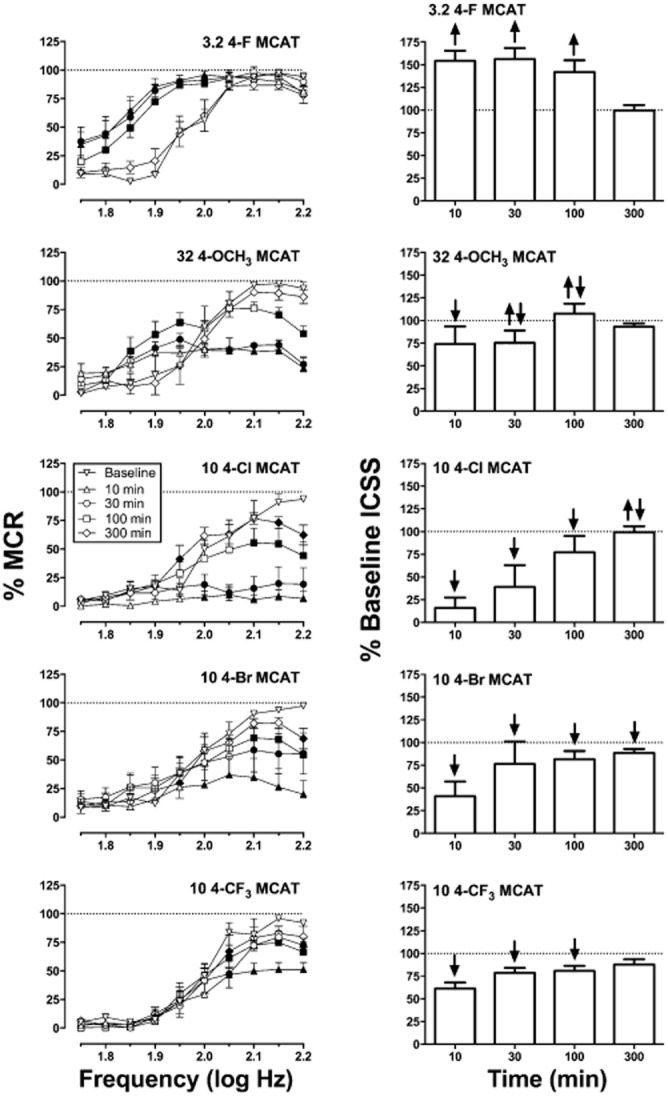
Time courses of drug effects on ICSS in rats. The title of each panel shows the drug and test dose in units of mg kg−1. Left panels show full ICSS frequency–rate curves. Time points are indicated in the key in units of minutes. Filled symbols represent frequencies at which reinforcement rates were statistically different from baseline rates as determined by two-way anova followed by Holm-Sidak post hoc test, P < 0.05. Right panels show summary ICSS data for drug effects across all frequencies. Abscissae: time after treatment in min. All data show mean ± SEM for five rats (4-OCH3 MCAT and 4-F MCAT) or six rats (all other drugs). Other details as in Figure 3.
Correlational analysis
Figure 5 shows that in vitro selectivity for DAT- versus SERT-mediated release correlated with in vivo efficacy to facilitate ICSS (Figure 5A). Figure 5, together with Table 2, also shows results of QSAR analysis. Significant correlations were found between steric bulk (Es) and both in vitro selectivity for DAT versus SERT (Figure 5B) and in vivo efficacy to facilitate ICSS (Figure 5C). For both of these QSAR correlations, the most striking outlier was 4-OCH3 MCAT, which had lower DAT selectivity and produced weaker ICSS facilitation than would have been predicted based on the correlation with Es. There was no significant correlation between steric bulk and in vitro potency at either DAT or SERT individually, and lipophilicity (πp) and electronic (σp) parameters did not correlate significantly with any in vitro or in vivo endpoint (Table 2).
Figure 5.
