Abstract
Background and Purpose
Pattern separation, that is, the formation of distinct representations from similar inputs, is an important hippocampal process implicated in cognitive domains like episodic memory. A deficit in pattern separation could lead to memory impairments in several psychiatric and neurological disorders. Hence, mechanisms by which pattern separation can be increased are of potential therapeutic interest.
Experimental approach
5-HT1A receptors are involved in spatial memory. Herein we tested the ‘biased’ 5-HT1A receptor agonists F15599, which preferentially activates post-synaptic heteroreceptors, and F13714, which preferentially activates raphe-located autoreceptors, in rats in a novel spatial task assessing pattern separation, the object pattern separation (OPS) task.
Key Results
The acetylcholinesterase inhibitor donepezil, which served as a positive control, significantly improved spatial pattern separation at a dose of 1 mg·kg−1, p.o. F15599 increased pattern separation at 0.04 mg·kg−1, i.p., while F13714 decreased pattern separation at 0.0025 mg·kg−1, i.p. The selective 5-HT1A receptor antagonist WAY-100635 (0.63 mg·kg−1, s.c.) counteracted the effects of both agonists. These data suggest that acute preferential activation of post-synaptic 5-HT1A heteroreceptors improves spatial pattern separation, whereas acute preferential activation of raphe-located 5-HT1A autoreceptors impairs performance.
Conclusions and Implications
We successfully established and validated a novel, simple and robust OPS task and observed a diverging profile of response with ‘biased’ 5-HT1A receptor agonists based on their targeting of receptors in distinct brain regions. Our data suggest that the post-synaptic 5-HT1A receptor consists of a potential novel molecular target to improve pattern separation performance.
Tables of Links
| LIGANDS |
|---|
| Donepezil |
| F15599 |
| WAY-100635 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guideto PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Pattern separation, or the formation of distinct representations from similar inputs, is an important cognitive process that may be fundamental to episodic memory storage (Clelland et al., 2009; Kheirbek et al., 2012). If the pattern separation process functions properly, similar stimuli or experiences get transformed into discrete, non-overlapping representations. The concept of pattern separation derives from neural-network (computational) theories on hippocampal functioning (Marr, 1971). The dentate gyrus (DG) is an area within the hippocampal formation that is thought to function as a pattern separator. As such, the DG takes similar patterns of neural activity and converts them into distinct representations. This is thought to be achieved via dispersion of entorhinal cortical inputs onto the granule cells within the DG, which subsequently sparsely send the information to CA3 pyramidal cells (Kheirbek et al., 2012). In a study in which the DG of rats was lesioned, these rats were unable to discriminate between two objects when these were spatially close together, but the performance normalized when the objects were placed further apart. This supports a role for the DG in spatial pattern separation (Hunsaker et al., 2008).
In line with these studies, activating cells in the DG could lead to an improvement of pattern separation. 5-HT1A receptors are present in the DG (Radley and Jacobs, 2002; Banasr et al., 2004), and might be especially interesting with regard to the process of spatial pattern separation because these receptors have shown to be involved in spatial memory (Koenig et al., 2008). The 5-HT1A receptor has drawn attention as a target for pharmacotherapy for a variety of CNS disorders (Newman-Tancredi, 2011). 5-HT1A agonists have been developed and investigated to function as anxiolytics (Akimova et al., 2009) and antidepressants (Blier and Ward, 2003; De Vry et al., 2004), but also to ameliorate female sexual dysfunction (Stahl et al., 2011) and Parkinson's disease (Newman-Tancredi et al., 2002; Jones et al., 2010; Huot et al., 2011). Furthermore, 5-HT1A receptor agonists are used as add-ons to atypical antipsychotics for the treatment of psychotic disorders like schizophrenia. The atypical antipsychotics clozapine, ziprasidone, aripiprazole and lurasidone, all have, among other pharmacological properties, 5-HT1A partial agonist activity (Newman-Tancredi, 2011).
5-HT1A receptors are expressed as both 5-HT1A autoreceptors, located in the raphe nucleus, and post-synaptic 5-HT1A heteroreceptors, located in various brain regions, including cortex, hypothalamus and septum/hippocampus (Newman-Tancredi, 2011). Activation of 5-HT1A receptors in these different brain regions exerts different effects. For example, activating post-synaptic 5-HT1A heteroreceptors is thought to mediate antidepressant effects (Blier, 2001); hypothalamic 5-HT1A receptors are involved in neuroendocrine control and thermoregulation (Green, 2006); and septum/hippocampal 5-HT1A receptors control ACh release and hence influence certain aspects of cognitive function like learning and memory (Ögren et al., 2008). Activation of raphe-located 5-HT1A autoreceptors, leads to inhibition of 5-HT release and hence is implicated in the delay of onset of antidepressant drugs (Blier, 2001; Newman-Tancredi, 2011). Current 5-HT1A agonists may not have the most favourable therapeutic profile, since they activate both raphe-located autoreceptors and post-synaptic heteroreceptors. In contrast, ‘biased’ agonists that preferentially activate either raphe-located 5-HT1A autoreceptors or post-synaptic 5-HT1A heteroreceptors may yield a more favourable therapeutic profile accompanied by a lower incidence of side effects (Newman-Tancredi, 2011).
The preferential targeting by biased agonists of raphe-located 5-HT1A autoreceptors or post-synaptic heteroreceptors is not due to differences in the receptor protein itself. Only a single intronless 5-HT1A receptor gene has been identified in humans and rats (Fargin et al., 1988; Albert et al., 1990). Therefore, the distinct responses to 5-HT1A ‘biased’ agonists are probably attributable to regional coupling differences of the 5-HT1A receptors to certain G-protein subtypes, regulators of G-protein signalling, or transcriptional regulation (Newman-Tancredi, 2011). For example, raphe 5-HT1A autoreceptors preferentially couple to Gαi3 subtypes, whereas hippocampal 5-HT1A receptors couple preferentially to Gαo subtypes (la Cour et al., 2006). In addition, the existence of agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A autoreceptors in the dorsal raphe nucleus of native rats has been shown (Valdizán et al., 2010). The effects of agonist-dependent coupling and transduction at the raphe-located 5-HT1A autoreceptors could also contribute to the pharmacological profile of these ‘biased’ agonists.
