Abstract
Background and Purpose
μ-Opioid receptors, pro-opiomelanocortin and pro-enkephalin are highly expressed in the nucleus tractus solitarii (NTS) and μ receptor agonists given to the NTS dose-dependently increased BP. However, the molecular mechanisms of this process remain unclear. In vitro, μ receptors heterodimerize with α2A-adrenoceptors. We hypothesized that α2A-adrenoceptor agonists would lose their depressor effects when their receptors heterodimerize in the NTS with μ receptors.
Experimental Approach
We microinjected μ-opioid agonists and antagonists into the NTS of rats and measured changes in BP. Formation of μ receptor/α2A-adrenoceptor heterodimers was assessed with immunofluorescence and co-immunoprecipitation methods, along with proximity ligation assays.
Key Results
Immunofluorescence staining revealed colocalization of α2A-adrenoceptors and μ receptors in NTS neurons. Co-immunoprecipitation revealed interactions between α2A-adrenoceptors and μ receptors. In situ proximity ligation assays confirmed the presence of μ receptor/α2A-adrenoceptor heterodimers in the NTS. Higher levels of endogenous endomorphin-1 and μ receptor/α2A-adrenoceptor heterodimers were found in the NTS of hypertensive rats, than in normotensive rats. Microinjection of the μ receptor agonist [D-Ala2, MePhe4, Gly5-ol]-enkephalin (DAMGO), but not that of the α2A-adrenoceptor agonist guanfacine, into the NTS of normotensive rats increased μ receptor/α2A-adrenoceptor heterodimer formation and BP elevation. The NO-dependent BP-lowering effect of α2A-adrenoceptor agonists was blunted following increased formation of μ receptor/α2A-adrenoceptor heterodimers in the NTS of hypertensive rats and DAMGO-treated normotensive rats.
Conclusions and Implications
Increases in endogenous μ receptor agonists in the NTS induced μ receptor/α2A-adrenoceptor heterodimer formation and reduced the NO-dependent depressor effect of α2A-adrenoceptor agonists. This process could contribute to the pathogenesis of hypertension.
Tables of Links
| TARGETS |
|---|
| GPCRs |
| α2A-adrenoceptors |
| μ receptors |
| LIGANDS | |
|---|---|
| CTAP | α-Methylnoradrenaline |
| DAMGO | L-NAME |
| Endomorphin-1 | NO |
| Guanfacine | Noradrenaline |
| Hydralazine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Opioid peptides have long been considered to be analgesic neuropeptides in the CNS. However, μ-opioid receptors (μ receptors) are also expressed in the nuclei that control BP, such as the parabrachial nucleus (PBN), the nucleus raphe magnus and the nucleus tractus solitarii (NTS) of the brainstem. Endogenous opioid ligands, including pro-opiomelanocortin and pro-enkephalin, are also highly expressed in the PBN and NTS (Mansour et al., 1988). Microinjection of the μ receptor agonist [D-Ala2, MePhe4, Gly5-ol]-enkephalin (DAMGO) into the NTS induces BP elevation and baroreflex impairment (Hassen and Feuerstein, 1987). Activation of μ receptors in the CNS prevents the sympathoinhibitory decompensation that occurs during an acute reduction of central blood volume (Evans and Ludbrook, 1990). These results suggest that endogenous opioid agonists participate in both cardiovascular regulation and analgesia in the CNS.
In the brain, the activation of α2-adrenoceptors appears to inhibit noradrenaline release (Vieira-Coelho et al., 2009) and these receptors are present in high density in the NTS (Glass et al., 2001). Altered α2-adrenoceptor function within the NTS contributes to baroreflex dysfunction in hypertensive animals (Hayward et al., 2002). Three different α2-adrenoceptor subtypes exist: α2A, α2B and α2C. α2A-adrenoceptors are the predominant subtype in the NTS (Glass et al., 2001). The antihypertensive and bradycardic effects of α2 agonists depend on α2A-adrenoceptors (Altman et al., 1999). The deletion of a gene encoding α2A-adrenoceptors leads to increases in BP and heart rate (HR) as well as cardiac failure (Brede et al., 2002).
The NTS is the primary integrative centre for cardiovascular control and other autonomic functions in the CNS (Cheng et al., 2012). Pharmacological ablation of the NTS induces lethal sympathetic hyperactivity and hypertension (Lu et al., 2013). The NTS both integrates convergent information and is itself the site of substantial modulation. Various neuromodulators participate in cardiovascular modulation in the NTS, including opioids, noradrenaline and NO (Tseng et al., 1996; Cheng et al., 2012; Lu et al., 2013). In addition, the predominant endogenous μ-opioid peptide endomorphin-1 (Martin-Schild et al., 1999), as well as μ receptors and α2A-adrenoceptors, are all expressed in the NTS (Glass and Pickel, 2002).
In transfected HEK293 cells, μ receptors and α2A-adrenoceptors form heterodimers and the binding of agonists to the μ receptors of μ receptor/α2A-adrenoceptor heterodimers triggers conformational changes in α2A-adrenoceptors and decreases α2A-adrenoceptor-mediated activity (Vilardaga et al., 2008). Therefore, we hypothesized that the high levels of μ receptors and α2A-adrenoceptors expressed in the NTS would form μ receptor/α2A-adrenoceptor heterodimers, and this μ receptor/α2A-adrenoceptor heterodimer formation would lead to α2A-adrenoceptor dysfunction, which in turn would lead to BP elevation.
The current study sought to determine (i) whether μ receptors interacted with α2A-adrenoceptors to form heterodimers in the NTS; (ii) whether μ receptor/α2A-adrenoceptor heterodimer formation was correlated with the development of high BP; (iii) what mechanism(s) triggered μ receptor/α2A-adrenoceptor heterodimer formation in the NTS; and (iv) how μ receptor/α2A-adrenoceptor heterodimer formation in the NTS elevated BP. Our results demonstrated that increased levels of endogenous μ receptor agonists in the NTS induced μ receptor/α2A-adrenoceptor heterodimer formation and attenuated the NO-dependent depressor effect of α2A-adrenoceptor agonists. Such increases in endogenous opioids are likely to contribute to the pathogenesis of hypertension.
Methods
Animals
All animal care and experimental procedures complied with the Guidebook for the Care and Use of Laboratory Animals (The Chinese-Taipei Society of Laboratory Animal Sciences, Taipei, Taiwan) and were approved by the Institutional Animal Care and Use Committee of Kaohsiung Veterans General Hospital (VGH-KS). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 83 animals were used in the experiments described here.
All of the experimental animals were killed at the end of the experiments by pentobarbital overdose (200 mg·kg−1). Male normotensive rats [6- and 20-week-old Wistar-Kyoto (WKY) rats], prehypertensive 6-week-old spontaneous hypertensive rats (SHRs) and hypertensive 20-week-old SHRs (weighing approximately 250 to 300 g) were obtained from the National Laboratory Animal Center (Taipei, Taiwan) and housed in the animal room of VGH-KS.