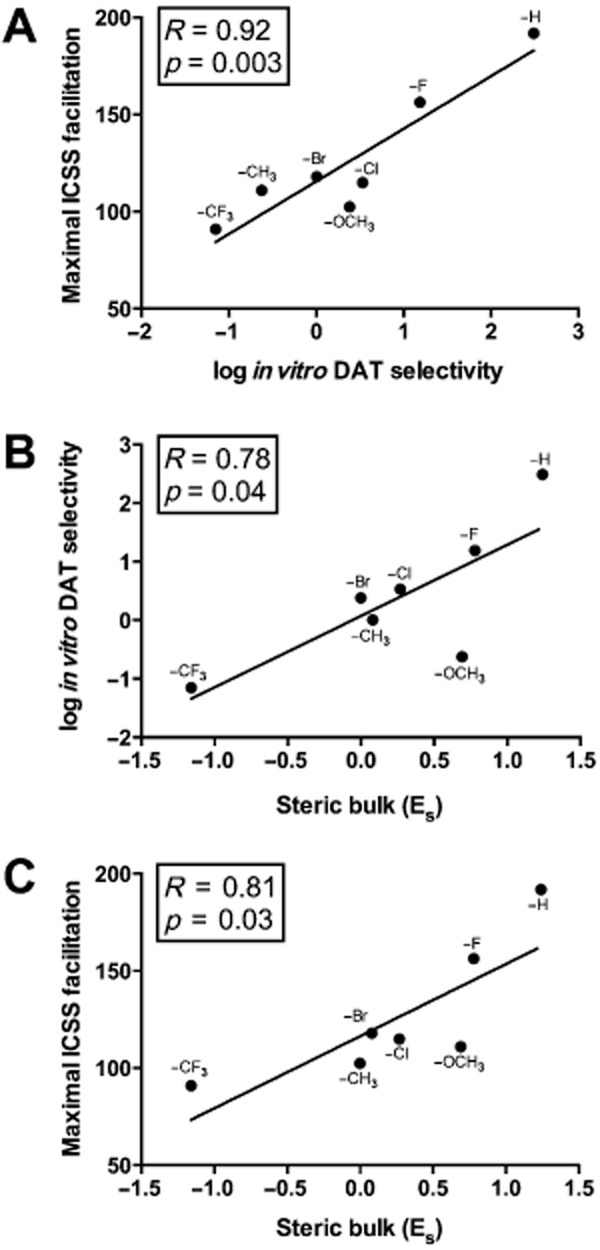
Correlational analysis across experimental endpoints and structural parameters from Table 1. Panel A shows the correlation between in vitro selectivity for DAT- versus SERT-mediated monoamine release (abscissa) and maximum in vivo ICSS facilitation (ordinate). Panel B shows the correlation between steric bulk (Es, abscissa) and in vitro selectivity for DAT- versus SERT-mediated monoamine release (ordinate). Panel C shows the correlation between steric bulk (Es, abscissa) and maximum in vivo ICSS facilitation (ordinate). All points show mean data for five to six rats.
Table 2.
Results of correlational analysis between physicochemical parameters, in vitro potencies at DAT and SERT, and maximal ICSS facilitation
| Physicochemical parametera | Log DAT EC50 | Log SERT EC50 | Log DAT selectivity | Maximal ICSS facilitation |
|---|---|---|---|---|
| Es | R = −0.71 | R = 0.58 | R = 0.78* | R = 0.81* |
| P = 0.07 | P = 0.17 | P = 0.04 | P = 0.03 | |
| σp | R = 0.37 | R = −0.10 | R = −0.29 | R = −0.24 |
| P = 0.42 | P = 0.84 | P = 0.53 | P = 0.61 | |
| πp | R = 0.26 | R = −0.64 | R = −0.52 | R = −0.66 |
| P = 0.57 | P = 0.12 | P = 0.23 | P = 0.11 |
Significant correlation.
Functional steric bulk, Es; electron-withdrawing capacity, σp; lipophilicity, πp. Bold styling is used to emphasize the significant correlations between Es and (i) log DAT selectivity and (ii) maximal ICSS facilitation.
Discussion and conclusions
This study compared in vitro neurochemical and in vivo behavioural effects produced by MCAT and six para-substituted analogues. There were three main findings. First, all seven compounds functioned as substrates for both DAT and SERT, and selectivity for DAT versus SERT varied >4000-fold across compounds. Second, all drugs dose-dependently altered ICSS, and efficacy to produce an abuse-related facilitation of ICSS also varied across compounds. In agreement with a previous study using a different set of compounds (Bauer et al., 2013), in vitro selectivity for DAT versus SERT correlated with in vivo efficacy to facilitate ICSS. Finally, QSAR analysis identified a significant correlation between the steric parameter (Es) of the para substituents and both in vitro DAT versus SERT selectivity and in vivo facilitation of ICSS. These results suggest that steric bulk of the para substituent of the MCAT scaffold is a key determinant of both in vitro selectivity for DAT versus SERT and in vivo expression of abuse-related behavioural effects in an ICSS procedure.