The 5-HT1A ‘biased’ agonists, which were used in the present studies were F15599 and F13714. Different studies have demonstrated that F15599 preferentially activates post-synaptic 5-HT1A heteroreceptors over raphe-located autoreceptors (Newman-Tancredi et al., 2009; Assié et al., 2010; Depoortère et al., 2010). F13714 exerts an opposite pharmacological profile with more pronounced activity at raphe-located autoreceptors, and only modest activation at post-synaptic heteroreceptors (Assié et al., 2006; Newman-Tancredi, 2011). The effects of the ‘biased’ 5-HT1A agonists F15599 and F13714 were investigated in a novel pattern separation task in rodents, the object pattern separation (OPS) task. Our focus is on the cognitive aspect of pattern separation and we developed a task in which the spatial distance of two initially symmetrical placed objects is gradually changed along a straight line. The displacement of the object in the test trial can vary with regard to the distance from the starting position. That is, instead of only maximal displacement of an object in the test trial [as in an object location task (OLT)], the displacement of an object in the OPS task can gradually increase with regard to the starting position. This task allows measurement of improvements as well as impairments in spatial pattern separation. The dose dependency of responses to F15599 and F13714 were investigated, as well as the receptor specificity of their effects. The latter was tested in studies where the agonists were either administered together or in combination with the selective 5-HT1A antagonist WAY-100635. Furthermore, the AChE inhibitor donepezil was tested as a positive control.
Methods
Animals
All animal care and experimental procedures complied with the Dutch Experiments on Animals Act (EAA, amended 1996) and the European Directive (20I0/63/EU) of the European Parliament and of the Council of the European Union (86/609EEC) on the protection of animals used for scientific purposes (22 September 2010), and were approved after careful evaluation by the ethical committee of Maastricht University (licensed animal ethical committee: Min.VWS, GZBIVVB 981845, 16 April 1998). The assigned official protocol number provided to these studies was DEC 2012-062. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 48 animals were used in the experiments described here.
For the initial OPS study, 24 4-month-old male Wistar rats (average weight at the beginning of the study: 393 g) were used. For the drug testing OPS studies, 24 3-month-old male Wistar rats (average weight at the beginning of the study: 361 g) were used. For all studies, the rats were obtained from Charles River Laboratories International, Inc. (Sulzfeld, Germany). Rats were housed individually in standard Tecniplast IVC system greenline cages on sawdust bedding. The animals were on a reversed 12/12 h light/dark cycle (lights on from 19:00 to 7:00 h); and food and water were available ad libitum. The rats were housed and tested in adjacent rooms. A radio, playing softly, provided background noise to mask noises in the room. All testing was performed between 9:00 and 18:00 h under low illumination (20 lux).
OPS
Apparatus and objects
The OPS was performed in a similar apparatus and with a similar procedure as described elsewhere for object recognition (Ennaceur and Delacour, 1988; Prickaerts et al., 2012). The apparatus consisted of a circular arena, 83 cm in diameter. Half of the 40 cm high wall was made of gray polyvinyl chloride, the other half of transparent polyvinyl chloride. Fluorescent red tubes and a light bulb provided a constant low illumination on the floor of the apparatus. Two different sets objects were used: (1) a metal block (10.0 × 5.0 × 7.5 cm) with two holes in it (diameter 1.9 cm), and (2) an aluminium block with a square base and a tapering top (13.0 × 8.0 × 8.0 cm). A rat could not displace the objects.
OPS task
The OPS allows the assessment of spatial pattern separation. It is a modified version of the OLT (Bruno et al., 2011; Vanmierlo et al., 2011), which in turn was developed from the object recognition task (ORT; Ennaceur and Delacour, 1988; Şik et al., 2003). Two identical objects are used in both the first trial (T1) and the second trial (T2). During T1, the apparatus contains two identical objects placed (and oriented) symmetrically on a horizontal line in the arena (see Figure 1, placement is ‘1L’ and ‘1R’). A rat is always placed in the apparatus facing the wall at the middle of the front (transparent) segment. After the first exploration period of 3 min (T1), the rat is put back in its home cage. Subsequently, after a 1 h interval, the rat is placed back in the apparatus for the second trial of 3 min (T2), but now one of the two similar objects is randomly displaced along a straight line on one of five possible locations in either direction (as opposed to the OLT where the object is only displaced to one new location) according to a randomization and a location scheme (see Figure 1). In other words, a new spatial arrangement is used. The times spent exploring each object during T1 and T2 are recorded manually using a personal computer. Subsequently, it can be determined how much time the rats spent exploring the displaced and/or the stationary object. Pattern separation is scored as the relative time spent on the displaced object. Usually, rats show a good pattern separation/spatial memory performance when the displacement is maximal (Figure 1, ‘position 5’) and a 1 h retention interval is interposed between T1 and T2. However, when the displacement of the object along a straight line is intermediate (Figure 1, ‘position 3’) between the starting position (like in T1, ‘position 1’) and the maximum displacement position (‘position 5’), pattern separation is attenuated.
Figure 1.
