Abstract
Background and Purpose
Schizandrin (SCH) has been reported to prevent or reduce learning and memory defects. However, it is not known whether SCH ameliorates cognitive impairments induced by oestrogen deficiency. In the present study, we investigated the effect of SCH on memory in ovariectomized (OVX) and non-OVX rats.
Experimental Approach
A passive avoidance test was used to evaluate the effect of SCH on memory. Field EPSPs were recorded in hippocampal slices using an electrophysiological method. In OVX rats, biochemical parameters in the bilateral hippocampus were measured; these included superoxide dismutase (SOD), malondialdehyde (MDA) and AChE. Also, the number of NADPH-diaphorase (NADPH-d) positive neurons was counted by NADPH-d histochemistry staining technique.
Key Results
Oral SCH improved the memory and facilitated the induction of long-term potentiation in non-OVX and OVX rats; this effect was more obvious in OVX rats. Similarly, SCH perfusion enhanced synaptic transmission in hippocampal slices from both non-OVX and OVX rats. However, SCH perfusion reduced the ratio of paired-pulse facilitation only in OVX but not in non-OVX rats. In addition, SCH decreased AChE activity and MDA level and increased SOD activity and the number of NADPH-d-positive neurons in OVX rats.
Conclusions and Implications
SCH improves memory in OVX rats and its potential mechanisms may include a reduction in the loss of hippocampal NADPH-d positive neurons, an increase of antioxidant properties and a potentiation of synaptic transmission that possibly involves to enhance cholinergic function. Overall, our findings indicate that SCH has potential as a therapeutic strategy for the cognitive dysfunctions associated with the menopause.
Tables of Links
| TARGETS |
|---|
| AChE |
| Neuronal (n) NOS |
| LIGANDS | ||
|---|---|---|
| ACh | Glutathione (GSH) | Oestrogen |
| Dexamethasone | Nitric oxide (NO) | Scopolamine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
A number of previous studies have shown that the oestrogen plays an important role in the preservation of cognitive functions within the mammalian brain (Sherwin, 2003). Cognitive impairment due to natural or surgical menopause is always associated with oestrogen deficiency (Walf et al., 2009). Generally, the effect of oestrogen on learning and memory functions has been conducted using ovariectomized (OVX) rats or mice in experimental studies. In addition, hormone replacement therapy, also called menopausal hormone therapy, has been proven to be effective in protecting against the oestrogen deficiency-induced memory and learning dysfunctions (Miller et al., 1999; Heikkinen et al., 2002; Talboom et al., 2008). However, the conventional application of oestrogen has major defects in that it increases the risk of venous thromboembolism (Olié et al., 2010) and breast cancer (Stahlberg et al., 2004), hence there is a strong motive to seek a new therapeutic strategy.
The dried fruit of Schisandra chinensis, one of the most famous and frequently used herbal medicines, has been used to treat amnesia in China, Korea and Japan as an astringent, sedative and tonic (Park et al., 2009; Ma et al., 2011). Schizandrin (SCH, an antioxidant lignan), the major bioactive constituent of S. chinensis fruits, has been shown to prevent or ameliorate the cognitive impairment induced by scopolamine, Aβ1-42 and dexamethasone (Egashira et al., 2008; Hu et al., 2012; Xu et al., 2012) and its potential mechanisms include improving antioxidation (Hu et al., 2012) and inhibiting AChE activity (Egashira et al., 2008). However, it is not known whether SCH prevents or ameliorates the learning and memory impairments produced by a deficiency in oestrogen.
Previous studies have shown that the induction of long-term potentiation (LTP), an electrophysiological model of learning and memory (Lynch, 2004), was reduced in OVX animals. However, oestrogen treatment could facilitate the formation of LTP and improve the basic synaptic transmission in OVX animals (Warren et al., 1995; Córdoba Montoya and Carrer, 1997). Additionally, NO is able to facilitate synaptic transmission and contributes to the formation of LTP in the hippocampus (Schuman and Madison, 1991). Chien et al. (2005) reported that inhibition of NO synthesis induces impairments in learning and memory. NO formation by neuronal NOS has been shown to depend on oestrogen-mediated association of neuronal NOS and oestrogen-treated triggered the expression of NOS in OVX animals (Panzica et al., 2006).
Because NADPH-diaphorase (NADPH-d) has been characterized biochemically and immunochemically as NOS (Wallace and Bisland, 1994), we further investigated the effect of SCH on the induction of tetanic LTP, basal synaptic transmission and the number of NADPH-d positive neurons in OVX rats. We hoped to advance the understanding of the potential therapeutic value of SCH for the cognitive dysfunctions associated with the menopause.
Methods
Animals, groups and treatments
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Animal procedures undertaken were approved by the Committee on Animal Care and Usage of South China Normal University and every effort was made to minimize animal suffering. Female Sprague Dawley rats (200–250 g), 3 months of age, were obtained from the Sun Yat-sen University (Guangzhou, China). The animals were housed at 22 ± 3°C, 55 ± 5% humidity and 12 h light/dark cycle from 08:00 to 20:00 h with free access to water and food. All animals were given 3 days to adapt to this new environment before any procedure was initiated.
Animals in the present study included non-OVX and OVX rats. In non-OVX rats, animals were randomly divided into control and drug-treated groups. Drug-treated groups were administered 10 or 30 mg·kg−1 SCH, p.o., once daily between 08:00–10:00 h for 28 consecutive days. The animals in the control group received 0.4% DMSO instead of SCH.
The OVX model was as described previously (Yang et al., 2012; Cai et al., 2013). In brief, rats were anaesthetized with sodium pentobarbital (35 mg·kg−1) via i.p. injection (without tail pinch reflex and the eyelid reflex) and then both ovaries were removed. Rats were randomly divided into sham-operated (sham), OVX, OVX + oestradiol benzoate (EB) and OVX + SCH (10 and 30 mg·kg−1). In the sham group, an equivalent amount of adipose tissue around the ovary was removed. Two weeks later, the continuously non-periodic change of vaginal cells for 7 days, determined using a vaginal smear examination, served as the successful criterion of ovariectomy. The oral administration of SCH or vehicle was started at 42 days after surgery. Each rat in the EB group was injected with EB (20 μg·kg−1, s.c.) once daily.
Passive avoidance test
A step-through type passive avoidance test was used to evaluate the effect of SCH on the memory activity of non-OVX and OVX rats. As described previously (Cai et al., 2013), the apparatus consisted of a chamber with two compartments (illuminated and darkened compartments), which were connected by a guillotine door. The experiment consisted of training and testing sessions. During the training session, the rat was placed in the illuminated compartment, facing away from the closed guillotine door, for 1 min before the door was raised. The latency to enter the darkened compartment was recorded (day 28 and day 70 in non-OVX and OVX rats respectively). After the rat entered the darkened compartment, the door was closed and an electric shock (0.5 mA, 3 s) was delivered from the steel-rod floor. On the next day (day 29 or day 71) and seventh day (day 35 or day 77) after training, the test session was performed. The rat was again placed in the illuminated compartment, with the guillotine door open. The retention latency to enter the darkened compartment was recorded for up to 300 s. If a rat did not enter the dark chamber during the 300 s, it was assigned a latency value of 300 s. No shock was delivered during the testing session.
Superoxide dismutase (SOD), malondialdehyde (MDA) and AChE assessments
After completion of the behavioural test, OVX rats (each group n = 12) were anaesthetized with sodium pentobarbital (50 mg·kg−1, i.p.) and killed by rapid decapitation and brains were removed. The bilateral hippocampus was homogenized and samples were centrifuged at 20 000 g and 3500 g (at 4°C); the supernatants were tested.
SOD activity was determined using a spectrophotometric indirect method based on the ability of the enzyme to inhibit O2−-dependent auto-oxidation of pyrogallol (Varija et al., 2009) and the MDA level was evaluated by the thiobarbituric acid reacting substance (TBARS) method (Nakhai et al., 2007). MDA reacted with thiobarbituric acid in the acidic condition at a high temperature and formed a red-complex TBARS. The absorbance of TBARS was determined at 532 nm.
Protein concentration was measured by the Coomassie blue method using BSA as a standard and AChE activity was tested as described previously (Pereira et al., 2012). The brain tissue was homogenized with Tris-citrate buffer (pH 7.4). The rate of acetylthiocholine hydrolysis was assessed with phosphate buffer [PB; pH 7.5 and 0.22 mM 5,5′-Dithio bis-(2-nitrobenzoic acid) (DTNB)]. The hydrolysis of the substrate was monitored by the formation of thiolate dianion of DTNB at 412 nm. The linearity of the protein concentration was determined, as above. AChE activity was expressed as μmol of thiocholine released h−1 mg−1 protein.