Drug effects on in vitro monoamine release
The present results confirm and extend previous research showing that MCAT and many of its analogues release monoamines through DAT and SERT but differ in their selectivity for DAT versus SERT. Thus, results with MCAT in this study were similar to results reported previously using the same in vitro procedures with rat brain homogenates (DAT EC50 = 20 ± 3 nM; SERT EC50 = 4 ± 1 μM; selectivity for DAT over SERT = 400; Cozzi et al., 2013). Results here are also consistent with a previous study using HEK cells expressing human transporters, which found higher selectivity for DAT with MCAT than with 4-F MCAT (Eshleman et al., 2013). 4-CH3 MCAT was slightly more selective for DAT than 4-F MCAT in the study with human transporters expressed in HEK cells (Eshleman et al., 2013), which contrasts with results in rat brain homogenates (Baumann et al., 2012; present study) and suggests some potential for species differences in 4-CH3 MCAT effects between human and rat transporters. However, these studies differed in other procedural variables as well. Finally, the SERT selectivity of 4-CF3 MCAT agrees with the SERT selectivity of fenfluramine (Rothman et al., 2001), which has a trifluoromethyl substituent on the phenyl ring of a phenethylamine scaffold closely related to the MCAT scaffold used here.
The present study extends these previous results by including 4-OCH3 MCAT and two halogenated MCAT analogues, 4-Cl MCAT and 4-Br MCAT. Like 4-CH3 MCAT, all three compounds had roughly similar potencies for increasing monoamine release through DAT and SERT, and all three compounds had lower selectivity for DAT versus SERT than 4-F MCAT. Consistent with the present results with halogenated MCAT analogues, previous studies with halogenated amphetamine analogues also showed that para-fluoroamphetamine had greater selectivity for DAT and more robust amphetamine-like behavioural effects than para-chloroamphetamine (Marona-Lewicka et al., 1995; Baumann et al., 2011).
Drug effects on ICSS
The present ICSS results confirm and extend previous research showing that MCAT and many of its analogues modulate ICSS, but differ in the degree to which they facilitate low rates of ICSS and/or depress high rates of ICSS. We showed previously that MCAT, which has high selectivity for DAT versus SERT, produced exclusive facilitation of ICSS across a broad range of doses (Bonano et al., 2014), and the present study found that 4-F MCAT, which also has moderate selectivity for DAT, produced a similar profile of effects. MCAT and 4-F MCAT also produced similar locomotor-activating effects in mice (Marusich et al., 2012) and fully substituted for the discriminative stimulus effects of stimulant drugs of abuse, such as cocaine and methamphetamine, in both rats and rhesus monkeys (Gatch et al., 2013; Kohut et al., 2013). Conversely, 4-CF3 MCAT, a SERT-selective compound, produced exclusive depression of ICSS, a profile of effects which parallels the effects previously reported for fenfluramine, another 5-HT-selective releaser (Bauer et al., 2013). Lastly, 4-CH3 MCAT, which is a relatively nonselective monoamine releaser, has been reported to produce mixed effects on ICSS consisting of both facilitation of low ICSS rates and depression of high ICSS rates (Robinson et al., 2012; Bonano et al., 2014). In the present study, similar profiles of mixed ICSS effects were also produced by the other MCAT analogues 4-OCH3 MCAT, 4-Cl MCAT and 4-Br MCAT, which also have low selectivity for DAT. These effects on ICSS are consistent with the relatively weak magnitude of locomotor stimulation reported in rodents for 4-OCH3 MCAT (Marusich et al., 2012) and 4-Br MCAT (Foley and Cozzi, 2003).