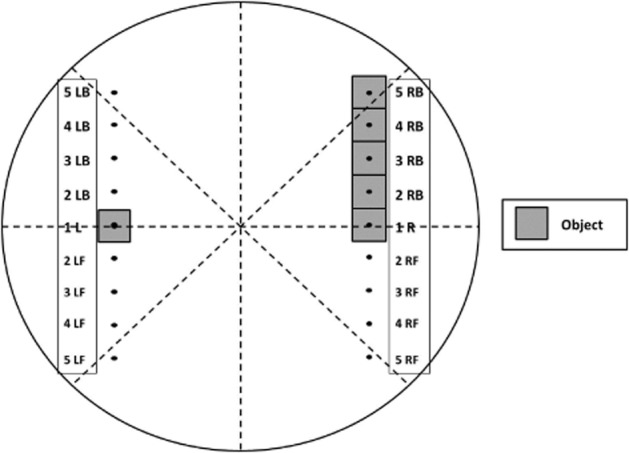
The OPS task. Schematic representation of the top-view of the arena with the possible new locations for an object in T2 (indicated on the right). In the schema, L and R refer, respectively, to ‘left’ and ‘right’. Furthermore, B and F refer to ‘back’ and ‘front’, respectively, indicative of the general direction of the displacement of an object. The number indications (1–5) represent the five possible locations to where an object can be displaced. Therefore, position 5 (in each direction) represents the farthest possible displacement, and position 1 represents no displacement. In T1, the placement of objects is always ‘1L’ and ‘1R’, in T2 (after a 1 h interval); one of the objects could get displaced to one of the new locations along the straight line (positions 1–5). Figure was adapted from Van Hagen et al. (2014).
Exploration was defined as follows: directing the nose to the object at a distance of no more than 2 cm and/or touching the object with the nose. Sitting on, or leaning to, an object was not considered as exploratory behaviour. In order to avoid the presence of olfactory cues, the objects were thoroughly cleaned after each trial with a 70% ethanol solution. All combinations and locations of objects were used in a balanced manner to reduce possible biases due to preferences for particular locations, sides or objects. There was no indication that the rats showed a preference towards either one of the two objects or sides (left/right) in the apparatus (data not shown). The experimenter was always blind to the conditions that were being tested.
Animal handling and OPS testing
Prior to the drug testing studies, the animals were handled daily, adapted to the procedures, and allowed to explore the apparatus with two objects for five min on two separate days. Over two weeks, the rats were adapted to injections of saline (i.p. and p.o. 2.0 mL·kg−1, 30 min before T1) and tested until they showed stable and good discrimination performance at a 1 h interval (Akkerman et al., 2012b).
First, a discrimination curve was made in which naïve animals were tested on all five possible new locations in the OPS test. After establishing this discrimination curve, the optimal location to measure pattern separation was selected. This had to be a location in which both discrimination performance impairment and improvement could be assessed (to avoid ceiling and floor effects), that is, a location with an intermediate performance (see Figure 2, the intermediate position is position 3).
Figure 2.
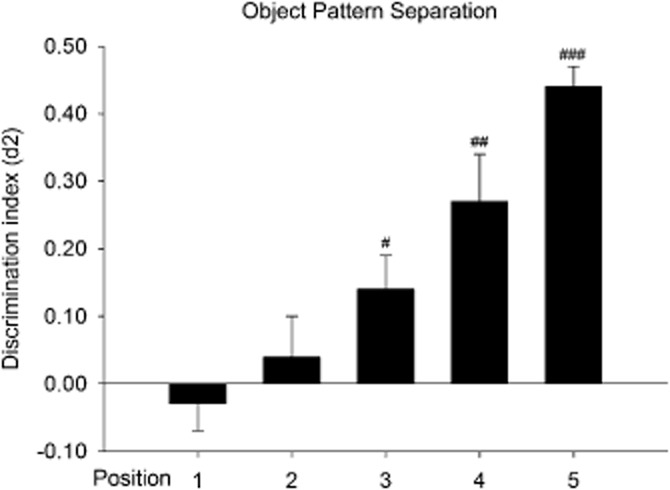
Means + SEM for the d2 index in the OPS on all five positions for the pilot study with untreated Wistar rats. Positions 3, 4 and 5 showed significant object location discrimination which increased with increasing distance from position 1 along the vertical axis (position 3 < 4 < 5), which is indicative of spatial pattern separation. On position 3, an increase or a decrease in pattern separation performance can be measured, that is, performance after an experimental manipulation could resemble the performance on positions 5 or 1 respectively. When compared with performance on position 1, positions 4 and 5 differed significantly with respect to pattern separation performance. #P < 0.05; ##P < 0.01; ###P < 0.001, significantly different from chance performance/zero. n = 10–14 animals per group.
In the next experiment, vehicle (saline) and the reference drug donepezil (an AChE inhibitor) were tested with objects on the starting position and the position with the intermediate performance. Following these experiments, the 5-HT1A receptor ‘biased’ agonists were tested only on the intermediate position.
Treatment
Vehicle and donepezil
The effects of vehicle and donepezil treatment were tested with objects on positions 1 and 3 (the intermediate position) in the OPS. Vehicle and 1 mg·kg−1 donepezil were injected i.p. and p.o., respectively, 30 min before T1 (dose, route and time of administration of donepezil were derived from De Bruin et al., 2011). A 1 h interval between T1 and T2 was interposed. A within design was used in which all experimental conditions contained 23–24 rats. One rat was excluded from the vehicle condition (position 3) due to too low exploration activity during T2 (Akkerman et al., 2012a).
Dose–response curves for F15599 and F13714
To establish the effects of F15599 and F13714 on pattern separation, dose–response curves were made with objects on position 3 in the OPS. A 1 h interval between T1 and T2 was used. F15599 or F13714 was injected i.p. 30 min before T1. The timing of administration followed earlier work that indicated that brain presence and duration of action of these drugs entirely covered the time period of T1 and T2 in the OPS task (Assié et al., 2010). The tested doses for F15599 were 0.0, 0.0025, 0.01, 0.04 and 0.16 mg·kg−1. For F13714 the tested doses were 0.0, 0.000625, 0.0025, 0.01 and 0.04 mg·kg−1. A within design was used in which all experimental conditions contained 23 rats.