NADPH-d histochemistry staining technique
After performing the passive avoidance test, eight OVX rats from each group were randomly chosen for counting NADPH-d positive neurons. Briefly, animals were anaesthetized (sodium pentobarbital, 50 mg·kg−1, i.p.) and transcardially perfused with 0.9% saline, followed by ice-cold fixative containing 4% paraformaldehyde dissolved in 0.1 M PB (pH 7.4) for 15 min. Brains were removed and thoroughly rinsed in PB. The brain was coronally sliced at a thickness of 40 μm. The prepared slices were placed (1–2 min) in diluent of 0.3% Triton-X 100 in PB, shaken and then stained by incubating the slices in a solution containing equal proportions of nitro-blue tetrazolium (0.4 mg·mL−1 in buffer) and NADPH (1 mg·mL−1 in buffer) for 1.0–1.5 h at 37°C. After the sections had been rinsed in buffer, the neuronal NOS possessing NADPH-d activity was stained by this NADPH-d technique (Hope et al., 1991).
For cell counting, five slices were selected randomly from each rat brain. Two independent researchers who were blind to the experimental conditions carried out the cell counting. The NADPH-d positive neurons were counted using a BX51T microscope (Olympus, Tokyo, Japan).
Electrophysiological recordings
The electrophysiological recordings included experiments I and II, and each experiment contained non-OVX and OVX rats. In experiment I, the rats were treated with SCH, p.o. Non-OVX rats were divided into three groups (baseline, control and 30 mg·kg−1 SCH ) and OVX rats were randomly divided into baseline, sham, OVX, OVX + EB and OVX + SCH (30 mg·kg−1) groups, each group n = 10. The hippocampal slice preparation from non-OVX or OVX rats was started at the end of oral drug or vehicle treatment. In contrast, in experiment II, the hippocampal slices from non-OVX or OVX rats were perfused with different dosages of SCH (50, 100 and 200 μg·mL−1) or vehicle for 30 min.
Hippocampal slice preparation
The method of hippocampal slice preparation and the recording technique were slightly modified from those described previously (Huang et al., 2013; Yao et al., 2013). Briefly, rats were anaesthetized (sodium pentobarbital, 50 mg·kg−1, i.p.) and killed by rapid decapitation. The bilateral hippocampus was removed and then coronally sliced thickness at a of 400 μm. Acute hippocampal slices were maintained in an interface recording chamber containing preheated artificial CSF (aCSF) of the following composition (mM): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4 and 11 glucose and maintained at 31 ± 0.5°C, pH 7.35–7.45. Slices were continuously perfused with this solution at a rate of 1.0–1.5 mL·min−1 while the surface of the slice was exposed to warm, humidified 95% O2/5% CO2. Recordings began after 100 min of incubation.
Field EPSPs (fEPSPs) recordings
The amplitude of the fEPSPs was recorded from CA1 stratum pyramidal cells using a single glass pipette filled with 2 M NaAc (yielding a resistance of 2–5 MΩ). Stimulation electrodes (twisted nichrome wires, 0.1 mm) were positioned at Schaffer collateral-commissural projections. The stimulating intensity of the test pulses (0.2–1.0 mA, 0.1 ms in duration) was initially adjusted so as to produce about 30 to 50% maximal fEPSPs amplitude and kept at the same level in the following recordings. After the establishment of a 15–20 min stable baseline, high-frequency stimulation (HFS) was given in experiment I. HFS consisted of 10 trains of five pulses at 100 Hz bursts presented at 200 ms intervals. Unlike in experiment I, in experiment II test compounds were introduced into the perfusion line by switching from control aCSF to SCH-containing aCSF after the establishment of a 15–20 min stable baseline. Data were collected and analysed by LTP230D software (WinLTP Ltd., Bristol, Britain).
In the paired-pulse facilitation (PPF) experiment, the paired-pulse stimulus (PPS) was applied at intervals of 50 ms. Recordings were obtained after the baseline had been stable for 15–20 min and then the effect of SCH on the PPF was tested by bath application for 30 min.
Statistical analysis
All statistical calculations were carried out using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) and data are expressed as mean ± SD. The passive avoidance result is expressed as box plots showing the median, 25% and 75% interquartile range and the amplitude of fEPSPs is expressed as percentage changes from the baseline level and was statistically analysed by a two-way anova with treatment and time as factors. Other data were statistically analysed using a one-way anova. Student's paired t-test was used to compare two independent groups. Differences between the groups were considered to be statistically significant when the two-tailed P–value was < 0.05.
Drugs
SCH (purity ≥ 98%) was obtained from the Guangdong Institute for Drug Control (Guangzhou, China) and other chemical reagents were bought from Sigma Co. Ltd (St. Louis, MO, USA). SCH was dissolved in 0.4% DMSO.
Results
SCH improves the memory activity of non-OVX rats in the step-through passive avoidance test
As compared with the control group, there were no significant differences in the median latency time at the day of behavioural training (day 28) in the three groups (P = 0.078; P = 0.098), shown in Figure 1A. Although 10 mg·kg−1 SCH slightly lengthened the median latency time at days 29 and 35 in comparison with the control animals, there were no obvious differences (P = 0.058; P = 0.055), as shown in Figure 1B and C. However, the median latency times in the 30 mg·kg−1 SCH group were significantly prolonged compared to that in the control group at the days of behavioural testing (P = 0.042; P = 0.046). These data demonstrate that oral application of SCH prolongs the latency time of the step-through type passive avoidance test in non-OVX rats, in a dose–dependent manner. Given that the better effect on improving memory activity was obtained from 30 mg·kg−1 SCH, the dose of 30 mg·kg−1 was thus used to examine the effect of SCH on the tetanus-induced LTP.
Figure 1.
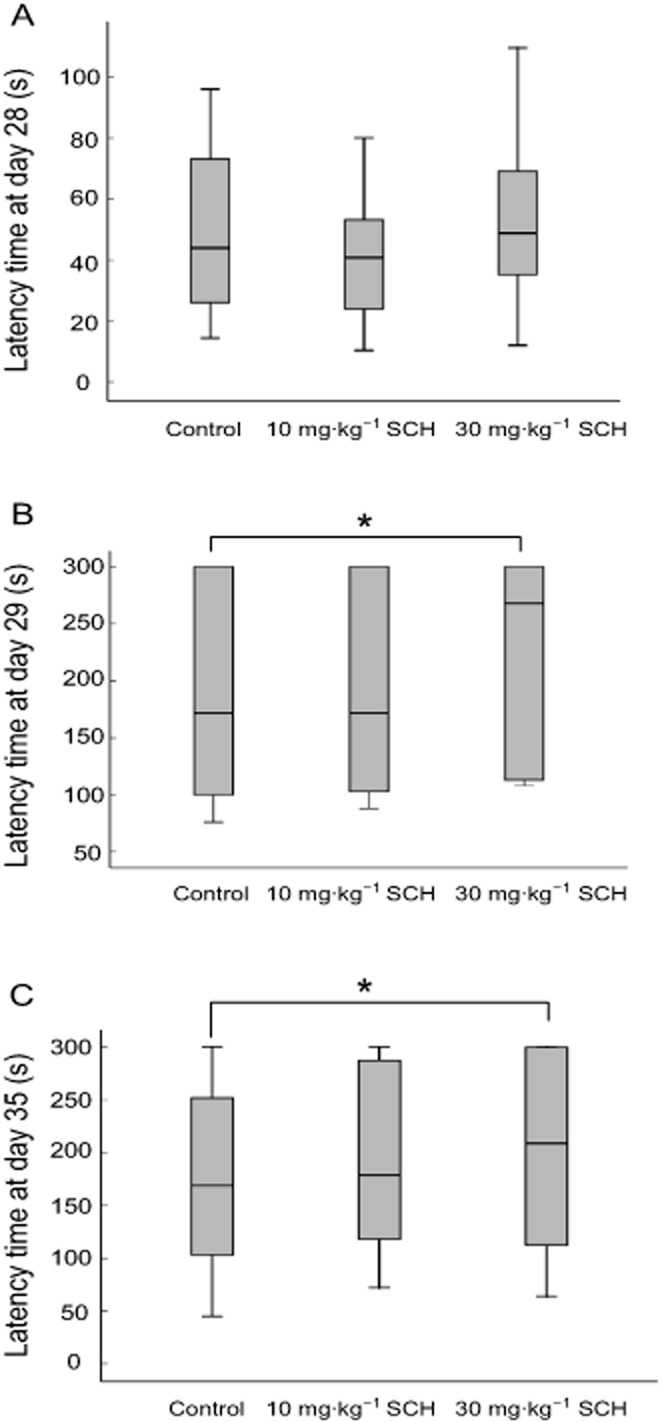
Effects of SCH on the latency time of passive avoidance test at days 28 (A), 29 (B) and 35 (C) in non-OVX rats. The box plots show the median and interquartile range. *P < 0.05. Day 28, the day of behavioural training; Days 29 and 35, the days of behavioural testing. n = 20 for all groups.