Intra-NTS microinjection
Rats were anaesthetized with urethane (1.0 g·kg−1 i.p., supplemented with 300 mg·kg−1 i.v., if necessary). A polyethylene catheter was placed in the femoral vein for drug administration. The BP was measured via a femoral artery catheter using a pressure transducer and polygraph (Gould Electronics, Cleveland, OH, USA). The HR was monitored continuously using a tachograph preamplifier (13-4615-65, Gould Electronics). A tracheostomy was performed to maintain airway patency during the experiment. For brainstem nuclei microinjection, the anaesthetized rats were placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA) with the head flexed downward at a 45° angle. The dorsal surface of the medulla was exposed by limited craniotomy and the rats were rested for at least 1 h before the experiments. Single-barrel glass catheters (0.031 inch outer diameter, 0.006 inch internal diameter; Richland Glass Co, Vineland, NJ, USA) that had external tip diameters of 40 μm were prepared. To verify that the needle tip of the glass catheter was exactly in the NTS, L-glutamate (0.154 nmol·60 nL−1) was microinjected, which would induce a characteristically abrupt decrease in the BP (ΔBP ≥ −35 mmHg) if the needle tip was located precisely in the medial site of the intermediate one-third of the NTS with the following coordinates: anteroposterior, 0.0 mm; mediolateral, 0.5 mm and vertical, 0.4 mm (with the obex as a reference) (Tseng et al., 1996). The doses for each microinjection (all in 60 nL volumes) were DAMGO 0.3 nmol; guanfacine 0.3 nmol; ; D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) 0.18 nmol; L-NAME 33 nmol. All these drugs were from Sigma Chemical (St. Louis, MO, USA) and were dissolved in 0.9% saline.
Co-immunoprecipitation assay
Rat brainstems were removed and immediately frozen on a −20°C cold pad. The NTS was dissected using a micropunch (1 mm inner diameter) from a 1 mm thick brainstem slice at the level of the obex, under a microscope. The size of the punched tissue from a single NTS of one rat was ∼1 mm3. Total protein was prepared by homogenizing the NTS tissue in lysis buffer containing 20 mM imidazole hydrochloride (pH 6.8), 100 mM KCl, 2 mM MgCl2, 20 mM EGTA (pH 7.0), 300 mM sucrose, 1 mM NaF, 1 mM NaVO3, 1 mM Na2MoO4, 0.2% Triton X-100 and proteinase inhibitor cocktail (Roche, Barcelona, Spain) for 1 h at 4°C. The NTS lysate was incubated with 5 μL of rabbit anti-α2A-adrenoceptor (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or goat anti-μ receptor (1:50; Santa Cruz Biotechnology) antibodies. The catch and release immunoprecipitation system (Millipore Corporation, Billerica, MA, USA) was utilized according to the manufacturer's instructions. The eluted proteins were subjected to immunoblotting analysis using anti-α2A-adrenoceptor (1:1000; Santa Cruz Biotechnology) or anti-μ receptor (1:500; Santa Cruz Biotechnology) antibodies. The specificity of the rabbit anti-α2A-adrenoceptor and goat anti-μ receptor antibodies was confirmed in pilot experiments (Supporting Information Fig. S1).
Immunofluorescence staining
Anaesthetized rats were perfused with 0.9% saline, then with 4% formaldehyde, and finally with a 30% sucrose solution. The brainstem was then cut in the microtome at thickness of 20 μm. To examine the interaction between α2A-adrenoceptors and μ receptors, brainstem sections were incubated with rabbit anti-α2A-adrenoceptor (1:100; Santa Cruz Biotechnology) and goat anti-μ receptor (1:100; Santa Cruz Biotechnology) antibodies overnight. After washing with PBS, each section was incubated with Alexa Fluor 568 goat anti-rabbit IgG (1:50; Invitrogen, Carlsbad, CA, USA) and Alexa Fluor 488 donkey anti-goat IgG (1:100; Invitrogen) antibodies at 25°C for 2 h. After washing with PBS, each section was incubated with DAPI (1:100,000; Invitrogen) at 25°C for 10 min.
To examine the endomorphin-1 levels in NTS from SHRs, the sections were incubated with primary rabbit anti-endomorphin-1 (1:100; Abcam, Cambridge, UK) antibodies overnight at 4°C. After washing with PBS, the sections were incubated with Alexa Fluor 488 donkey anti-rabbit IgG antibody (1:200; Invitrogen) at 37°C for 2 h. The stained sections were examined using a confocal laser scanning microscope (Carl Zeiss LSM 5 PASCAL, Heidelberg, Germany).
Fluorescence in situ α2A-adrenoceptor and μ receptor proximity ligation assay
The Duolink in situ proximity ligation assay (PLA; OLINK Bioscience, Uppsala, Sweden) was utilized to detect the formation of μ receptor/α2A-adrenoceptor heterodimers. The pilot study of PLA specificity was performed on PC-12 and SH-SY5Y cell lines. Only the SH-SY5Y cell line, which expresses both μ receptor and α2A-adrenoceptor, could detect the signals of μ receptor/α2A-adrenoceptor heterodimers (Supporting Information Fig. S1). The NTS of prehypertensive and hypertensive SHRs and normotensive WKY rats were studied to determine the formation of α2A-adrenoceptor and μ receptor heterodimers in situ. The rats were perfused first with saline, then with 4% formaldehyde, and finally with 30% sucrose. The brainstem was then cut in the microtome at thickness of 5 μm. Primary antibody diluent (OLINK Bioscience) with two primary antibodies (1:100 rabbit anti-α2A-adrenoceptor antibodies and 1:100 for goat anti-μ receptor antibodies) was added to the sections and incubated overnight at 4°C. Then, the PLA probe anti-rabbit plus and anti-goat minus (OLINK Bioscience), which were secondary antibody with specific oligonucleotides, were applied to the sections and incubated for 2 h at 37°C. Samples were treated with a ligation solution (allowing nearby oligonucleotide probe pairs to form a closed circle) and an amplification solution containing polymerase and fluorescently labelled oligonucleotides was also added, allowing rolling-circle amplification and the detection of a discrete fluorescent spot. All of the procedures were performed according to the manufacturer's instructions. The images of the sections were acquired using a confocal laser scanning microscope (Carl Zeiss LSM 5 PASCAL). Images for each slide with the PLA were analysed with LSM 5 PASCAL software (Version 3.5, Carl Zeiss), which automatically counts the number of spots per areas. All the experiments were repeated at least three times by in situ PLA.
Measurement of NO in the NTS
The NTS protein lysate were deproteinized using a Microcon YM-30 (Millipore, Bedford, MA, USA). The degradation products of NO (as nitrate) in the samples was determined using the purge system of a Sievers NO analyzer (NOA 280i; Sievers Instruments, Boulder, CO, USA) and a modified procedure based on chemiluminescence (Tseng et al., 1996). Samples (10 μL) were injected into a reflux column containing 0.1 mol·L−1 VCl3 in 1 mol·L−1 HCl at 80°C to reduce any nitrates or nitrites (NOx) to NO. NO then combined with O3 produced by the analyzer to form NO2. The resulting emission from the excited NO2 was detected by a photomultiplier tube and recorded digitally (mV). The values were then plotted on a standard curve of NaNO3 concentrations determined simultaneously. Measurements were collected in triplicate for each sample.
Data analysis
All data are expressed as means ± SEM. The paired Student's t-test (for comparisons of cardiovascular parameters before and after treatment), Student's t-test (for control and study group comparisons) or one-way anova with Scheffé post hoc comparisons was applied to compare the differences between groups. Differences with P < 0.05 were considered statistically significant.
Results
Heterodimers are formed between μ receptors and α2A-adrenoceptors in the NTS
The presence of μ receptors and α2A-adrenoceptors in the NTS was confirmed via immunofluorescence staining. Both μ receptors and α2A-adrenoceptors were expressed in the NTS of WKY rats and these receptors were colocalized in the neuronal cells of the NTS (Figure 1B). To provide additional support for the interaction between α2A-adrenoceptors and μ receptors, co-immunoprecipitation experiments were performed. These tests revealed protein–protein interactions between μ receptors and α2A-adrenoceptors in the neuronal cells of the NTS in both normotensive and hypertensive rats (Figure 1C). The results of the PLA also showed that the μ receptors and α2A-adrenoceptors form heterodimers in the NTS (Figure 1D).
Figure 1.