In order to demonstrate that the effects of F15599 and F13714 on pattern separation were indeed mediated via the 5-HT1A receptors, experiments were performed (and separately analysed) in which the selective (‘non-biased’) 5-HT1A antagonist WAY-100635 was co-administered with the effective dose of F15599 or F13714. In accordance with previous studies, a dose of 0.63 mg·kg−1 WAY-100635 was administered s.c., 45 min before T1 (or 15 min before the F15599 or F13714 administration; Assié et al., 2006; 2010). The effective doses of F15599 and F13714 were 0.04 mg·kg−1 (improving) and 0.0025 mg·kg−1 (impairing), respectively, which were administered and dissolved as already described.
Combination of effective doses of F15599 and F13714
In this experiment, the effective doses of both F15599 and F13714 were combined. The rationale was that when the post-synaptic 5-HT1A heteroreceptors and raphe-located 5-HT1A autoreceptors were both stimulated with the optimum doses of their respective ‘biased’ agonists, that the net effect would be equal to that of vehicle-treated rats. In other words, co-administration of these optimum doses should lead to cancellation of either improving or impairing effects on pattern separation. Therefore, 0.04 mg·kg−1 F15599 and 0.0025 mg·kg−1 F13714 were dissolved and administered as described earlier. A within design was used in which all experimental conditions contained 23 rats.
Data analysis
The OPS provides measures for exploration time and discrimination (for an ORT study with the same parameters, see Prickaerts et al., 1997). The measures were the times spent by rats in exploring each object location during T1 and T2. The time spent in exploring the two symmetrically placed objects in T1 were represented by ‘a1’ and ‘a2’ respectively. The time spent in exploring the stationary and the moved object in T2 were represented by ‘a3’ and ‘b’ respectively. From these exploration times, the following variables were calculated: e1, e2 and d2 (discrimination index between objects for trial 2 = (b – a3)/e2; Table 1). Total exploration times in both trials should be sufficient in order to be able to reliably assess pattern separation (Akkerman et al., 2012a). If an animal did not show sufficient exploration time in T1, T2 or both, the animal was excluded from the dataset. The d2 index is a relative measure of discrimination corrected for exploratory activity. The d2 index can range from −1 to 1, with −1 or 1 indicating complete preference for the familiar or novel object location, respectively, and 0 signifying no preference for either object location.
Table 1.
Derived measures in the object recognition task
| Trial number | Exploration time (s) | Discrimination index |
|---|---|---|
| T1 | e1 = a1 + a2 | Not applicable |
| T2 | e2 = a3 + b | d2 = (b − a3)/e2 |
e1 is the measure of the time spent in exploring both identical object locations (a1 and a2) during T1, and e2 is the measure of the time spent in exploring both the familiar (a3) and new object location (b) in T2; d2 corresponds to the ability to discriminate between the old and new object location during T2. The d2 index is corrected for exploration time during T2.
One-sample t-statistics were performed in order to assess whether the d2 index, for each experimental condition separately, differed significantly from zero. Experimental conditions were also compared using repeated-measures anovas, one-way anovas or paired-samples t-statistics, depending on the experimental design. When the overall anova was significant, a post hoc analysis with Bonferroni t-tests was performed. An α level of 0.05 was considered significant. A d2 value that is significantly different from zero (as indicated by one-sample t-statistics) signifies an intermediate effect, and as such, already indicates recognition of the familiar object. When a d2 index shows both a difference from zero and a between group effect (as indicated by anova or paired-samples t-statistics), it is called a full effect (see table 4 in Akkerman et al., 2012a).
Materials
F15599 (3-Chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methylpyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone) and F13714 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylaminopyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone), respectively, fumarate and glycolate salts (for the chemical structures of F15599 and F13714; see Assié et al., 2010), were synthesized by Neurolixis, Inc. (San Diego, CA, USA) Donepezil was a generous gift from Solvay Pharmaceuticals (Weesp, the Netherlands). WAY-100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide) maleate was purchased from Tocris Bioscience (Abingdon, UK). All compounds were dissolved in sterile physiological saline (B. Braun Melsungen AG, Melsungen, Germany) to produce an injection volume of 2 mL·kg−1. All doses refer to the weight of the salt.
Results
OPS study
One-way anova revealed no differences between the different object positions on the level of exploration in T1 (F4,55 = 1.33; P = 0.272) or T2 (F4,55 = 2.46; P = 0.056). There was an effect of object position on the d2 index (F4,55 = 13.885; P = 0.000). Post hoc analyses revealed that the d2 index of position 1 was lower than the d2 indices of position 4 (P = 0.002) and position 5 (P = 0.000). One-sample t-statistics revealed that the d2 indices of object positions 3, 4 and 5 were different from zero (position 3: t13 = 2.65; P = 0.020, position 4: t9 = 4.09; P = 0.003, position 5: t12 = 13.78; P = 0.000), indicating that recognition of the familiar object location was present in these three displacement conditions (see Figure 2).
Position 3 (see Figures 1 and 2) seemed to be the most appropriate position for the assessment of pattern separation, since on position 3, animals had an ‘intermediate performance’ when compared with positions 1 and 5 (see Figure 2). On positions 3, performance can vary bidirectionally, that is, both performance impairment (i.e. discrimination performance is no longer statistically different from zero, that is, more resemblance with position 1), and performance improvement (i.e. discrimination performance more resembles performance on position 5) can be measured depending on the experimental condition that is being tested. In subsequent studies, position 3 was therefore used in order to assess pattern separation.
Vehicle and donepezil
Paired-samples t-tests revealed differences between some of the different treatment conditions and object positions on the level of exploration in T1 (position 1: vehicle and donepezil: t23 = 2.51; P = 0.019; position 3: vehicle and donepezil: t22 = 4.30; P = 0.000). No differences in exploration time were found in T2. Furthermore, paired samples t-tests showed differences (or a trend) between the discrimination performances of different conditions. Differences were found between the following d2 indices: position 3: vehicle and donepezil: t22 = −2.47; P = 0.022, vehicle: position 1 and position 3: t22 = −1.72; P = 0.099; donepezil: position 1 and position 3: t23 = −4.51; P = 0.000.