Chronic SCH treatment facilitates tetanus-induced LTP in the CA1 region of hippocampal slices from non-OVX rats
HFS was given after the 15 min baseline recordings. As shown in Figure 2C, HFS caused a fast potentiation in the CA1 response evoked, which persisted for at least 75 min in the control group. In contrast, the amplitude of the fEPSPs displayed no significant changes in the baseline group without HFS (Figure 2). As compared with the control group, the enhancement of the fEPSPs' amplitude in the SCH group 15 min after HFS was significantly improved (P = 0.035), shown in Figure 2B and C. These results suggest that 30 mg·kg−1 SCH, p.o., facilitates the induction of tetanic LTP in the hippocampal Schaffer-CA1 pathway of non-OVX rats.
Figure 2.
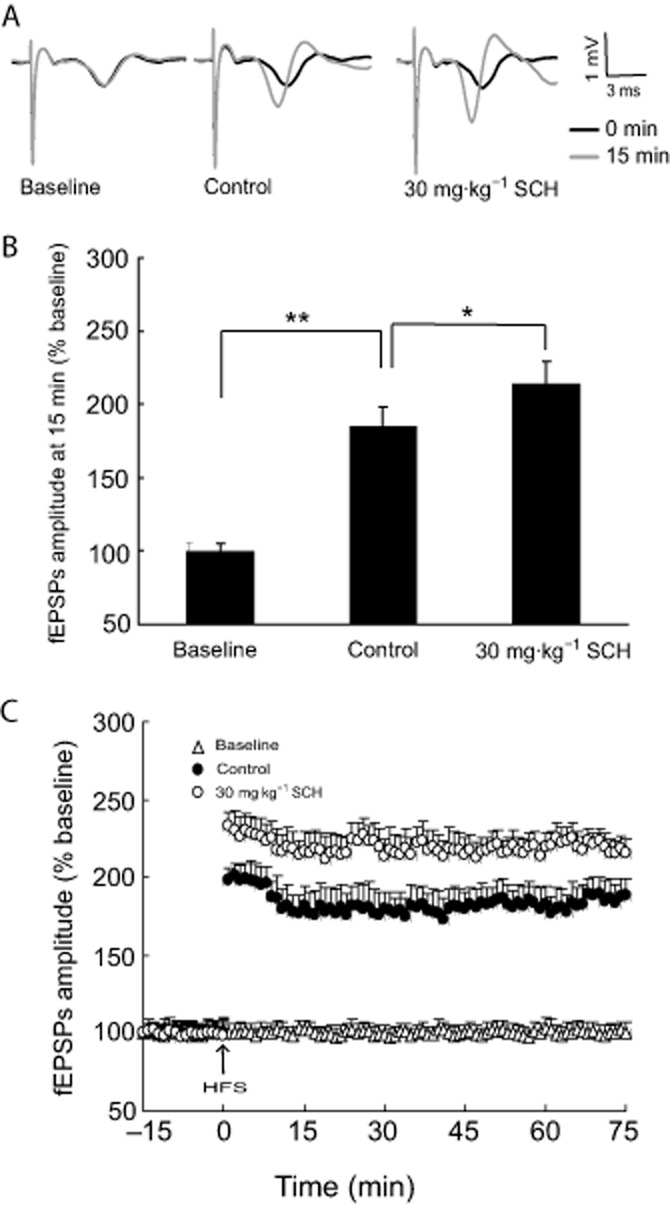
SCH 30 mg·kg−1, p.o., facilitates the induction of tetanic LTP in the hippocampal Schaffer-CA1 pathway of non-OVX rats. (A) Representative records of evoked potentials in hippocampal CA1 region. Black, traces before HFS; gray, traces at 15 min after HFS. (B) Noted that the enhancement in the amplitude of the fEPSPs at 15 min after HFS. (C) Effects of SCH on the amplitude of the fEPSPs after HFS during the 75 min recordings. HFS consisted of 10 trains of five pulses at 100 Hz bursts presented at 200 ms intervals. *P < 0.05 and **P < 0.01. Each group n = 10.
Acute SCH treatment enhances basal synaptic transmission in hippocampal slices from non-OVX rats
In the preliminary experiments, we observed that 75 min after washout of SCH (50, 100 and 200 μg·mL−1) on the fEPSPs' amplitude was restored to control levels and remained at the baseline (about 100%) during the 120 min recordings (data not shown). Therefore, we only show the data of the fEPSPs' amplitude at 75 min in the following experiments. The dose–response relationship for the effect of SCH on the basal synaptic transmission in the Schaffer-CA1 pathway is shown in Figure 3A; 50, 100 and 200 μg·mL−1 acute SCH perfusions for 30 min enhanced the basal synaptic transmission in non-OVX rats, and the maximum effect was observed with the dose of 200 μg·mL−1. Therefore, 200 μg·mL−1 SCH was chosen for use in the next experiment. As shown in Figure 3B, the fEPSPs' amplitude was reversibly enhanced by acute perfusion with 200 μg·mL−1 SCH-containing aCSF. The maximal increase in fEPSPs' amplitude was obtained at about 15 min after SCH application and persisted for about 75 min, and then returned to the baseline level after washout.
Figure 3.
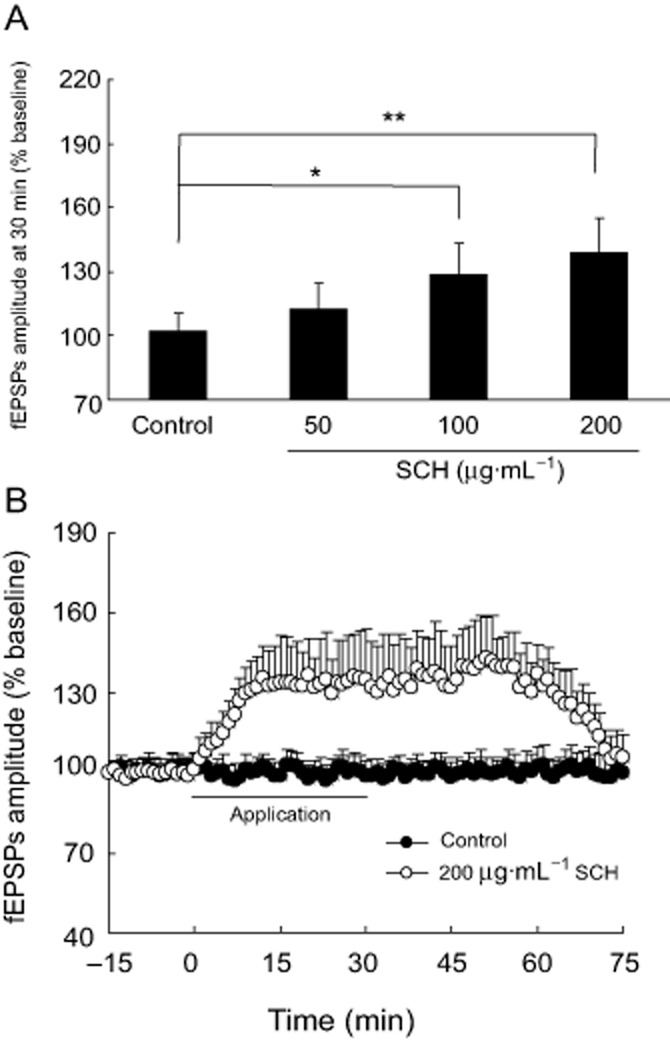
Perfusion of SCH enhances the basal synaptic transmission of hippocampal Schaffer-CA1 pathway in non-OVX rats. (A) Dose–response relationship of the effect of SCH (50, 100 and 200 μg·mL−1) on the basal synaptic transmission is shown. (B) Perfusion with 200 μg·mL−1 SCH reversibly enhanced the fEPSPs' amplitude during the 75 min recordings. The intensity of the stimulating test pulses (0.2–1.0 mA, 0.1 ms in duration) was initially adjusted so as to produce about 30 to 50% maximal fEPSPs' amplitude. *P < 0.05 and **P < 0.01. Each group n = 10.
Acute SCH perfusion does not alter the ratio of PPF in the CA1 region of hippocampal slices from non-OVX rats
Initially, we examined the effect of PPS on the hippocampal slice from non-OVX rats. As shown in Figure 4C, in the control group, we observed a rapid growth in the second fEPSPs' amplitude after PPS at an interval of 50 ms and then obtained a PPF and a stable paired-pulse ratio (PPR) during a 90 min recording period. Interestingly, with the perfusion of 200 μg·mL−1 SCH for 30 min, the first fEPSPs were slightly enhanced and persisted for about 75 min and then returned to the baseline level after washout. Because the second fEPSPs amplitude also displayed a slight increase, the PPR in the hippocampal slice from non-OVX rats did not obviously change with the perfusion of 200 μg·mL−1 SCH (P = 0.055), as shown in Figure 4.
Figure 4.