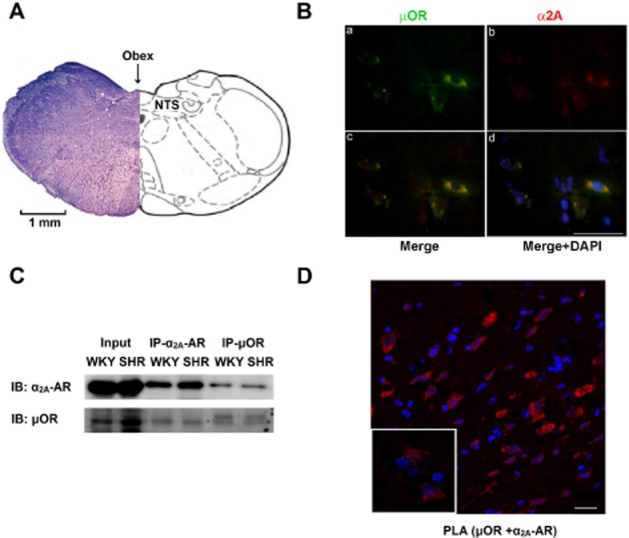
Formation of μ receptor and α2A-adrenoceptor heterodimers in the NTS. (A) Cross-section of the medulla oblongata rostral to the obex, indicating the location of the NTS. (B) Representative immunofluorescence images of the NTS showing that μ receptors (μOR; a, green) and α2A-adrenoceptors (α2A; b, red) were colocalized (c, d) in the neuronal cells of the NTS of WKY rats. (C) Confirmation of the μ receptor (μOR) and α2A-adrenoceptor (α2A-AR) interaction using co-immunoprecipitation from an NTS protein lysate. (D) Confirmation of μ receptor/α2A-adrenoceptor heterodimer formation in the NTS of SHRs using in situ PLA. Red, μ receptor/α2A-adrenoceptor heterodimers; blue, DAPI. Scale bar: 20 μm.
Increases in endomorphin-1 in the NTS of hypertensive rats
We compared the levels of the endogenous μ receptor agonist, endomorphin-1, in the NTS of normotensive rats; prehypertensive, 6-week-old SHRs; and hypertensive, 20-week-old SHRs. The level of endomorphin-1 in the NTS of hypertensive SHRs was higher than that in prehypertensive SHRs and WKY rats (Figure 2A, P = 0.001, n = 6). No difference was observed in the endomorphin-1 levels between prehypertensive SHRs and WKY rats.
Figure 2.
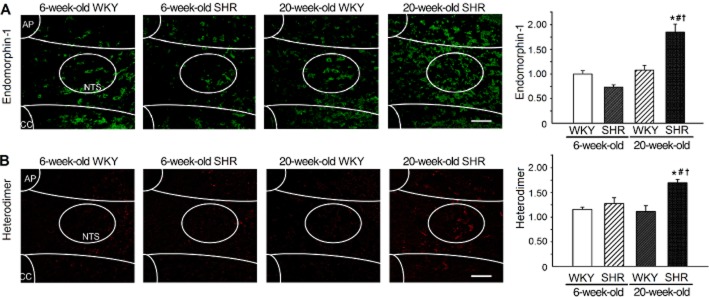
The NTS of hypertensive SHRs contains more endomorphin-1 and μ receptor/α2A-adrenoceptor heterodimers than found in normotensive rats. (A) Immunofluorescence images (green) and the summary results of endomorphin-1 in the NTS of normotensive rats and hypertensive, 20-week-old SHRs. (B) The PLA images (red) and the summary results of μ receptor/α2A-adrenoceptor heterodimers in the NTS of normotensive rats and hypertensive, 20-week-old SHRs. Scale bar: 20 μm. All values are means ± SEM (n = 6). *P < 0.05, significantly different from 6-week-old WKY rats. #P < 0.05, significantly different from 6-week-old SHRs. †P < 0.05, significantly different from 20-week-old WKY rats.
Increases in μ receptor/α2A-adrenoceptor heterodimers in the NTS of hypertensive rats
To test the association between μ receptor/α2A-adrenoceptor heterodimer formation and the development of hypertension in rats, we compared the number of μ receptor/α2A-adrenoceptor heterodimers in the NTS of normotensive rats, prehypertensive SHRs and hypertensive SHRs. The number of heterodimers in the NTS of hypertensive SHRs was higher than that of the prehypertensive SHRs and WKY rats (Figure 2B). The NTS of hypertensive SHRs contained approximately 40% more μ receptor/α2A-adrenoceptor heterodimers than those of age-matched WKY rats (Figure 2B, P = 0.039, n = 6).
Elevation of endogenous μ-opioids in the NTS of SHRs induces hypertension and μ receptor/α2A-adrenoceptor heterodimer formation
Hypertensive SHRs had the highest level of endomorphin-1 in their NTS. We blocked the μ receptors with the μ receptor-specific antagonist CTAP. The BP of the hypertensive SHRs began to decrease gradually to reach a minimum approximately 30 min after intra-NTS microinjection of CTAP. However, BP did not fall after the intra-NTS CTAP microinjection in normotensive rats (Figure 3A). The effect of endogenous μ receptor agonists in the induction of μ receptor/α2A-adrenoceptor heterodimer formation was then assessed. Thirty minutes after the intra-NTS CTAP microinjection, a 35% reduction in μ receptor/α2A-adrenoceptor heterodimers was observed in the NTS of hypertensive SHRs (Figure 3B and C, P = 0.017, n = 6).
Figure 3.
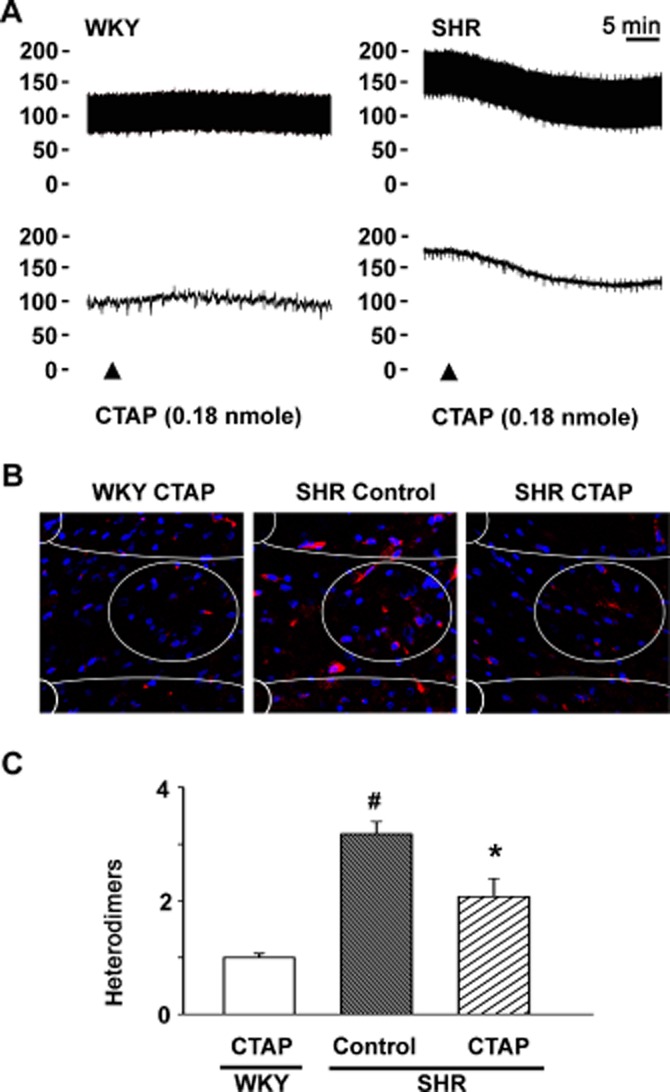
Effects of blocking μ receptors in the NTS of SHRs on μ-opioid receptor/α2A-adrenoceptor heterodimer formation and hypertension. (A) Representative BP recordings of an intra-NTS microinjection of the μ receptor antagonist CTAP in WKY rats and hypertensive SHRs. (B) The PLA images (red) and (C) summary results of μ receptor/α2A-adrenoceptor heterodimers in the NTS of WKY rats and hypertensive SHRs, 30 min after intra-NTS CTAP microinjection. Scale bar: 20 μm. Values shown are means ± SEM (n = 6). #P < 0.05, significantly different from WKY rats. *P < 0.05, significantly different from control SHRs.