One-sample t-statistics revealed that only the d2 indices of the vehicle and donepezil conditions on position 3 were different from zero (position 3 vehicle: t22 = 2.77; P = 0.011; position 3 donepezil: t23 = 6.64; P = 0.000), indicating intermediate effects, that is, that recognition of the familiar object location was present in these treatment conditions (see Figure 3).
Figure 3.
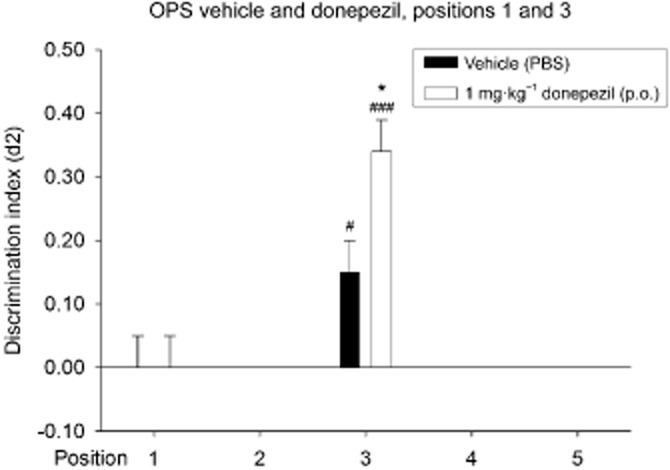
Means + SEM for the d2 index in the OPS for vehicle and donepezil treatment on positions 1 and 3. On position 1, both treatments with vehicle and with 1 mg·kg−1 donepezil showed no significant discrimination. On position 3, significant discrimination was found in both the vehicle and donepezil-treated rats. Treatment with 1 mg·kg−1 donepezil on Position 3 did show significantly better discrimination when compared with vehicle treatment on position 3. #P < 0.05; ###P < 0.001, significantly different from chance performance/zero. *P < 0.05, significantly different from the corresponding vehicle condition,. n = 23–24 animals per group.
Dose–response curve for F15599
A repeated-measures anova revealed differences between treatment conditions on the level of exploration in both T1 (F4,88 = 5.17; P = 0.001) and T2 (F4,88 = 2.58; P = 0.043). Post hoc analyses revealed lower exploration times in T1 in the 0.16 mg·kg−1 F15599 condition when compared with the vehicle (P = 0.001), 0.0025 mg·kg−1 F15599 (P = 0.047) and 0.01 mg·kg−1 F15599 (P = 0.019) conditions. In T2, post hoc analyses showed a trend towards higher exploratory behaviour in the vehicle condition when compared with the 0.01 mg·kg−1 F15599 condition (P = 0.087). Furthermore, repeated-measures anova indicated differences between treatment conditions for the d2 indices (F4,88 = 3.82; P = 0.007). Post hoc analyses revealed higher object location discrimination (or a trend) in the 0.04 mg·kg−1 F15599 condition when compared with the vehicle (P = 0.079), 0.01 mg·kg−1 F15599 (P = 0.013) and 0.16 mg·kg−1 F15599 (P = 0.032) conditions. This indicates a full effect of the d2 index of 0.04 mg·kg−1 F15599 on OPS performance (i.e. improvement of OPS performance).
One-sample t-statistics showed that the discrimination performance was different from zero in the vehicle (t22 = 2.77; P = 0.011), 0.0025 mg·kg−1 F15599 (t22 = 2.32; P = 0.030) and 0.04 mg·kg−1 F15599 (t22 = 7.55; P = 0.000) conditions, indicating intermediate effects, that is, that recognition of the familiar object location was present in these treatment conditions (see Figure 4).
Figure 4.
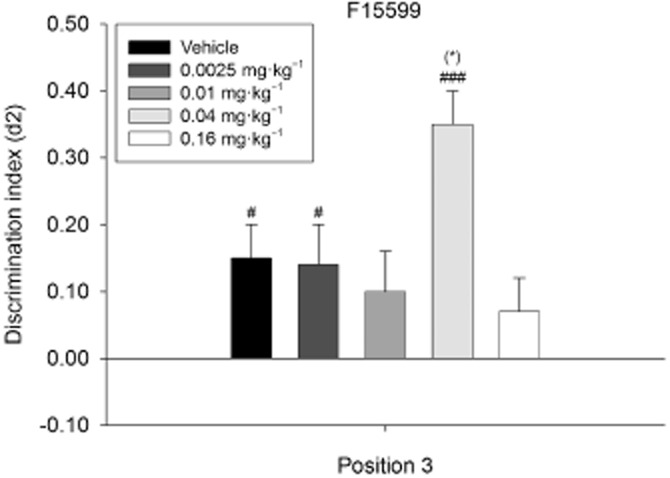
Means + SEM for the d2 index in the OPS for F15599 treatment on position 3. The dose–response curve of F15599 on position 3 in the OPS task. Administration of 0.04 mg·kg−1 F15599 led to the best discrimination performance. #P < 0.05; ###P < 0.001, significantly different from chance performance/zero. (*)P = 0.079, different from the vehicle condition,. n = 23 animals per group.
Effective dose F15599 in combination with WAY-100635
For the combination study of 0.04 mg·kg−1 F15599 and WAY-100635, one-way anova revealed no differences between treatment conditions on the level of exploration in T1 (F3,78 = 1.74; P = 0.166). In T2, one-way anova did reveal differences between treatment conditions on the level of exploration (F3,78 = 5.34; P = 0.002). Post hoc analyses revealed higher exploration times in T2 in the vehicle and WAY-100635 condition when compared with the vehicle (P = 0.006) and 0.04 mg·kg−1 F15599 and WAY-100635 (P = 0.007) conditions. Furthermore, one-way anova indicated differences between treatment conditions for the d2 indices (F3,78 = 3.06; P = 0.034). Post hoc analyses revealed a tendency towards higher object location discrimination in the 0.04 mg·kg−1 F15599 condition when compared with the vehicle (P = 0.140) and 0.04 mg·kg−1 F15599 and WAY-100635 (P = 0.057) conditions. This indicates only tendencies towards full effects on the d2 indices.