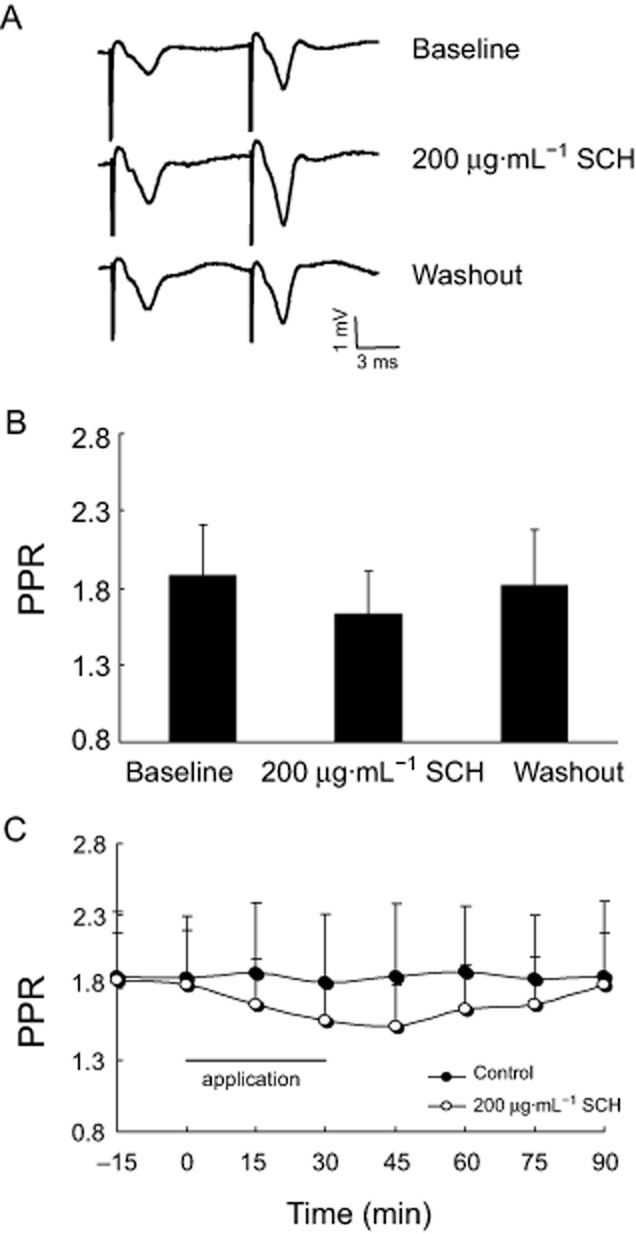
The effect of SCH perfused in vitro on the ratio of PPF in the brain slices from non-OVX rats. (A) Representative records of PPS-evoked potentials after the application of 200 μg·mL−1 SCH. (B) Note that 200 μg·mL−1 SCH slightly decreased the ratio of PPF. (C) Time course of PPR in the hippocampal slice from non-OVX rats after perfusion of SCH during a period of 90 min. PPS at an interval of 50 ms was applied. Recordings were collected after a stable baseline had been established for 15 min and then the effect of SCH on PPF was tested by bath application for 30 min. Each group n = 10.
SCH improves the memory of OVX rats in the step-through passive avoidance test
Figure 5A shows that there were no significant differences in the median latency time at day 70 among the five groups tested (P = 0.060–0.098). However, the median latency time was shortened at days 71 and 77 in the OVX group (P = 0.021, P = 0.003) as compared with the sham group, shown as in Figure 5B and C. Interestingly, the median latency time at days 71 and 77 in the OVX rats was significantly prolonged by the administration of 20 μg EB (P = 0.025; P = 0.032) or SCH, 10 and 30 mg·kg−1 (P = 0.004–0.033), but the effect of SCH 30 mg·kg−1 was greater than that of 10 mg·kg−1. These results suggest that treatment with EB or SCH could reduce the ovariectomy-induced memory impairment in the step-through passive avoidance test, with the response to SCH being dose-dependent. Because the improvement in memory was more obvious with 30 mg·kg−1 SCH, this dose of SCH was used to determine its effect on electrophysiological recordings in the hippocampal Schaffer-CA1 pathway.
Figure 5.
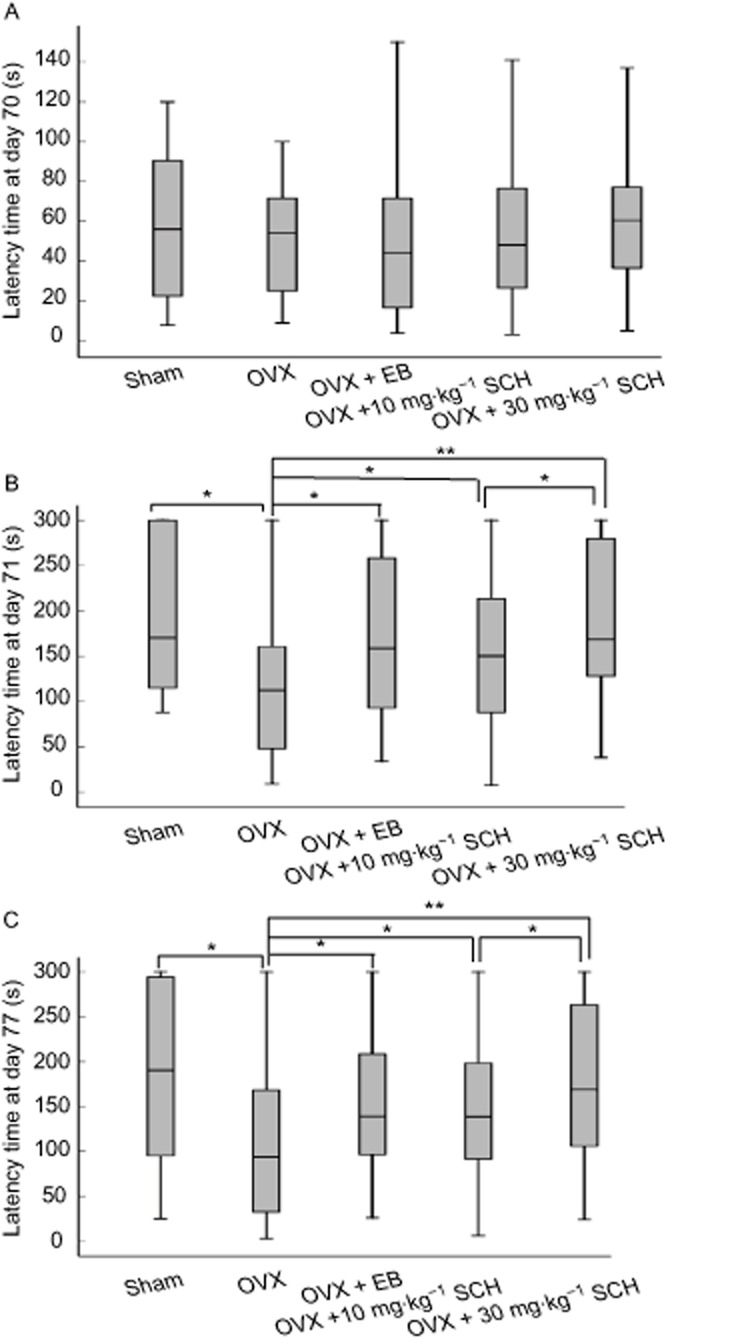
Effects of SCH on the latency time of passive avoidance test at days 70 (A), 71 (B) and 77 (C) in OVX rats. The box plots show the median and interquartile range. *P < 0.05 and **P < 0.01. Day 70, the day of behavioural training; days 71 and 77, the days of behavioural testing. OVX, ovariectomized group; EB, OVX plus 20 μg·kg−1 EB treatment; 10 and 30 mg·kg−1 SCH, OVX plus 10 or 30 mg·kg−1 SCH treatments respectively. n = 20 for all groups.
Chronic SCH treatment facilitates the production of LTP in the hippocampal Schaffer-CA1 pathway of OVX rats
In the baseline group, shown in Figure 6, the fEPSPs' amplitude displayed no significant change in the absence of HFS during the 75 min recordings. However, in the sham group, HFS given after 15 min baseline recordings resulted in a fast potentiation in the fEPSPs' amplitude (185.2 ± 14.8 at 15 min after HFS), which persisted for more than 75 min and then formed a LTP. Interestingly, in the OXV group, the fEPSPs' amplitude at 15 min after HFS was only 127.3 ± 11.3, which was obviously lower than that in the sham animals (P = 0.007). Nevertheless, the 30 mg·kg−1 SCH-treated group displayed significantly improved fEPSPs' amplitude after HFS in OVX rats, in which the fEPSPs amplitude at 15 min was 149.2 ± 10.9. As compared with the OVX group, the fEPSPs' amplitude at 15 min in the SCH-treated group was markedly improved (P = 0.045) and the same result was observed with the EB-treated group. These results suggest that 30 mg·kg−1 SCH, p.o., facilitates the formation of tetanus-induce LTP in OVX rats.
Figure 6.