Correlational analysis confirmed a significant positive correlation between in vitro selectivity for DAT versus SERT and ICSS facilitation, and the correlation obtained with this set of MCAT analogues agrees with a similar correlation between selectivity for DAT and ICSS facilitation produced by a structurally distinct set of amphetamine-based monoamine releasers (Bauer et al., 2013). Insofar as magnitude of ICSS facilitation is predictive of abuse potential (Negus and Miller, 2014), these results suggest that selectivity for DAT versus SERT is a key determinant of abuse potential for a wide range of monoamine releasers.
QSAR
To explore molecular mechanisms that contribute to monoamine releaser abuse potential, this study employed QSAR analysis to evaluate structural determinants of both selectivity for DAT versus SERT and abuse-related ICSS effects. Of the physicochemical parameters evaluated (steric bulk, Es; electron-withdrawing capacity, σp; lipophilicity, πp), only Es correlated with both selectivity for DAT and ICSS facilitation, suggesting that steric attributes of the para substituent play a key role in determining selectivity of releasers for DAT versus SERT. More specifically, insofar as large Es values indicate low functional steric bulk of the substituent, and increasingly negative Es values indicate progressively greater magnitudes of steric bulk, these results suggest that small substituents that produce little steric hindrance promote selectivity for DAT, whereas larger substituents that produce greater steric hindrance promote selectivity for SERT. Additional aspects of steric hindrance that underlie selectivity for DAT versus SERT and ICSS behavioural effects are considered in the companion paper, together with molecular models that explore structural interactions that might account for interactions between MCAT analogues and both DAT and SERT (Sakloth et al., 2014).
In contrast to the significant correlations obtained for Es values, neither lipophilic (πp) nor electronic (σp) parameters of the para substituent correlated with in vitro or in vivo drug effects. The non-significant correlations of these values with DAT versus SERT selectivity or with ICSS facilitation suggest that, within the ranges studied here, these parameters are less important than steric hindrance as determinants of abuse-related neurochemical and behavioural effects of MCAT analogues. However, more extreme lipophilic and/or electronic values beyond these ranges might influence neurochemical and behavioural effects, and lipophilic and electronic parameters might also influence other aspects of pharmacology, such as pharmacokinetics.
Altogether, QSAR analysis of these seven MCAT analogues provided evidence for a key role of steric bulk of the para substituent in influencing drug interaction with monoamine transporters. Results from this study support our hypothesis that MCAT analogues containing para substituents with low steric bulk display DAT selectivity, produce abuse-related facilitation of ICSS, and have high abuse potential. Conversely, MCAT analogues containing para substituents with greater steric bulk display SERT selectivity, produce abuse-limiting depression of ICSS, and have lower clinical abuse potential.
Acknowledgments
This research was supported by the National Institutes of Health grants F30DA037649 (J. S. B.), R01DA033930 (R. A. G. and S. S. N.), R21DA017675 (N. V. C.) and by the National Institute on Drug Abuse, Intramural Research Programme Grant DA000523-07 (M. H. B.).
Glossary
Abbreviations
- 4-CH3 MCAT
mephedrone
- 4-F MCAT
flephedrone
- 4-OCH3 MCAT
methedrone
- 5-HT
serotonin
- ICSS
intracranial self-stimulation
- MCAT
methcathinone
- QSAR
quantitative structure–activity relationship
Author contributions
J. S. B. completed ICSS studies and data analyses and assumed primary responsibility for compiling and writing the paper. All other authors contributed to manuscript preparation and review of initial drafts. In addition, R. A. G., R. K., F. S. and M. L. Barnier synthesized compounds for study (MCAT, 4-F MCAT, 4-OCH3 MCAT, 4-Cl MCAT, 4-Br MCAT, 4-CH3 MCAT) and oversaw conduct and interpretation of QSAR analysis. N. V. C. synthesized 4-CF3 MCAT for study. J. S. P. and M. H. B. completed in vitro release assays and provided methods and results for in vitro studies. M. L. Banks and S. S. N. aided in experimental design.