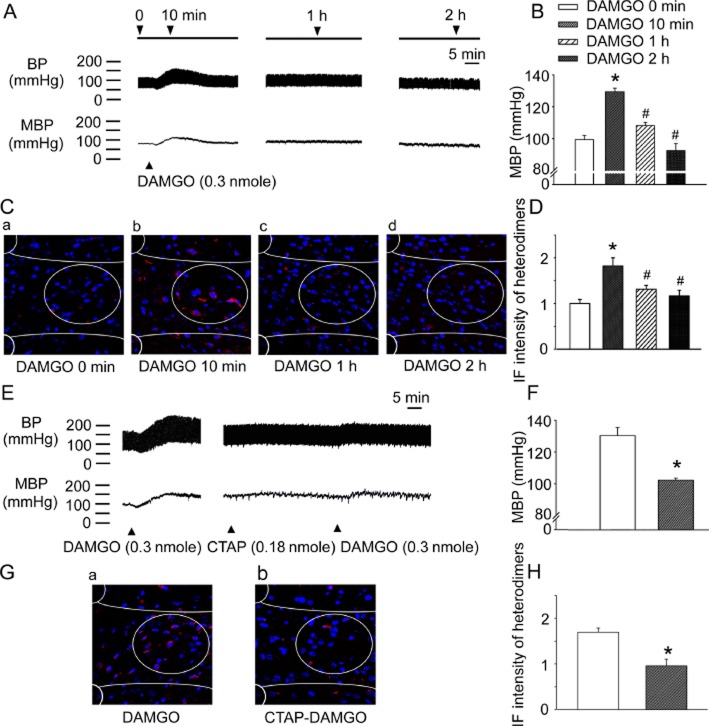
Exogenous μ receptor agonist given to the NTS induces hypertension and heterodimer formation
A μ receptor-specific agonist, DAMGO, was microinjected into the NTS of WKY rats (Supporting Information Fig. S2) and the changes in BP and the amount of μ receptor/α2A-adrenoceptor heterodimers in the NTS were measured. The pressor effect of intra-NTS DAMGO microinjection was most prominent 10 min after administration (Figure 4A and Supporting Information Fig. S3A; P = 0.0001, n = 6) and BP elevation diminished gradually, although BP was still elevated 1 h post-DAMGO microinjection (Figure 4B; P = 0.0001, n = 6). Moreover, DAMGO-induced μ receptor/α2A-adrenoceptor heterodimer formation was most marked 10 min after the DAMGO microinjection (Figure 4B and Supporting Information Fig. S3B, P = 0.0001, n = 6). The heterodimer levels decreased gradually but remained higher than control levels at 1 h (Figure 4D; 10 vs. 0 min, P = 0.0001; 10 min vs. 1 h, P = 0.012; 10 min vs. 2 h, P = 0.001, n = 6) and the increases in μ receptor/α2A-adrenoceptor heterodimers in the NTS of WKY rats were correlated with BP elevation after DAMGO microinjection (Supporting Information Fig. S3C). CTAP pretreatment blocked the DAMGO-induced pressor effect (Figure 4E and 4F, P = 0.0001, n = 6). CTAP pretreatment also inhibited DAMGO-induced μ receptor/α2A-adrenoceptor heterodimer formation (Figure 4G and 4H, P = 0.005, n = 6). These results indicate that the μ receptor agonist DAMGO triggers μ receptor/α2A-adrenoceptor heterodimer formation in the NTS and also raised BP.
Figure 4.
A microinjection of exogenous μ receptor agonist into the NTS of WKY rats induces μ receptor/α2A-adrenoceptor heterodimer formation and BP elevation. (A) Representative BP recordings and (B) the summary results of BP recordings at different time points after intra-NTS DAMGO injection. (C) Representative PLA images (red) and (D) the summary results of μ receptor/α2A-adrenoceptor heterodimers at different time points after intra-NTS. DAMGO microinjection. (E) The representative BP recordings and (F) summary results demonstrate that CTAP blocked the BP-elevating effect of intra-NTS DAMGO administration. (G) The representative PLA images (red) and (H) summary results demonstrate that CTAP pretreatment reduced the DAMGO-induced formation of μ receptor/α2A-adrenoceptor heterodimers. Values shown are means ± SEM (n = 6). *P < 0.01, significantly different from the control group. #P < 0.05, significantly different from the 10 min group. Scale bar: 20 μm.
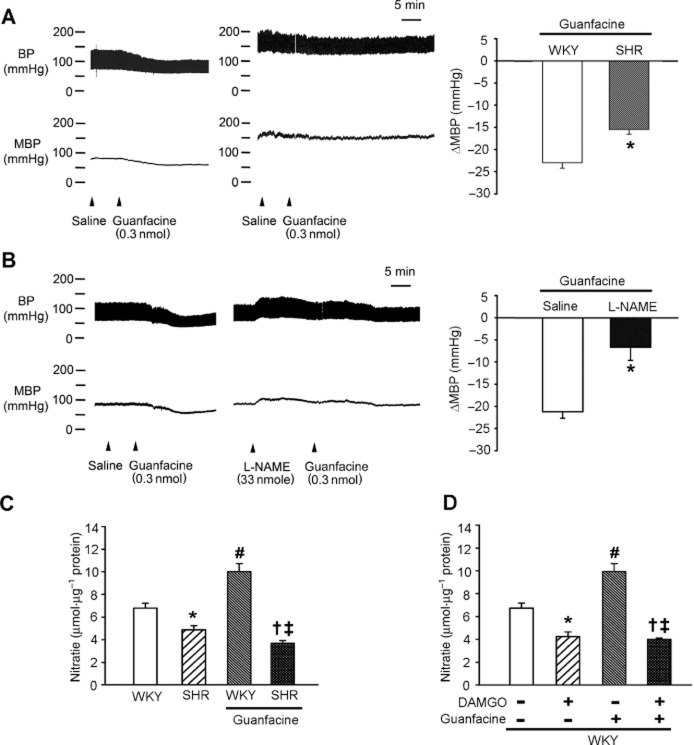
Formation of μ receptor/α2A-adrenoceptor heterodimer impairs the NO-dependent depressor effect of α2A-adrenoceptor agonists in the NTS
We also investigated how μ receptor/α2A-adrenoceptor heterodimers affect BP regulation in the NTS. The BP of WKY rats was lowered by activating their α2A-adrenoceptors with an intra-NTS microinjection of the α2A-adrenoceptor-specific agonist guanfacine (Figure 5 and Supporting Information Figs. S2 and S3A). The BP-lowering effect of intra-NTS guanfacine was significantly attenuated in hypertensive SHRs (Figure 5A; P = 0.016, n = 6). These data indicate that the α2A-adrenoceptor agonist lost its BP-lowering effect in the NTS of SHRs, where more μ receptor/α2A-adrenoceptor heterodimer formation was observed.
Figure 5.