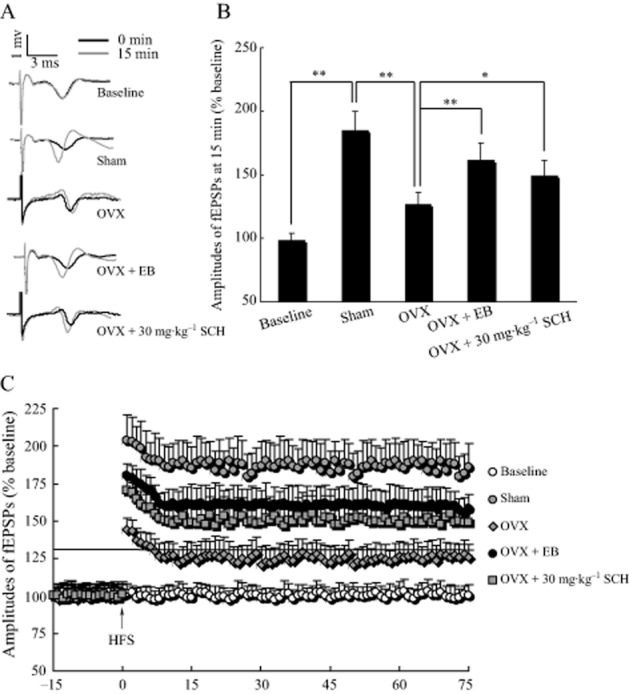
SCH 30 mg·kg−1, p.o., facilitates the induction of tetanic LTP in the hippocampal Schaffer-CA1 pathway of OVX rats. (A) Representative records of the potentials evoked in the hippocampal CA1 region from baseline, in sham, OVX, OVX + EB and OVX + SCH groups. Black, traces before tetanic stimulation; gray, traces 15 min after HFS. (B) Note that ovariectomy obviously lowered the fEPSPs' amplitude at 15 min after HFS, while 30 mg·kg−1 SCH markedly improved the fEPSPs' amplitude after HFS in OXV rats. *P < 0.05 and **P < 0.01. (C) Effects of 30 mg·kg−1 SCH on the fEPSPs' amplitude after HFS in the hippocampal Schaffer-CA1 pathway of OVX rats. HFS consisted of 10 trains of five pulses at 100 Hz, bursts presented at 200 ms intervals. Each group n = 10.
Acute SCH perfusion in vitro promotes the basic synaptic transmission of hippocampal Schaffer-CA1 in OVX rats
The effect of SCH on basal synaptic transmission was investigated in the hippocampal slice from OVX rats. As shown in Figure 7B, the fEPSPs' amplitude was reversibly enhanced by perfusion with 100 μg·mL−1 SCH-containing aCSF. SCH 100 μg·mL−1 induced a rapid increase in synaptic transmission that persisted for about 75 min and then returned to the baseline level after washout. The maximum fEPSPs' amplitude was seen at approximately 15 min after the application of SCH. The dose–response relationship for the effect of SCH on basal synaptic transmission is shown in Figure 7A; the different concentrations of SCH perfused enhanced the basal synaptic transmission in a dose-dependent manner with 100 μg·mL−1 SCH inducing a maximum effect of the three concentrations tested. We thus used this dose to examine the effect of SCH on the ratio of PPF.
Figure 7.
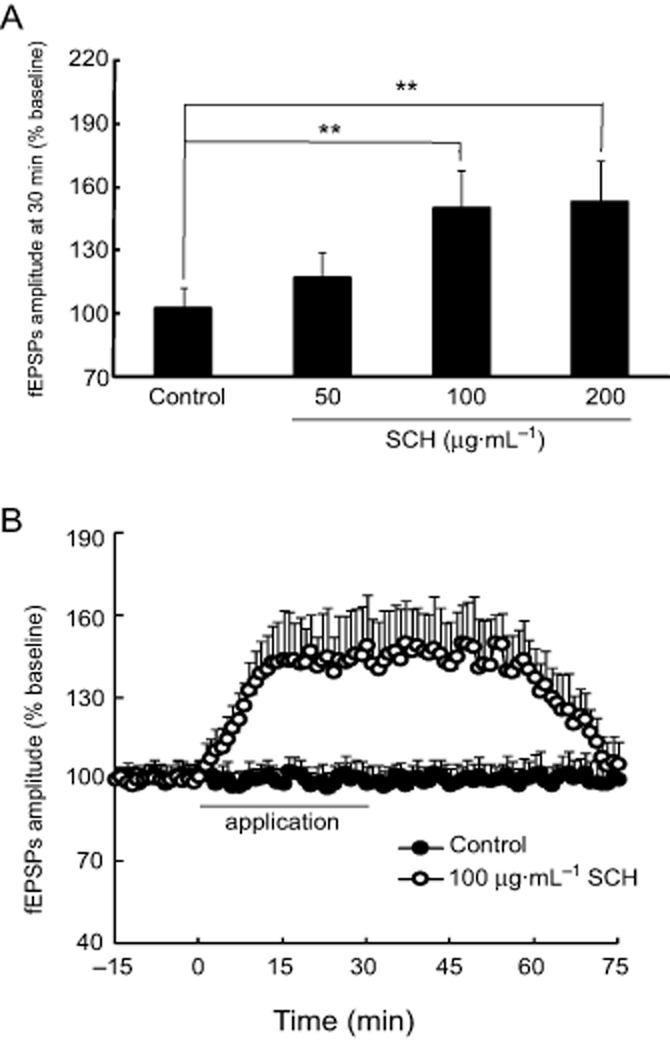
A perfusion of SCH enhances the basal synaptic transmission in the hippocampal Schaffer-CA1 pathway of OVX rats. (A) The dose–response relationship for the effect of SCH (50, 100 and 200 μg·mL−1) on the fEPSPs' amplitude. (B) SCH 100 μg·mL−1 reversibly enhanced the fEPSPs' amplitude in the hippocampal slices from OVX rats during a period of 75 min recordings. The intensity of the stimulating test pulses (0.2–1.0 mA, 0.1 ms in duration) was initially adjusted so as to produce about 30 to 50% maximal fEPSPs' amplitude. **P < 0.01. Each group n = 10.
Acute SCH perfusion decreases the ratio of PPF in OVX rats
We firstly examined the effect of PPS on the hippocampal brain slice from OVX rats. As shown in Figure 8C, in the OVX group, there was a rapid growth in the second fEPSPs' amplitude after PPS at an interval of 50 ms and then the PPF and PPR became stable during the 90 min recording period. Interestingly, after the perfusion of SCH at 100 μg·mL−1 for 30 min, the first fEPSPs revealed a rapid onset and persisted for about 75 min and then returned to the baseline level after washout. In spite of the second fEPSPs' amplitude being slightly enhanced, a more obvious improvement in the first fEPSPs was observed and is thus associated with a significant reduction in PPR (P = 0.007) (Figure 8A and B). Hence, 100 μg·mL−1 SCH decreases the ratio of PPF in the basic synaptic transmission of hippocampal slices from OVX rats.
Figure 8.
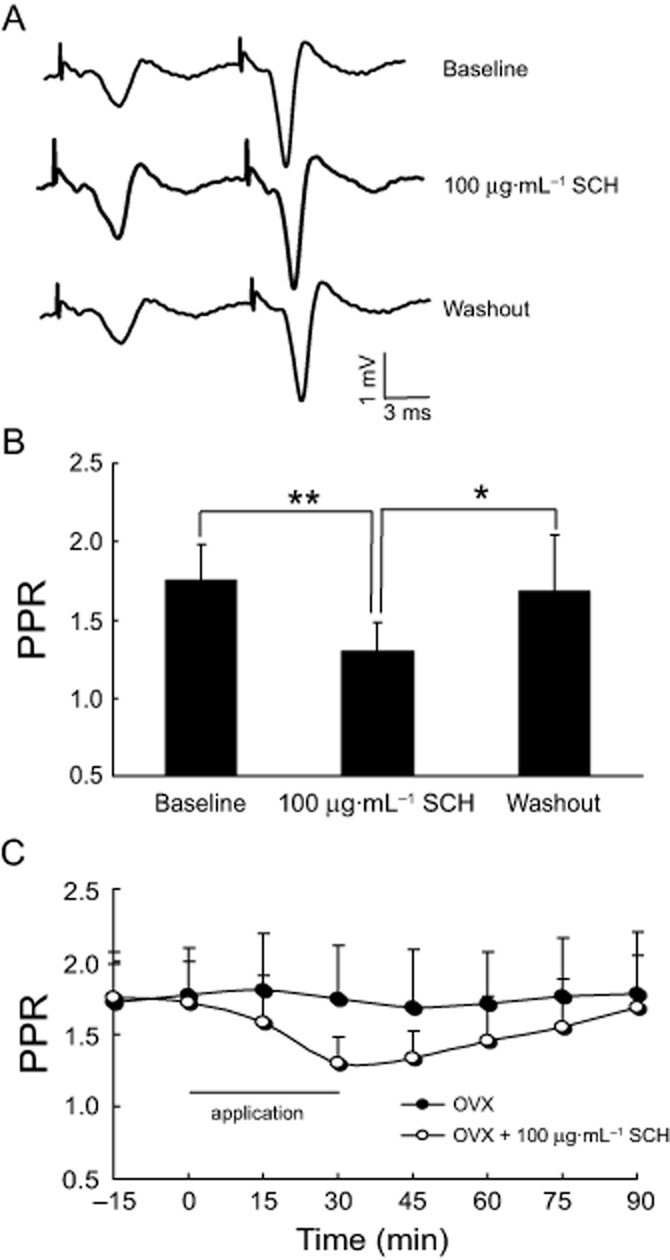
Perfusion of 100 μg·mL−1 SCH in vitro reduces the ratio of PPF in OVX rats. (A) Representative records of PPS-evoked potentials with 100 μg·mL−1 SCH applied for 30 min. (B) Note that 100 μg·mL−1 SCH obviously decreased the ratio of PPF. (C) Time course of the ratio of PPF in the hippocampal slices from OVX rats treated with SCH during a period of 90 min. PPS was applied at intervals of 50 ms. *P < 0.05 and **P < 0.01. Each group n = 10.