One-sample t-statistics showed that the discrimination performance was different from zero in the vehicle (t22 = 2.77; P = 0.011), vehicle and WAY-100635 (t15 = 2.29; P = 0.037), and 0.04 mg·kg−1 F15599 (t23 = 6.98; P = 0.000) conditions, indicating intermediate effects, that is, that recognition of the familiar object location was present in these treatment conditions (see Figure 5).
Figure 5.
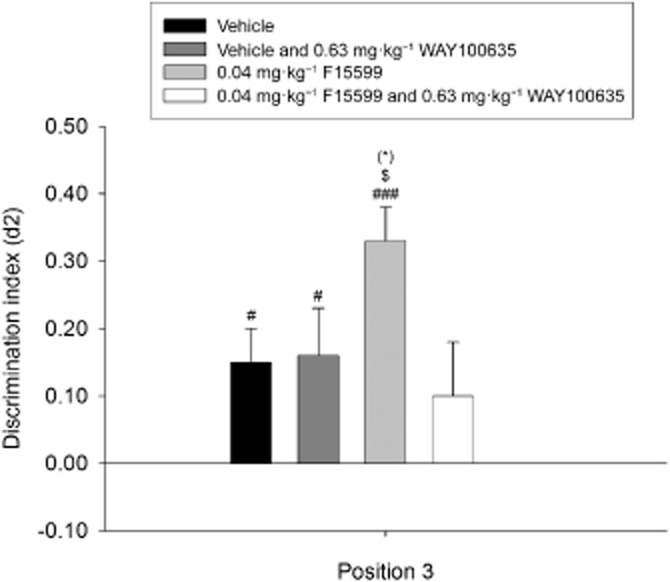
Means + SEM for the d2 index in the OPS for the effective dose of F15599 in combination with WAY-100635 treatment on position 3. Co-administration of the effective dose of F15599 with WAY-100635 on position 3 in the OPS task. Co-administration of 0.63 mg·kg−1 WAY-100635 with 0.04 mg·kg−1 F15599 led to a reduction in discrimination performance, indicative of the importance of 5-HT1A receptor activation. WAY-100635 did not exert any effects on discrimination performance when co-administered with vehicle (saline). #P < 0.05; ###P < 0.001, significantly different from chance performance/zero. (*)P = 0.14, different from the vehicle condition. $P = 0.057, significantly different from the 0.04 mg·kg−1 F15599 and 0.63 mg·kg−1 WAY-100635 condition:. n = 16–24 animals per group.
Dose–response curve for F13714
A repeated-measures anova revealed differences between treatment conditions on the level of exploration in both T1 (F4,88 = 5.93; P = 0.000) and T2 (F4,88 = 2.82; P = 0.030). Post hoc analyses revealed lower exploration times in T1 in the 0.04 mg·kg−1 F13714 condition when compared with the vehicle (P = 0.000) and 0.0025 mg·kg−1 F13714 (P = 0.028) conditions. In T2, post hoc analyses showed trends towards higher exploratory behaviour in the 0.000625 mg·kg−1 F13714 condition when compared with the vehicle (P = 0.055) and 0.04 mg·kg−1 F13714 (P = 0.065) conditions. Furthermore, repeated-measures anova indicated no differences between treatment conditions for the d2 indices (F4,88 = 1.23; P = 0.305), indicative of no full effects on d2 indices.
One-sample t-statistics, however, did show differences (or a trend) in discrimination performance as indicated by differences from zero, in the vehicle (t22 = 2.77; P = 0.011), 0.000625 mg·kg−1 F13714 (t22 = 1.86; P = 0.076) and 0.04 mg·kg−1 F13714 (t22 = 3.56; P = 0.002) conditions only, indicative of intermediate effects, that is, that recognition of the familiar object location was present in these treatment conditions (see Figure 6).
Figure 6.
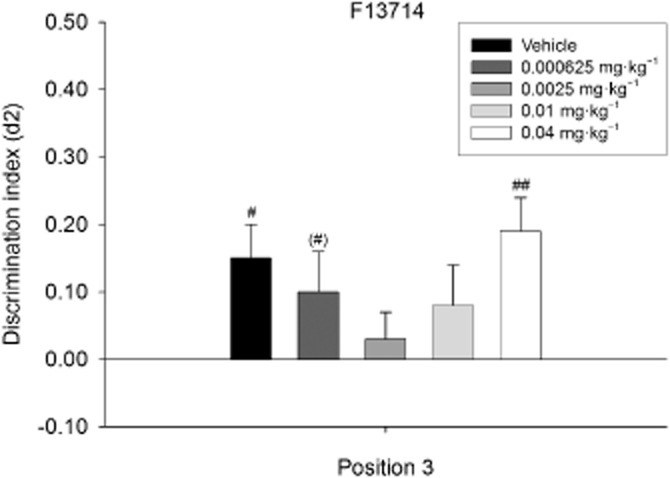
Means + SEM for the d2 index in the OPS for F13714 treatment on position 3. The dose–response curve of F13714 on position 3 in the OPS task. Administration of 0.0025 mg·kg−1 F13714 led to the greatest impairment of discrimination performance, as indicated by one-sample t-tests. (#)P = 0.08; #P < 0.05; ##P < 0.01, significantly different from chance performance/zero. n = 23 animals per group.
Effective dose F13714 in combination with WAY-100635
For the combination study of 0.0025 mg·kg−1 F13714 and WAY-100635, one-way anova revealed no differences between treatment conditions on the level of exploration in T1 (F3,78 = 0.25; P = 0.864). In T2, one-way anova did reveal differences between treatment conditions on the level of exploration (F3,78 = 3.66; P = 0.016). Post hoc analyses revealed higher exploration times in T2 in the vehicle and WAY-100635 condition when compared with the vehicle condition (P = 0.013). Furthermore, one-way anova indicated no differences between treatment conditions for the d2 indices (F3,78 = 1.24; P = 0.302), indicative of no full effects on d2 indices.