Formation of μ receptor/α2A-adrenoceptor heterodimers impairs the NO-dependent BP-lowering effect of a α2A-adrenoceptor agonist in the NTS. (A) Representative BP recordings and the summary results (means ± SEM; n = 6) of the different BP-lowering effects of guanfacine (the α2A-adrenoceptor agonist) in the NTS of WKY rats and SHRs. *P < 0.05, significantly different from WKY. (B) Representative BP recordings and the summary results (means ± SEM; n = 6) of the effect of the NOS inhibitor L-NAME on the BP-lowering effect of α2A-adrenoceptor activation in the NTS. *P < 0.05, significantly different from saline. (C) A bar graph (means ± SEM; n = 6) illustrating the different NO production in the NTS of WKY rats and SHRs, with or without guanfacine treatment. *P < 0.05, significantly different from WKY; #P < 0.05, significant effect of guanfacine in WKY; †P < 0.05, significant effect of guanfacine in SHR; ‡P < 0.05, significantly different from WKY + guanfacine group. (D) A bar graph (means ± SEM; n = 6) illustrating the differences of NO production in the NTS of WKY rats with or without DAMGO, guanfacine or both types of treatment. *P < 0.05, significant effect of DAMGO; #P < 0.05, significant effect of guanfacine; †P < 0.05, significantly different from DAMGO alone; ‡P < 0.05, significantly different from guanfacine alone
Our previous studies demonstrated that NO is one of the key BP-regulatory molecules in the NTS and the NO production in the NTS of SHRs is lower than that of WKY rats. We tested whether μ receptor/α2A-adrenoceptor heterodimer-associated α2A-adrenoceptor dysfunction was caused by a reduction of NO synthesis in the NTS. The depressor effect of guanfacine in the NTS of WKY rats was significantly blocked by pretreatment with L-NAME (Figure 5B; P = 0.002, n = 6). The NO production in the NTS increased after guanfacine microinjection (Figures 5C and D; P = 0.001, n = 6). The NO production in the NTS of SHRs was approximately 60% lower than that of the NTS of WKY rats after the same intra-NTS guanfacine microinjection dose (4.24 ± 0.41 vs. 9.93 ± 0.72, P = 0.0001, n = 6; Figure 5D).
Because the intra-NTS microinjection of DAMGO induced μ-opioid receptor/α2A-adrenoceptor heterodimer formation (Figure 4C), the induction of NO by guanfacine was inhibited in the DAMGO-pretreated NTS of WKY rats (Figure 5D; P = 0.001, n = 6). These results indicated that the depressor effect of α2A-adrenoceptor agonists given to the NTS was NO-dependent and that μ receptor/α2A-adrenoceptor heterodimers impair the induction of NO synthesis by α2A-adrenoceptor agonists.
Discussion
The present study demonstrated that (i) endomorphin-1 levels were increased in the NTS of hypertensive SHRs; (ii) μ receptors form heterodimers with α2A-adrenoceptors in the NTS; (iii) high levels of μ receptor agonists trigger μ receptor/α2A-adrenoceptor heterodimer formation in the NTS, which might contribute to the development of hypertension; and (iv) μ receptor/α2A-adrenoceptor heterodimer formation impairs the NO-dependent depressor effect of α2A-adrenoceptor agonists in the NTS. Previous reports support these findings, indicating that μ receptors can form heterodimers with α2A-adrenoceptors (Jordan et al., 2003) and that μ receptor/α2A-adrenoceptor heterodimer formation impairs the function of α2A-adrenoceptors (Vilardaga et al., 2008). Our results also suggest that μ receptor/α2A-adrenoceptor heterodimer formation in the NTS contributes to the genesis of hypertension by impairing the ability of α2A-adrenoceptor agonists to lower BP.
Studies in the 1970s and 1980s first proposed that GPCRs might exist as dimers and the first evidence of this dimerization was provided in 1996 (Hebert et al., 1996). Recent evidence indicating the existence of GPCR dimers (Casado et al., 2009) has challenged the traditional concept of monomeric GPCRs as functional units. The discovery of GPCR heterodimers provided evidence of novel pharmacological properties that are distinct from those of either monomeric receptor, including alterations in ligand binding, receptor activation, desensitisation and trafficking (George et al., 2002). Emerging experimental and clinical data indicate that increased levels of GPCR heterodimers are associated with certain diseases. For example, angiotensin II-induced hypersensitivity in pre-eclampsia is caused by increased levels of angiotensin AT1 receptor/bradykinin B2 receptor heterodimers (AbdAlla et al., 2001); decreased responsiveness to morphine-induced analgesia is caused by increased levels of μ-/δ-opioid receptor heterodimers in spinal cord neurons (George et al., 2000); the reduced bronchodilator response to β2-adrenoceptor agonists in asthma is caused by increased levels of prostaglandin EP1 receptor/β2-adrenoceptor heterodimers in airway smooth muscle (McGraw et al., 2006); and psychosis might be caused by inhibiting 5-HT2A agonist-induced signalling via 5-HT2A receptor/mGlu receptor heterodimers in cortical pyramidal neurons (Gonzalez-Maeso et al., 2008).
The current study demonstrated that μ receptors are able to form heterodimers with α2A-adrenoceptors in the NTS of rats. Research on GPCR dimers has been dominated by techniques involving the use of recombinant cell lines expressing mutant receptors such as FRET, BRET and protein complementation assays. These techniques cannot be applied in vivo or even in primary cell cultures (Teitler and Klein, 2012). The current study used a newly developed method, PLA, to detect heterodimer formation in vivo. PLA can detect nearby proteins within tens of nanometres (Leuchowius et al., 2009) and uses primary antibodies raised in different host species to detect the native receptors/proteins of interest in vivo. Oligonucleotide-conjugated secondary antibodies are then used to detect the primary antibodies. If two secondary antibodies are close enough (<40 nm), then the complementary oligonucleotides (linked to each of the secondary antibodies) would hybridize to form a template. The template can then be amplified repeatedly, yielding a powerful and specific signal amplification (Fredriksson et al., 2002). Each spot on an array indicates an independent protein–protein interaction or dimerization. PLA has the advantage that it can detect the dimerization or protein–protein interaction without overexpression of recombinant proteins, has a high sensitivity with less background and provides the unique ability to quantify dimerization signals. However, PLA also has a drawback. As an antibody-based assay, its success requires specific and high-affinity antibody-binding conditions. This requirement emphasizes the need for positive and negative controls for each protein–protein or ligand–receptor interaction tested.
How GPCR dimers form under pathophysiological conditions remains unclear. Some studies have demonstrated that GPCR dimers were formed from the endoplasmic reticulum but most studies have reported that GPCR dimers were formed by ligand induction (George et al., 2002). The current study provided three major results: (i) higher concentrations of endomorphin-1 and μ receptor/α2A-adrenoceptor heterodimers were found in the NTS of hypertensive SHRs (Figure 2); (ii) blockade of the μ receptors, with the selective antagonist CTAP, reduced the number of μ receptor/α2A-adrenoceptor heterodimers (Figure 3); and (iii) microinjecting the μ receptor agonist DAMGO into the NTS increased μ receptor/α2A-adrenoceptor heterodimer formation (Figure 4). These results suggest that high levels of endogenous μ receptor agonists would induce heterodimer formation.
The current study also revealed that (i) blockade of μ receptors in the NTS with CTAP markedly reduced BP and μ receptor/α2A-adrenoceptor heterodimers in hypertensive SHRs (Figure 3C) (ii) and microinjecting the μ receptor agonist DAMGO into the NTS of WKY rats elevated BP as well as increasing the number of μ receptor/α2A-adrenoceptor heterodimers (Figure 4). These results suggest that high levels of endogenous μ receptor agonists in the NTS could cause hypertension.
Furthermore, neither reducing BP, using oral hydralazine (16 mg·kg−1·day−1) in hypertensive rats, nor elevating BP, via bilateral intra-NTS L-NAME microinjection in normotensive rats, had any effect on the number of μ receptor/α2A-adrenoceptor heterodimers in the NTS (Supporting Information Figs. S4 and S5). These results suggest that μ receptor/α2A-adrenoceptor heterodimer formation was the cause rather than a consequence, of hypertension.