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer RP. Fluoromethcathinone, a new substance of abuse. Forensic Sci Int. 2009;185:10–20. doi: 10.1016/j.forsciint.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, Felice LJD, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic ‘bath salts’ cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata A, Rogers KS. Electronic representation of the lipophilic parameter pi. J Med Chem. 1971;14:269–274. doi: 10.1021/jm00286a001. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, 3rd, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Brandt SD, Daley PF, Partilla JS, Rothman RB, Tulzer A, et al. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. Eur J Pharmacol. 2013;699:180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97:20–26. doi: 10.1016/j.lfs.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JW, Warren JT, Hill JA, Welch RM. Stereospecific radioimmunoassays for d-pseudoephedrine in human plasma and their application to bioequivalency studies. J Pharm Sci. 1981;70:624–631. doi: 10.1002/jps.2600700613. [DOI] [PubMed] [Google Scholar]
- Foley KF, Cozzi NV. Novel aminopropiophenones as potential antidepressants. Drug Dev Res. 2003;60:252–260. [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug discrimination and in vivo structure–activity relationships. In: Glennon RA, Young R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. New York: Wiley; 2011. pp. 163–165. [Google Scholar]
- Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Hall C, Heyd C, Butler C, Yarema M. Bath salts’ intoxication: a new recreational drug that presents with a familiar toxidrome. CJEM. 2014;16:171–176. doi: 10.2310/8000.2013.131042. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav. 1994;47:981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Blough BE, Rothman RB, Mello NK. Effects of methcathinone and 3-Cl-methcathinone (PAL-434) in cocaine discrimination or self-administration in rhesus monkeys. Int. J. Neuropsychopharmacol. 2013;16:1985–1998. doi: 10.1017/S146114571300059X. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Leffler AM, Smith PB, Armas A, de Dorman FL. The analytical investigation of synthetic street drugs containing cathinone analogs. Forensic Sci Int. 2014;234:50–56. doi: 10.1016/j.forsciint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Lespagnol A, Hallot J. Preparation of p-methoxyephedrone. 1954. pp. 45–46. Bulletin de la Societe de Pharmacie de Lille.
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE. Psychostimulant-like effects of p-fluoroamphetamine in the rat. Eur J Pharmacol. 1995;287:105–113. doi: 10.1016/0014-2999(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in ‘bath salts’ on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott SD, Power JD, Kavanagh P, O'Brien J. The analysis of substituted cathinones. Part 2: an investigation into the phenylacetone based isomers of 4-methylmethcathinone and N-ethylcathinone. Forensic Sci Int. 2011;212:13–21. doi: 10.1016/j.forsciint.2011.06.030. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J. Med. Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234:76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Clark RD, Partilla JS, Baumann MH. (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 2003;305:1191–1199. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- Sakloth F, Kolanos R, Mosier PD, Bonano JS, Banks ML, Partilla JS, et al. Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol. 2014 doi: 10.1111/bph.13043. doi: 10.1111/bph.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of ‘bath salts’ and ‘legal highs’ (synthetic cathinones) in the United States. Clin. Toxicol. (Phila.) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Taft RW. Linear free energy relationships from rates of esterification and hydrolysis of aliphatic and ortho-substituted benzoate esters. J Am Chem Soc. 1952;74:2729–2732. [Google Scholar]
- Taft RW. Linear steric energy relationships. J Am Chem Soc. 1953;75:4538–4539. [Google Scholar]
- Trepanier DL, Sprancmanis V. 5,6-Dihydro-4H-1,3,4-oxadiazines. II. Structural requirements for effective hydrazido-hydroxyl interaction. J Org Chem. 1964;29:673–677. [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201:191–195. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. In: Olmstead MC, editor. Animal Models of Drug Addiction. Totowa, NJ: Humana Press; 2011. pp. 3–56. [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wolff ME. The quantitative analysis of structure–activity relationships. In: Wolff ME, editor. Burger's Medicinal Chemistry. 4th edn. New York: Wiley; 1980. pp. 393–418. [Google Scholar]
- Yoshida T, Shimizu M, Harada M, Hitaoka S, Chuman H. Reassessment of Hammett σ as an effective parameter representing intermolecular interaction energy-links between traditional and modern QSAR approaches. Bioorg Med Chem Lett. 2012;22:124–128. doi: 10.1016/j.bmcl.2011.11.047. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus effects of S(−)-methcathinone (CAT): a potent stimulant drug of abuse. Psychopharmacology (Berl) 1998;140:250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]
- Zuba D, Byrska B. Prevalence and co-existence of active components of ‘legal highs’. Drug Test. Anal. 2013;5:420–429. doi: 10.1002/dta.1365. [DOI] [PubMed] [Google Scholar]