Chronic SCH treatment increases antioxidant effect and cholinergic function in OVX rats
As shown in Figure 9, the hippocampal MDA levels, SOD and AChE activities were obtained after the behavioural experiments in the five groups. As compared with the sham group, SOD activity in the hippocampus was significantly reduced by ovariectomy (P = 0.044); meanwhile, the hippocampal MDA level and AChE activity were markedly increased (P = 0.005; P = 0.007). These results demonstrate that the antioxidant activity and cholinergic function were reduced by the oestrogen deficiency.
Figure 9.
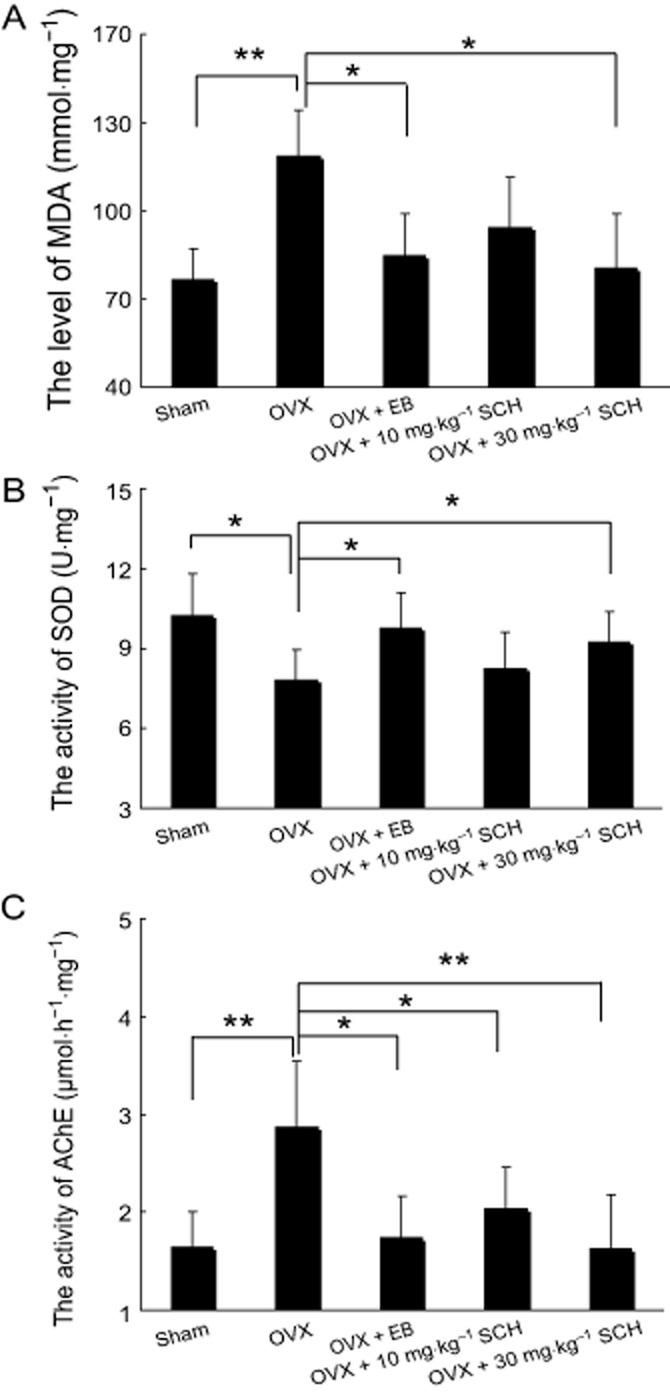
Effects of oral SCH on the level of MDA (A) and activities of SOD (B) and AChE (C) in the hippocampus of OVX rats. *P < 0.05 and **P < 0.01. OVX, ovariectomized group; EB, OVX plus 20 μg·kg−1 EB; 10 and 30 mg·kg−1 SCH, OVX plus 10 or 30 mg·kg−1 SCH respectively. n = 12 for all groups.
However, SOD activity in OVX rats was significantly increased by the administration of 20 μg EB (P = 0.047) and the level of MDA and activity of AChE were decreased accordingly (P = 0.038; P = 0.007). The 30 mg·kg−1 SCH-treated group also exhibited similar effects (P = 0.046; P = 0.008), even though the SOD activity and MDA level displayed no obvious differences between the sham and the 10 mg·kg−1 SCH groups in our experiments (P = 0.056; P = 0.053). Our findings suggest that chronic SCH treatment improves the antioxidant activity and reduces AChE activity in OVX rats in a dose-dependent manner.
Chronic SCH reduces the loss of NADPH-d positive neurons induced by ovariectomy in the hippocampal CA1 region
The results from the evaluation of the number of NADPH-d positive neurons in the hippocampal CA1 area are shown in Figure 10. The number of NADPH-d positive neurons in the OVX group was noticeably reduced in the hippocampal CA1 region (P = 0.006) as compared with the sham group, demonstrating the reduction in oestrogen resulted in a loss of NADPH-d positive neurons in the CA1 area. With the administration of 20 μg EB significantly increased the number of NADPH-d positive neurons (P = 0.022). Likewise, oral treatment with SCH 30 mg·kg−1 also ameliorated the loss of NADPH-d positive neurons caused by ovariectomy (P = 0.015). Although 10 mg·kg−1 SCH slightly increased in the number of NADPH-d positive neurons, this was not significant compared with the OVX group (P = 0.055). Our findings demonstrate that oral SCH treatment reduces the ovariectomy-induced loss of NADPH-d positive neurons in a concentration-dependent manner.
Figure 10.
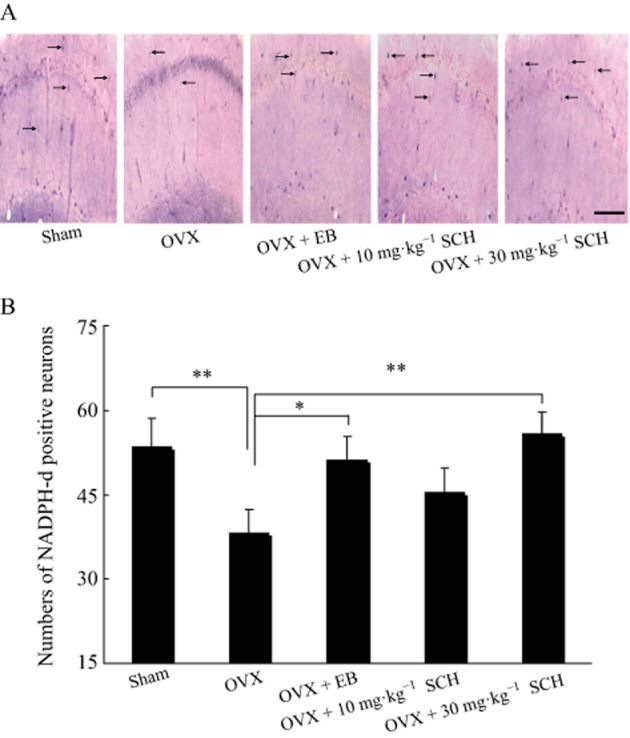
Oral SCH decreases the loss of NADPH-d positive neurons in the hippocampal CA1 region of OVX rats. (A) Photographs showing the distribution of NADPH-d positive neurons in the hippocampal CA1 area (×400, bar = 100 μm). The black arrows indicate the NADPH-d positive neurons. (B) Number of NADPH-d positive neurons in the hippocampal CA1 area. Sham, sham surgery; OVX, ovariectomized group; EB, OVX plus 20 μg·kg−1 EB; 10 and 30 mg·kg−1 SCH, OVX plus 10 or 30 mg·kg−1 SCH respectively. *P < 0.05 and **P < 0.01. n = 40 for all groups.