Our study suggests some mechanisms that account for the observations made decades ago regarding the role of μ receptor agonists in the NTS relevant to the pathogenesis of hypertension. For instance, activation of μ receptors with DAMGO in the NTS caused pressor responses (De Jong et al., 1983; Hassen and Feuerstein, 1987). The intraventricular administration of the μ receptor antagonist naloxone produced a dose-dependent reduction to BP in SHRs but not WKY rats (Delbarre et al., 1982), and the chronic infusion of naloxone prevented the development of hypertension (Quock et al., 1984).
Sympathetic hyperactivity has gradually been accepted as an important contributor to the pathogenesis of hypertension (Colombari et al., 2001). The NTS is the primary integrative centre for sympathetic and cardiovascular control in the CNS (Ho et al., 2008) and ablation of the NTS can result in BP elevation and lead to sustained or lethal hypertension (Lu et al., 2013). α2A-Adrenoceptors are highly expressed in the NTS and their blockade can attenuate baroreflex responses and induce hypertension (Bhuiyan et al., 2009). The current study found that (i) the NO-dependent depressor effect of α2A-adrenoceptor agonists was impaired by the μ receptor/α2A-adrenoceptor heterodimers induced by a μ receptor agonist in the NTS (Figure 5D); (ii) the level of heterodimers in the NTS was correlated with the progression of hypertension in SHRs (Figure 2B); and (iii) less NO production was observed in the NTS of hypertensive SHRs (Figure 5C). These results suggest that impairment of the NO-dependent, depressor effect of α2A-adrenoceptor agonists by the formation of μ receptor/α2A-adrenoceptor heterodimers in the NTS, contributes to the genesis of hypertension (Figure 6).
Figure 6.
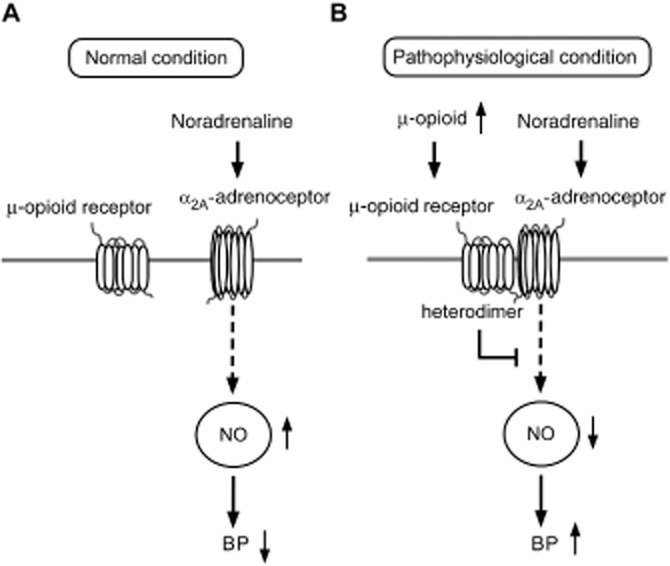
The proposed pathogenetic mechanisms of α2A-adrenoceptor and μ receptor heterodimer induced hypertension in the NTS. (A) Under normal conditions, endogenous noradrenaline binds to α2A-adrenoceptor and increases NO production in the NTS. The physiological concentration of NO in the NTS maintains BP within the normal range. (B) Under pathophysiological conditions that result from environmental factors or genetic abnormalities, the level of endogenous μ-opioid agonists increases in the CNS. These additional peptides can then induce additional μ receptor/α2A-adrenoceptor heterodimer formation. In turn, fewer functional and free α2A-adrenoceptor molecules remain in the NTS; subsequently, less NO is produced, ultimately leading to BP increases.
We found that the hypotensive effects of activating α2-adrenoceptors via an intra-NTS guanfacine microinjection were attenuated in hypertensive SHRs (Figure 5A), whereas Zandberg and de Jong did not find a difference between SHRs and WKY rats using a microinjection of α-methylnoradrenaline into the NTS (Zandberg and de Jong, 1979). This difference might reflect the activity of α-methylnoradrenaline as an agonist at β-adrenoceptors (De Jong and Nijkamp, 1976) and the higher affinity of guanfacine for α2A-adrenoceptors (Uhlen and Wikberg, 1991; Kanagy, 2005). The activation of central α2A-adrenoceptors should be more effective as an antihypertensive mechanism (Kanagy, 2005). In addition, the microinjection of an α2-adrenoceptor agonist in the NTS of SHRs is less effective in lowering BP because this strain has a significantly lower α2-adrenoceptor density and sensitivity in the NTS, compared with WKY rats (Nomura et al., 1985; Yamada et al., 1989). These observations are consistent with the diminished BP-lowering effect of intra-NTS α2-adrenoceptor agonist injection in hypertensive SHRs.
Interestingly, Taylor et al. demonstrated that SHRs were hypoalgesic in the hot plate test (Taylor et al., 2001). A significant relationship between hypertension and decreased pain sensitivity has also been observed in humans (Bruehl et al., 2010). This phenomenon is referred to as ‘hypertensive hypoalgesia’ (Campbell et al., 2003) and is believed to reflect a homeostatic feedback loop that maintains a stable BP in the presence of noxious stimuli. However, the underlying mechanisms of this phenomenon remain unclear. In healthy individuals, hypertensive hypoalgesia had an endogenous opioid-mediated component (Bruehl et al., 2010). The normalization of BP using antihypertensive drugs or a low-salt diet did not ameliorate the hypoalgesia of patients with hypertension. Furthermore, plasma β-endorphin levels were significantly higher among patients with hypertension (Campbell et al., 2003) and the β-endorphin levels in the pituitary, hypothalamus and spinal cord of SHRs were higher than those in normotensive rats (Guasti et al., 1996). These results suggest that increased endogenous opioids are an important pathogenic factor for both hypertension and hypoalgesia. Our results demonstrate that endomorphin-1 was increased in hypertensive SHRs (Figure 2A) and the activation of μ receptors induced μ receptor/α2A-adrenoceptor heterodimer formation in the NTS (Figure 4C). Increases in μ receptor/α2A-adrenoceptor heterodimers inhibited the depressor effect of α2A-adrenoceptors (Figure 5A). Therefore, our results might at least partially explain the underlying mechanism of hypertensive hypoalgesia.
In conclusion, under certain pathophysiological circumstances, the increased release of endogenous μ receptor agonists in the NTS will induce μ receptors to form heterodimers with α2A-adrenoceptors. Once formed into heterodimers with μ receptors, α2A-adrenoceptors lose their ability to exert NO-dependent depressor effects, following activation with appropriate agonists. The inappropriate release of μ-opioid peptides and the formation of μ receptor/α2A-adrenoceptor heterodimers might contribute to the pathogenesis of hypertension.
Funding
This work was supported by funding from the National Science Council (NSC98-2320-B-075B-001-MY3, NSC98-2321-B-075B-002, NSC99-2321-B-075B-002, NSC100-2321-B-075B-002) and Kaohsiung Veterans General Hospital (VGHKS 100-101) to Dr. C.-J. T. and NSC100-2320-B-037-021 to Dr. W.-Y. H.
Conflict of interest
None declared.
Author contributions
The study was conceived and designed by C.-J. T., P.-J. L. and M. H. G.-C. S. conducted most of the studies with assistance from W.-Y. H., B.-R. C. and P.-W. C. The paper was written by G.-C. S., with contributions from W.-H. C., T.-C. Y. and M.-C. H.