One-sample t-statistics showed that the discrimination performance was different from zero (or showed a trend) in the vehicle (t22 = 2.77; P = 0.011), vehicle and WAY-100635 (t15 = 2.29; P = 0.037), and 0.0025 mg·kg−1 F13714 and WAY-100635 (t15 = 1.82; P = 0.089) conditions, but not in the 0.0025 mg·kg−1 F13714 condition (t23 = 0.66; P = 0.515). This indicates that there were intermediate effects in all experimental conditions except the 0.0025 mg·kg−1 F13714 condition (see Figure 7).
Figure 7.
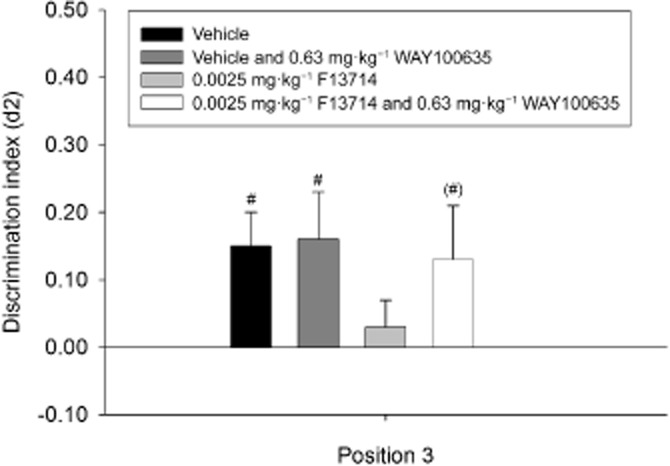
Means + SEM for the d2 index in the OPS for the effective dose of F13714 in combination with WAY-100635 treatment on position 3. Co-administration of the effective (impairing) dose of F13714 with WAY-100635 on position 3 in the OPS task. One-sample t-tests revealed that co-administration of 0.63 mg·kg−1 WAY-100635 with 0.0025 mg·kg−1 F13714 led to an improvement in discrimination performance, indicative of the importance of 5-HT1A receptor activation. WAY-100635 did not exert any effects on discrimination performance when co-administered with vehicle (saline). (#)P = 0.09; #P < 0.05, significantly different from chance performance/zero. n = 16–24 animals per group.
Combination of F15599 and F13714
For the combination of effective doses of F15599 (0.04 mg·kg−1) and F13714 (0.0025 mg·kg−1), a repeated-measures anova revealed differences between treatment conditions on the level of exploration in T1 (F3,66 = 4.19; P = 0.009) but not in T2 (F3,66 = 1.72; P = 0.171). Post hoc analyses revealed higher exploration times in T1 in the 0.0025 mg·kg−1 F13714 condition when compared with the 0.04 mg·kg−1 F15599 (P = 0.045), and 0.04 mg·kg−1 F15599 and 0.0025 F13714 (P = 0.034) conditions. Furthermore, repeated-measures anova indicated differences between treatment conditions for the d2 indices (F3,66 = 5.78; P = 0.001). Post hoc analyses revealed (a tendency towards) higher object location discrimination in the 0.04 mg·kg−1 F15599 condition when compared with the vehicle (P = 0.047), 0.0025 mg·kg−1 F13714 (P = 0.000) and the combination of 0.04 mg·kg−1 F15599 and 0.0025 mg·kg−1 F13714 (P = 0.098) conditions. This is indicative of full effects on d2 indices in these conditions. No difference was found between the vehicle and the combination of 0.04 mg·kg−1 F15599 and 0.0025 mg·kg−1 F13714 condition (P = 1.000).
One-sample t-statistics showed that the discrimination performance was different from zero (or showed a trend) in the vehicle (t22 = 2.77; P = 0.011), 0.04 mg·kg−1 F15599 (t22 = 7.55; P = 0.000), and the combination of 0.04 mg·kg−1 F15599 and 0.0025 mg·kg−1 F13714 (t22 = 1.84; P = 0.079) conditions, and as such, showed intermediate effects, and hence presence of recognition of the familiar object location in these treatment conditions (see Figure 8).
Figure 8.
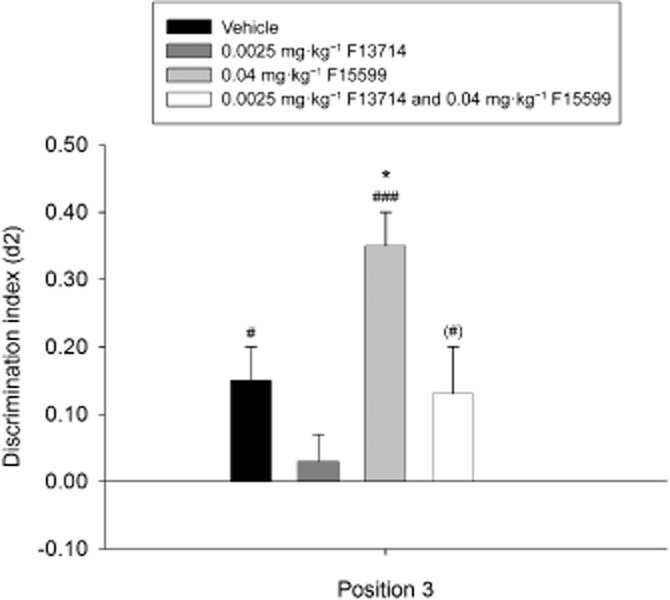
Means + SEM for the d2 index in the OPS for the effective doses of F15599 and F13714 combined on position 3. Co-administration of the effective doses of F15599 and F13714 on position 3 in the OPS task. Co-administration of 0.04 mg·kg−1 F15599 and 0.0025 mg·kg−1 F13714 led to a discrimination performance that resembled vehicle treatment on this position. In other words, combining the effective doses of these two compounds led to an increase of performance when compared with mono-treatment of 0.0025 mg·kg−1 F13714, and to a decrease of performance when compared with mono-treatment with 0.04 mg·kg−1 F15599. The vehicle and the combination of F13714 and F15599 conditions did not significantly differ in discrimination performance. (#)P = 0.08; #P < 0.05; ###P < 0.001, significantly different from chance performance/zero. *P < 0.05, significantly different from the vehicle condition,. n = 23 animals per group.