Discussion and conclusions
We observed that the latency time of the passive avoidance test was obviously prolonged in non-OVX rats after oral treatment with SCH at a dose of 30 but not 10 mg·kg−1. Oral administration of SCH also significantly improved the memory activity in OVX animals, at doses of 10 and 30 mg·kg−1. The vast majority of studies have shown that hippocampus-dependent learning and memory are impaired by ovariectomy (Su et al., 2010; Cai et al., 2013). Indeed, we found that the memory activity of OVX rats was attenuated in the step-through passive avoidance test, and that SCH improved this memory activity. These results are consistent with those from previous studies showing that SCH plays an obvious role in preventing or ameliorating the cognitive impairment induced by scopolamine, Aβ1-42 or dexamethasone (Egashira et al., 2008; Hu et al., 2012; Xu et al., 2012). Moreover, previous studies have shown that SCH improved the impairment of learning and memory in different concentrations such as 10 (Egashira et al., 2008), 36 (Hu et al., 2012) and 45 mg·kg−1 (Xu et al., 2012). The distinct dosage requirements may be related to the differences in the treatment paradigm and purity of SCH. Collectively, the enhancement of memory activity by SCH was more obvious in the OVX than in the non-OVX rats.
It was reported that orally administered SCH can be quickly absorbed into the blood and may then express its pharmacological effects within 1 h (Xu et al., 2005). SCH was found to distribute quickly from the blood into most tissues (heart, liver, fat, brain, etc.) and accumulates in these tissues. The very low biliary, urinary and faecal excretion of SCH indicates that it is mainly excreted in the form of metabolites (Zhan et al., 2014). This pharmacokinetic feature, such as its accumulation in the brain, may help to elucidate the role of SCH in the CNS, including its effects on learning and memory functions.
LTP, a long-lasting enhancement of synaptic transmission observed in the hippocampus, is believed to be a model of synaptic plasticity and underlies a cellular mechanism of learning and memory (Lynch, 2004; Whitlock et al., 2006). We found that the induction of LTP is reduced in OVX rats. Similarly, Warren et al. (1995) reported that HFS did not induce LTP when the oestrogen level is low. Córdoba Montoya and Carrer (1997) also showed that LTP was not produced in OVX animals. However, in our experiments, oral administration of 30 mg·kg−1 SCH for 4 weeks facilitated the induction of LTP in OVX rats and acute perfusion of SCH in vitro enhances the basic synaptic transmission in the hippocampal slice from OVX rats. In addition, we further found that SCH was more effective in OVX rats than in non-OVX rats. These findings demonstrate that SCH is able to facilitate the formation of tetanus-induced LTP and improve basic synaptic transmission in OVX and non-OVX rats.
PPF, a transient increase in the probability of vesicular release or a dynamic enhancement of transmitter release, is considered crucial in CNS information processing, such as synaptic transmission (Bornschein et al., 2013). PPF has been explained as a simple presynaptic mechanism in synaptic plasticity and synaptic transmission (Satake et al., 2012). In the present study, we analysed the potential mechanisms by which SCH enhances basic synaptic transmission using PPF. The result showed that SCH perfusion obviously reduces the ratio of PPF in the hippocampal slice from OVX rats rather than non-OVX rats, demonstrating that SCH might act through different mechanisms to enhance the basic synaptic transmission in the two different animal models (non-OVX and OVX rats). In OVX rats, SCH facilitates basic synaptic transmission possibly through a presynaptic mechanism, such as modulating the release of presynaptic neurotransmitter (Yao et al., 2013). Nevertheless, we do not know the exact underlying mechanism by which SCH enhances the basic synaptic transmission in non-OVX rats. We need to further investigate how SCH enhances the synaptic transmission of non-OVX animals in the future.
Our results further demonstrated that oral administation of SCH significantly decreases hippocampal AChE activity in OVX rats. Central cholinergic signalling has long been implicated in various cognitive processes, including memory acquisition, consolidation and attention (Leung et al., 2003). ACh is known generally as the major neurotransmitter in the hippocampus that can facilitate both memory and cognition (Hasselmo, 2006). Selective inhibition of central AChE activity is able to increase the ACh neurotransmission of the synaptic cleft in the brain (Tsukada et al., 1997). Additionally, many studies have shown that the animals subjected to ovariectomy present significantly increased AChE activity in the hippocampus (Monteiro et al., 2005; Huang and Zhang, 2010). Consistently, our results suggested that AChE is increased in the hippocampus of OVX rats, while oral SCH decreases this enhanced AChE activity. Furthermore, Giridharan et al. (2011) also reported that the ameliorating effects of SCH on memory deficits might be caused by the modulation of ACh levels mediated through an inhibitory effect on the AChE enzyme.
In the current study, we observed that SCH improves the fEPSPs' amplitude and reduces the ratio of PPF and AChE activity in the OVX rats. Considering that changes in the PPF ratio reflect alterations in synaptic efficacy, which is determined by the probability of neurotransmitter release (Chu et al., 2011), we thus assume that the effects of SCH on fEPSPs' amplitude and PPF in OVX rats are caused by its ability to improve the release of presynaptic neurotransmitters, such as ACh. Additionally, calcium plays a critical role in neurotransmitter release (Adler et al., 1991; Wan et al., 2012); therefore, in the future we need to determine the effect of SCH on the levels of both extracellular and intracellular calcium. Overall, we speculate that SCH reduces the activity of AChE and then enhances the release of ACh, thus resulting in an improvement in the basic synaptic transmission of OVX rats.
Oxidative stress participates in the processing of cognitive functions (Clausen et al., 2010). Enhancing or reducing the the enzymatic activity of endogenous antioxidants may partially mediate the changes in memory activity (Chiu et al., 2011). In the present study, we evaluated the effect of ovariectomy on the antioxidant enzymes by evaluating SOD activity and MDA levels in OVX animals. Our results show that ovariectomy evidently reduces SOD activity and increases the MDA level, which are in agreement with those from a previous study suggesting that SOD activity and MDA level are changed after ovariectomy (Huang and Zhang, 2010). Inconsistently, Monteiro et al. (2005) reported that ovariectomy did not alter SOD activity. These obviously conflicting results may be caused by the different time interval between ovariectomy and SOD assay. For instance, the time interval between the surgical operation and biochemical assay in our experiments was 42 rather than 30 days.
Furthermore, our results strongly show that oral SCH enhances antioxidation by increasing SOD activity and reducing the MDA level of OVX rats. We did not test the effect of SCH on GSH or GSH peroxidase (GSH-px) activities in OVX rats, whereas Hu et al. (2012) reported that SCH prevents the reduction in GSH and GSH-px activities induced by Aβ1-42. Consequently, our findings suggest that the ability of SCH to reverse ovariectomy-induced memory deficits may be, at least partially, mediated by its enhancement of antioxidant activity.
We further found that the number of NADPH-d positive neurons in the hippocampal CA1 region was significantly decreased by ovariectomy, while the treatment with SCH markedly reversed the loss of NADPH-d positive neurons induced by oestrogen deficiency. Neuronal NADPH-d is a NOS and NADPH-d histochemistry provides a specific histochemical marker for neurons producing NO (Hope et al., 1991). Biochemical studies have shown that the NADPH-d activity represents only a part of the total cellular diaphorase pool and NADPH-d activity can be demonstrated in cells expressing no constitutive NOS (Grozdanovic and Gossrau, 1995). However, only particular populations of neurons are stained by the NADPH-d histochemical technique and macrophages and endothelial cells are not stained. Thus, NADPH-d is a neuronal NOS (Hope et al., 1991) and NADPH-d has been characterized biochemically and immunochemically as NOS (Wallace and Bisland, 1994).
Furthermore, oxidative stress is able to change the quantity and structure of hippocampal neurons and synapses in the mammalian brain (Wang et al., 2012). SCH reduced the loss of NADPH-d positive neurons in the hippocampal CA1 area induced by ovariectomy at a dose of 30 rather than 10 mg·kg−1 in our study. Accordingly, SCH also displayed antioxidant activity in OVX rats at a dose of 30 but not 10 mg·kg−1. As oxidative stress has been shown to mediate hippocampal neuron death in rats after lithium-pilocarpine-induced status epilepticus (Liu et al., 2010), we suspect that the ability of SCH to prevent the loss of NADPH-d positive neurons in the hippocampal CA1 area may be a result of its antioxidant effect and that this then ameliorates the memory impairment in OVX rats.
In conclusion, our results demonstrate that SCH improves memory activity in non-OVX and OVX animals. The potential mechanisms by which SCH produces this effect in OVX rats may include reducing the loss of hippocampal NADPH-d positive neurons, increasing the activity of antioxidants, and improving synaptic transmission, possibly by enhancing cholinergic function. These findings suggest that SCH could have potential as a therapeutic agent for the cognitive dysfunctions associated with the menopause.
Author contributions
C-H. L. contributed to the conception and design of the study and drafted the manuscript. Z-J. J., C-Y. W. and X. X. contributed to the acquisition of data. J-F. Y., J-N. H. and Z-P. C. contributed to the analysis and interpretation of data. P. X. and C-H. L. critically revised the manuscript.
Conflict of interest
The authors declare no competing financial interests.