Glossary
Abbreviations
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- DAMGO
[D-Ala2, MePhe4, Gly5-ol]-enkephalin
- HR
heart rate
- NTS
nucleus tractus solitarii
- PBN
parabrachial nucleus
- PLA
proximity ligation assay
- SHR
spontaneous hypertensive rat
- WKY
Wistar-Kyoto rats
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Specificity antibody and PLA tests. PC12 and SH-SY5Y cell lines were used to determine the specificity of the antibodies used in the immunoblotting and PLA assays. The PC-12 cell line expresses α2A-adrenoceptors but not μ receptors. However, the SH-SY5Y cell line expresses both μ receptors and α2A-adrenoceptors. (A) The binding patterns of μ receptors and α2A-adrenoceptors were captured with film by applying immunoblotting detection using primary rabbit anti-α2A-adrenoceptor or goat anti-μ receptor antibodies on PC12 or SH-SY5Y cell lines. Our pilot immunoblotting tests revealed that the antibodies used to perform the whole-cell lysates were able to specifically recognize α2A-adrenoceptors and μ receptors. (B) PLA was performed using a combination of the same two primary antibodies followed by the PLA probes anti-rabbit plus and anti-goat minus secondary antibodies with specific oligonucleotides to detect μ receptor/α2A-adrenoceptor heterodimers. PLA signals were not captured for the PC12 cell line (which expresses α2A-adrenoceptors but not μ-opioid receptors). (C) The same experiment, conducted on the SH-SY5Y cell line (which expresses both α2A-adrenoceptors and μ receptors), showed a μ receptor/α2A-adrenoceptor heterodimer signal. Scale bar: 20 μm.
Figure S2 Representative traces of the cardiovascular dose responses after the intra-NTS microinjection of guanfacine and DAMGO. (A) Microinjections of 0.1, 0.3 and 1 nmol guanfacine into anaesthetized WKY rats’ NTS elicited a dose-dependent depressor effect. (B) Microinjections of 0.1, 0.3 and 1 nmol DAMGO elicited a dose-dependent hypertensive effect. A saline vehicle did not alter BP.
Figure S3 μ receptor agonist-induced μ receptor/α2A-adrenoceptor heterodimer formation in the NTS of WKY rats leading to BP elevation. (A) Microinjection of the μ receptor agonist DAMGO (0.3 nmol) into the NTS of WKY rats elicited a sustained BP elevation that lasted for more than 1 h. The microinjection of the α2A-adrenoceptor agonist guanfacine (0.3 nmol) into the NTS caused a depressor effect. (B) Immunofluorescence PLA images of the NTS of WKY rats 10 min after treatment with saline (a), DAMGO (b) or guanfacine (c). The level of μ receptor/α2A-adrenoceptor heterodimers increased markedly after DAMGO microinjection (b) but not after guanfacine microinjection (c). The summary data for immunofluorescence intensity indicated a 1.6-fold increase in μ receptor/α2A-adrenoceptor heterodimer formation in the NTS after DAMGO treatment (d). (C) The amount of μ receptor/α2A-adrenoceptor heterodimers in the NTS were correlated with the mean BP of WKY rats. (γ2 = 0.9241, P < 0.001). Red, heterodimers; blue, DAPI. The values are presented as the mean ± SEM (n = 5). * indicates P < 0.05 compared with the control value. Scale bar: 5 μm. MBP, mean BP.
Figure S4 Anti-hypertensive treatment did not change μ receptor/α2A-adrenoceptor heterodimer formation in the NTS of hypertensive SHRs. (A) Representative BP recordings 2 weeks after oral hydralazine (16 mg·kg−1·day−1) treatment of WKY rats and hypertensive SHRs; (B) Statistical and (C) PLA images (red) results of μ receptor/α2A-adrenoceptor heterodimers in the NTS of WKY rats and hypertensive SHRs with oral hydralazine treatment. Scale bar: 20 μm. Values are shown as the mean ± SEM (n = 6). *P < 0.05 compared with control SHRs.
Figure S5 Induced hypertension in WKY rats did not affect heterodimer formation in the NTS. (A) Representative BP recordings of the bilateral intra-NTS microinjection of L-NAME in a WKY rat; (B) PLA images (red) and (C) the statistical results of μ receptor/α2A-adrenoceptor heterodimers in the NTS of WKY rats 30 min post-L-NAME microinjection. Scale bar: 20 μm. Values are shown as the mean ± SEM (n = 6).
References
- AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: overview. Br J Pharmacol. 2013;170:1449–1458. [Google Scholar]
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, et al. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Bhuiyan ME, Waki H, Gouraud SS, Takagishi M, Cui H, Yamazaki T, et al. Complex cardiovascular actions of alpha-adrenergic receptors expressed in the nucleus tractus solitarii of rats. Exp Physiol. 2009;94:773–784. doi: 10.1113/expphysiol.2008.046490. [DOI] [PubMed] [Google Scholar]
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Magid E, Chont M, Gilliam W, et al. Hypoalgesia associated with elevated resting blood pressure: evidence for endogenous opioid involvement. J Behav Med. 2010;33:168–176. doi: 10.1007/s10865-009-9241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TS, Ditto B, Seguin JR, Sinray S, Tremblay RE. Adolescent pain sensitivity is associated with cardiac autonomic function and blood pressure over 8 years. Hypertension. 2003;41:1228–1233. doi: 10.1161/01.HYP.0000072802.84202.86. [DOI] [PubMed] [Google Scholar]
- Casado V, Cortes A, Mallol J, Perez-Capote K, Ferre S, Lluis C, et al. GPCR homomers and heteromers: a better choice as targets for drug development than GPCR monomers? Pharmacol Ther. 2009;124:248–257. doi: 10.1016/j.pharmthera.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Lu PJ, Hsiao M, Hsiao CH, Ho WY, Cheng PW, et al. Renin activates PI3K-Akt-eNOS signalling through the angiotensin AT1 and Mas receptors to modulate central blood pressure control in the nucleus tractus solitarii. Br J Pharmacol. 2012;166:2024–2035. doi: 10.1111/j.1476-5381.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR, Jr, Lopes OU. Role of the medulla oblongata in hypertension. Hypertension. 2001;38:549–554. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- De Jong W, Nijkamp FP. Centrally induced hypotension and bradycardia after administration of alpha-methylnoradrenaline into the area of the nucleus tractus solitarii of the rat. Br J Pharmacol. 1976;58:593–598. doi: 10.1111/j.1476-5381.1976.tb08628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong W, Petty MA, Sitsen JM. Role of opioid peptides in brain mechanisms regulating blood pressure. Chest. 1983;83:306–308. doi: 10.1378/chest.83.2_supplement.306. [DOI] [PubMed] [Google Scholar]
- Delbarre B, Casset-Senon D, Delbarre G, Sestillange P, Christin O. Naloxone effects on blood pressure, analgesia and diuresis in spontaneous hypertensive and normotensive rats. Neurosci Lett. 1982;30:167–172. doi: 10.1016/0304-3940(82)90291-9. [DOI] [PubMed] [Google Scholar]
- Evans RG, Ludbrook J. Effects of mu-opioid receptor agonists on circulatory responses to simulated haemorrhage in conscious rabbits. Br J Pharmacol. 1990;100:421–426. doi: 10.1111/j.1476-5381.1990.tb15822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, et al. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Pickel VM. Alpha(2A)-adrenergic receptors are present in mu-opioid receptor containing neurons in rat medial nucleus tractus solitarius. Synapse. 2002;43:208–218. doi: 10.1002/syn.10036. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Aicher SA, Milner TA, Pickel VM. Subcellular localization of alpha-2A-adrenergic receptors in the rat medial nucleus tractus solitarius: regional targeting and relationship with catecholamine neurons. J Comp Neurol. 2001;433:193–207. doi: 10.1002/cne.1135. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasti L, Cattaneo R, Daneri A, Bianchi L, Gaudio G, Regazzi MB, et al. Endogenous beta-endorphins in hypertension: correlation with 24-hour ambulatory blood pressure. J Am Coll Cardiol. 1996;28:1243–1248. doi: 10.1016/S0735-1097(96)00312-9. [DOI] [PubMed] [Google Scholar]