Glossary
Abbreviations
- aCSF
artificial CSF
- EB
oestradiol benzoate
- fEPSPs
field EPSPs
- GSH-px
GSH peroxidase
- LTP
long-term potentiation
- MDA
malondialdehyde
- NADPH-d
NADPH-diaphorase
- OVX
ovariectomized
- PPF
paired-pulse facilitation
- PPR
paired-pulse ratio
- PPS
paired-pulse stimulus
- SCH
schizandrin
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reacting substance
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornschein G, Arendt O, Hallermann S, Brachtendorf S, Eilers J, Schmidt H. Paired-pulse facilitation at recurrent Purkinje neuron synapses is independent of calbindin and parvalbumin during high-frequency activation. J Physiol. 2013;591(Pt 13):3355–3370. doi: 10.1113/jphysiol.2013.254128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZL, Wang CY, Gu XY, Wang NJ, Wang JJ, Liu WX, et al. Tenuigenin ameliorates learning and memory impairments induced by ovariectomy. Physiol Behav. 2013;118:112–117. doi: 10.1016/j.physbeh.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1. Eur J Neurosci. 2005;21:1679–1688. doi: 10.1111/j.1460-9568.2005.03993.x. [DOI] [PubMed] [Google Scholar]
- Chiu CS, Chiu YJ, Wu LY, Lu TC, Huang TH, Hsieh MT, et al. Diosgenin ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced by D-galactose. Am J Chin Med. 2011;39:551–563. doi: 10.1142/S0192415X11009020. [DOI] [PubMed] [Google Scholar]
- Chu HY, Wu Q, Zhou S, Cao X, Zhang A, Jin GZ, et al. SKF83959 suppresses excitatory synaptic transmission in rat hippocampus via a dopamine receptor-independent mechanism. J Neurosci Res. 2011;89:1259–1266. doi: 10.1002/jnr.22653. [DOI] [PubMed] [Google Scholar]
- Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31:425–433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Egashira N, Kurauchi K, Iwasaki K, Mishima K, Orito K, Oishi R, et al. Schizandrin reverses memory impairment in rats. Phytother Res. 2008;22:49–52. doi: 10.1002/ptr.2258. [DOI] [PubMed] [Google Scholar]
- Giridharan VV, Thandavarayan RA, Sato S, Ko KM, Konishi T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic Res. 2011;45:950–958. doi: 10.3109/10715762.2011.571682. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z, Gossrau R. Alpha-NADPH appears to be primarily oxidized by the NADPH-diaphorase activity of nitric oxide synthase (NOS) Acta Histochem. 1995;97:313–320. doi: 10.1016/s0065-1281(11)80196-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Puoliväli J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michacl GJ, Knigge KM, Vincent SR. Neuronal NADPH- diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Cao Y, He R, Han N, Liu Z, Miao L. Schizandrin, an antioxidant lignan from Schisandra chinensis, ameliorates Aβ1–42-induced memory impairment in mice. Oxid Med Cell Longev. 2012;2012:1–7. doi: 10.1155/2012/721721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JN, Wang CY, Wang XL, Wu BZ, Gu XY, Liu WX, et al. Tenuigenin treatment improves behavioral Y-maze learning by enhancing synaptic plasticity in mice. Behav Brain Res. 2013;246:111–115. doi: 10.1016/j.bbr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Huang YH, Zhang QH. Genistein reduced the neural apoptosis in the brain of ovariectomized rats by modulating mitochondrial oxidative stress. Br J Nutr. 2010;104:1297–1303. doi: 10.1017/S0007114510002291. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Shen B, Rajakumar N, Ma J. Cholinergic activity enhances hippocampal long-term potentiation in CA1 during walking in rats. J Neurosci. 2003;23:9297–9304. doi: 10.1523/JNEUROSCI.23-28-09297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Wang AH, Li LL, Huang YF, Xue P, Hao AJ. oxidative stress may mediate hippocampal neuron death in rats after lithium-pilocarpine-induced status epilepticus. Seizure. 2010;19:165–172. doi: 10.1016/j.seizure.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Phys Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma CH, Liu TT, Yang L, Zu YG, Wang SY, Zhang RR. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal Chim Acta. 2011;689:110–116. doi: 10.1016/j.aca.2011.01.012. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Monteiro SC, Matté C, Delwing D, Wyse AT. Ovariectomy increases Na+, K+-ATPase, acetylcholinesterase and catalase in rat hippocampus. Mol Cell Endocrinol. 2005;236:9–16. doi: 10.1016/j.mce.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Nakhai LA, Mohammadirad A, Yasa N, Minaie B, Nikfar S, Ghazanfari G, et al. Benefits of Zataria multiflora boiss in experimental model of mouse inflammatory bowel disease. Evid Based Complement Alternat Med. 2007;4:43–50. doi: 10.1093/ecam/nel051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olié V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr Opin Hematol. 2010;17:457–463. doi: 10.1097/MOH.0b013e32833c07bc. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Sica M, Gotti S, Martini M, Pinos H, et al. Effects of gonadal hormones on central nitric oxide producing systems. Neuroscience. 2006;138:987–995. doi: 10.1016/j.neuroscience.2005.07.052. [DOI] [PubMed] [Google Scholar]
- Park JY, Shin HK, Lee YJ, Choi YW, Bae SS, Kim CD. The mechanism of vasorelaxation induced by Schisandra chinensis extract in rat thoracic aorta. J Ethnopharmacol. 2009;121:69–73. doi: 10.1016/j.jep.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira VM, Bortolotto JW, Kist LW, Azevedo MB, Fritsch RS, Oliveira Rda L, et al. Endosulfan exposure inhibits brain AChE activity and impairs swimming performance in adult zebrafish (Daniorerio) Neurotoxicology. 2012;33:469–475. doi: 10.1016/j.neuro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Satake S, Inoue T, Imoto K. Paired-pulse facilitation of multivesicular release and intersynaptic spillover of glutamate at rat cerebellar granule cell-interneurone synapses. J Physiol. 2012;590(Pt 22):5653–5675. doi: 10.1113/jphysiol.2012.234070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Stahlberg C, Pedersen AT, Lynge E, Andersen ZJ, Keiding N, Hundrup YA, et al. Increased risk of breast cancer following different regimens of hormone replacement therapy frequently used in Europe. Int J Cancer. 2004;109:721–727. doi: 10.1002/ijc.20016. [DOI] [PubMed] [Google Scholar]
- Su J, Sripanidkulchai K, Wyss JM, Sripanidkulchai B. Curcuma comosa improves learning and memory function on ovariectomized rats in a long-term Morris water maze test. J Ethnopharmacol. 2010;130:70–75. doi: 10.1016/j.jep.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H, Kakiuchi T, Ando I, Ouchi Y. Functional activation of cerebral blood flow abolished by scopolamine is reversed by cognitive enhancers associated with cholinesterase inhibition: a positron emission tomography study in unanesthetized monkeys. J Pharmacol Exp Ther. 1997;281:1408–1414. [PubMed] [Google Scholar]
- Varija D, Kumar KP, Reddy KP, Reddy VK. Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J Med Res. 2009;129:587–592. [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MN, Bisland SK. NADPH- diaphorase activity in activated astrocytes represents inducible nitric oxide synthase. Neuroscience. 1994;69:905–919. doi: 10.1016/0306-4522(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Wan QF, Nixon E, Heidelberger R. Regulation of presynaptic calcium in a mammalian synaptic terminal. J Neurophysiol. 2012;108:3059–3067. doi: 10.1152/jn.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Han XQ, Zhao YN, Zhang PP, Yang L, Lei JQ, et al. The relation of learning deficits with oxidative stress in rats exposed to severe intermittent hypoxia. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:24–28. [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang G, Xie H, Wang R, Wang W, Li X, et al. Determination of schizandrin in rat plasma by high-performance liquid chromatography-mass spectrometry and its application in rat pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;828:55–61. doi: 10.1016/j.jchromb.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou X, Zhou XW, Zhang Z, Liao MJ, Gao Q, et al. Schizandrin prevents dexamethasone-induced cognitive deficits. Neurosci Bull. 2012;28:532–540. doi: 10.1007/s12264-012-1258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MY, Cai ZL, Xiao P, Li CH. Schisandra N-butanol extract improves synaptic morphology and plasticity in ovarectomized mice. Neural Regen Res. 2012;7:1365–1369. doi: 10.3969/j.issn.1673-5374.2012.18.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Huang JN, Li CH, Li HH, Yan WW, Cai ZL, et al. Cordycepin suppresses excitatory synaptic transmission in rat hippocampal slices via a presynaptic mechanism. CNS Neurosci Ther. 2013;19:216–221. doi: 10.1111/cns.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan SY, Shao Q, Fan XH, Li Z, Cheng YY. Tissue distribution and excretion of herbal components after intravenous administration of a Chinese medicine (Shengmai injection) in rat. Arch Pharm Res. 2014 doi: 10.1007/s12272-014-0376-7. doi: 10.1007/s12272-014-0376-7. [DOI] [PubMed] [Google Scholar]