- Hassen AH, Feuerstein G. mu-Opioid receptors in NTS elicit pressor responses via sympathetic pathways. Am J Physiol. 1987;252:H156–H162. doi: 10.1152/ajpheart.1987.252.1.H156. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Riley AP, Felder RB. alpha(2)-Adrenergic receptors in NTS facilitate baroreflex function in adult spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2002;282:H2336–H2345. doi: 10.1152/ajpheart.00167.2001. [DOI] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Ho WY, Lu PJ, Hsiao M, Hwang HR, Tseng YC, Yen MH, et al. Adenosine modulates cardiovascular functions through activation of extracellular signal-regulated kinases 1 and 2 and endothelial nitric oxide synthase in the nucleus tractus solitarii of rats. Circulation. 2008;117:773–780. doi: 10.1161/CIRCULATIONAHA.107.746032. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- Kanagy NL. Alpha(2)-adrenergic receptor signalling in hypertension. Clin Sci. 2005;109:431–437. doi: 10.1042/CS20050101. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchowius KJ, Weibrecht I, Landegren U, Gedda L, Soderberg O. Flow cytometric in situ proximity ligation analyses of protein interactions and post-translational modification of the epidermal growth factor receptor family. Cytometry A. 2009;75:833–839. doi: 10.1002/cyto.a.20771. [DOI] [PubMed] [Google Scholar]
- Lu WH, Hsieh KS, Lu PJ, Wu YS, Ho WY, Lai CC, et al. Hexamethonium reverses the lethal cardiopulmonary damages in a rat model of brainstem lesions mimicking fatal enterovirus 71 encephalitis. Crit Care Med. 2013;41:1276–1285. doi: 10.1097/CCM.0b013e3182771364. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Ohtsuji M, Nagata Y. Changes in the alpha-adrenoceptors in the medulla oblongata including nucleus tractus solitarii of spontaneously hypertensive rats. Neurochem Res. 1985;10:1143–1154. doi: 10.1007/BF00965888. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. C-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quock RM, Vaughn LK, Kouchich FJ. Influence of chronic naloxone treatment on development of hypertension in the spontaneously hypertensive rat. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:88–90. doi: 10.1007/BF00507060. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Roderick RE, St Lezin E, Basbaum AI. Hypoalgesia and hyperalgesia with inherited hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R345–R354. doi: 10.1152/ajpregu.2001.280.2.R345. [DOI] [PubMed] [Google Scholar]
- Teitler M, Klein MT. A new approach for studying GPCR dimers: drug-induced inactivation and reactivation to reveal GPCR dimer function in vitro, in primary culture, and in vivo. Pharmacol Ther. 2012;133:205–217. doi: 10.1016/j.pharmthera.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202:235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- Vieira-Coelho MA, Serrao MP, Afonso J, Pinto CE, Moura E. Catecholamine synthesis and metabolism in the central nervous system of mice lacking alpha-adrenoceptor subtypes. Br J Pharmacol. 2009;158:726–737. doi: 10.1111/j.1476-5381.2009.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- Yamada S, Ashizawa N, Nakayama K, Tomita T, Hayashi E. Decreased density of alpha 2-adrenoceptors in medulla oblongata of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1989;13:440–446. doi: 10.1097/00005344-198903000-00012. [DOI] [PubMed] [Google Scholar]
- Zandberg P, de Jong W. Hypotensive response in spontaneously hypertensive rats following microinjection of alpha-methylnoradrenaline in the caudal brain-stem. Neurosci Lett. 1979;14:119–122. doi: 10.1016/0304-3940(79)95356-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Specificity antibody and PLA tests. PC12 and SH-SY5Y cell lines were used to determine the specificity of the antibodies used in the immunoblotting and PLA assays. The PC-12 cell line expresses α2A-adrenoceptors but not μ receptors. However, the SH-SY5Y cell line expresses both μ receptors and α2A-adrenoceptors. (A) The binding patterns of μ receptors and α2A-adrenoceptors were captured with film by applying immunoblotting detection using primary rabbit anti-α2A-adrenoceptor or goat anti-μ receptor antibodies on PC12 or SH-SY5Y cell lines. Our pilot immunoblotting tests revealed that the antibodies used to perform the whole-cell lysates were able to specifically recognize α2A-adrenoceptors and μ receptors. (B) PLA was performed using a combination of the same two primary antibodies followed by the PLA probes anti-rabbit plus and anti-goat minus secondary antibodies with specific oligonucleotides to detect μ receptor/α2A-adrenoceptor heterodimers. PLA signals were not captured for the PC12 cell line (which expresses α2A-adrenoceptors but not μ-opioid receptors). (C) The same experiment, conducted on the SH-SY5Y cell line (which expresses both α2A-adrenoceptors and μ receptors), showed a μ receptor/α2A-adrenoceptor heterodimer signal. Scale bar: 20 μm.
Figure S2 Representative traces of the cardiovascular dose responses after the intra-NTS microinjection of guanfacine and DAMGO. (A) Microinjections of 0.1, 0.3 and 1 nmol guanfacine into anaesthetized WKY rats’ NTS elicited a dose-dependent depressor effect. (B) Microinjections of 0.1, 0.3 and 1 nmol DAMGO elicited a dose-dependent hypertensive effect. A saline vehicle did not alter BP.
Figure S3 μ receptor agonist-induced μ receptor/α2A-adrenoceptor heterodimer formation in the NTS of WKY rats leading to BP elevation. (A) Microinjection of the μ receptor agonist DAMGO (0.3 nmol) into the NTS of WKY rats elicited a sustained BP elevation that lasted for more than 1 h. The microinjection of the α2A-adrenoceptor agonist guanfacine (0.3 nmol) into the NTS caused a depressor effect. (B) Immunofluorescence PLA images of the NTS of WKY rats 10 min after treatment with saline (a), DAMGO (b) or guanfacine (c). The level of μ receptor/α2A-adrenoceptor heterodimers increased markedly after DAMGO microinjection (b) but not after guanfacine microinjection (c). The summary data for immunofluorescence intensity indicated a 1.6-fold increase in μ receptor/α2A-adrenoceptor heterodimer formation in the NTS after DAMGO treatment (d). (C) The amount of μ receptor/α2A-adrenoceptor heterodimers in the NTS were correlated with the mean BP of WKY rats. (γ2 = 0.9241, P < 0.001). Red, heterodimers; blue, DAPI. The values are presented as the mean ± SEM (n = 5). * indicates P < 0.05 compared with the control value. Scale bar: 5 μm. MBP, mean BP.
Figure S4 Anti-hypertensive treatment did not change μ receptor/α2A-adrenoceptor heterodimer formation in the NTS of hypertensive SHRs. (A) Representative BP recordings 2 weeks after oral hydralazine (16 mg·kg−1·day−1) treatment of WKY rats and hypertensive SHRs; (B) Statistical and (C) PLA images (red) results of μ receptor/α2A-adrenoceptor heterodimers in the NTS of WKY rats and hypertensive SHRs with oral hydralazine treatment. Scale bar: 20 μm. Values are shown as the mean ± SEM (n = 6). *P < 0.05 compared with control SHRs.
Figure S5 Induced hypertension in WKY rats did not affect heterodimer formation in the NTS. (A) Representative BP recordings of the bilateral intra-NTS microinjection of L-NAME in a WKY rat; (B) PLA images (red) and (C) the statistical results of μ receptor/α2A-adrenoceptor heterodimers in the NTS of WKY rats 30 min post-L-NAME microinjection. Scale bar: 20 μm. Values are shown as the mean ± SEM (n = 6).