Discussion
In these studies, we successfully established and validated a novel, simple and robust pattern separation task, the OPS task, and observed a diverging profile of response with acute treatment of ‘biased’ agonists targeted to specific 5-HT1A receptor subpopulations. The AChE inhibitor donepezil significantly improved spatial pattern separation at a dose of 1 mg·kg−1, p.o. F15599 increased pattern separation at 0.04 mg·kg−1, i.p., while F13714 decreased pattern separation at 0.0025 mg·kg−1, i.p. All drugs were given 30 min before training. The data suggest that preferential activation of post-synaptic 5-HT1A heteroreceptors (with F15599) has beneficial influence on spatial pattern separation, whereas activation of raphe-located 5-HT1A autoreceptors (with F13714) impairs this performance. The dose-reponse curves of both F15599 and F13714 (see Figures 4 and 6) clearly show that either the increase or the decrease (with F15599 and F13714, respectively) of OPS performance was lost at supraoptimal drug concentrations. This effect is very likely because the drugs lose their raphe-located autoreceptor or post-synaptic heteroreceptors selectivity when the doses are increased. Bell-shaped dose–response curves are often encountered in behavioural pharmacology and stress the importance of testing multiple drug doses in experiments. The 5-HT1A receptor antagonist WAY-100635 reversed the effects of F15599 and F13714, supporting a role for 5-HT1A receptors in their effects on spatial pattern separation. Likewise, when the effective doses of both compounds were co-adminstered, the impairing and/or improving effect was counteracted. This indicates that simultaneous activation of 5-HT1A post-synaptic heteroreceptors and raphe-located autoreceptors reverses the improvement and impairment of pattern separation induced by F15599 and F13714 respectively. This fine-tuning might explain the diverse effects on animal behaviour that have been found with different ‘traditional’ 5-HT1A receptor agonists (Newman-Tancredi, 2011). The dose, and raphe-located/post-synaptic receptor activation-ratio, probably contributes to this diversity of effects.
The rats in the drug testing studies were repeatedly tested in the OPS task. To secure sufficient wash-out, test sessions were only carried out once a week. By using this schedule, the rats do not habituate to the test and this contributes to proper activity of the rats in the OPS apparatus during the period of testing. Therefore, the incidental statistical significant differences in exploratory behaviour of the rats in T1 and/or T2 were not due to the rats being tested too frequently. Furthermore, the differences in exploratory behaviour were unlikely to be related to drug action. To elaborate on this, the differences between drug conditions were not stable over T1 and T2. The prolonged target engagement of 5-HT1A receptors in the brain by F15599 and F13714 has been demonstrated in rat (and cat) micro-PET experiments (Lemoine et al., 2010; 2012) in which loss of binding to the therapeutic target was not observed over the period of brain imaging scans (up to 90 min) consistent with a prolonged period of receptor labelling. If the effects on exploratory behaviour were due to the drug conditions, they would stay stable over both trials, considering these trials were only 1 h apart. In addition, no consistent pattern could be found in the changes in exploratory behaviour. Drug effects showed no dose–response relationship. There was no consistency in the direction of the effects on exploratory behaviour. That is, no dose seemed to consistently increase or decrease exploratory behaviour in the rats. Importantly, despite the statistical significant differences in exploratory behaviour in either T1, T2 or both, the mean exploration times of the animals were always sufficient (>17.5 s, data not shown) to draw reliable conclusions (Akkerman et al., 2012a). Sporadic changes in exploratory behaviour can occur during behavioural testing, and often the exact reasons remain unknown. Most importantly, no impairment in locomotor activity was found according to the exploratory behaviour in the ORT (i.e. sufficient exploration times). Therefore, we interpret any differences in the amount of exploratory behaviour to be incidental.
Different 5-HT1A receptor subpopulations show their own rate of desensitization upon frequent activation (e.g. Kreiss and Lucki, 1997). Likewise, different 5-HT1A receptor ligands show their own capability of desensitizing 5-HT1A receptors (e.g. Assié et al., 2006). Since the present studies only assessed the acute effects of F15599 and F13714, it remains to be shown to what extent these behavioural effects are retained when the compounds are administered (sub)chronically. F13714 desensitizes neurochemical responses of raphe-located 5-HT1A autoreceptors within just 3 days of treatment (Assié et al., 2006). Whether F15599 desensitizes post-synaptic heteroreceptors in the same way and whether this could result in a possible negative functional effect, remains to be investigated. Nevertheless, previous studies have indicated that cortical 5-HT1A heteroreceptors are resistant to desensitization with various serotonergic drugs (Hensler, 2003).
Several clinical studies have demonstrated that adjunctive therapy with 5-HT1A receptor partial agonists improves the cognitive symptoms of patients with schizophrenia [cognitive impairments associated with schizophrenia (CIAS)] (Meltzer and Sumiyoshi, 2008). However, different 5-HT1A receptor agonists exert varying effects on patients, possibly due to differences in receptor selectivity and agonist efficacy. A possible strategy to gain better outcomes is to only target specific 5-HT1A receptor subpopulations in certain relevant brain areas, while avoiding irrelevant ones. Indeed, in a rodent model of CIAS, F15599 was tested in a paradigm in which cognitive impairment was induced by the administration of the non-competitive NMDA receptor antagonist phencyclidine (PCP). F15599 improved performance in a reversal learning task in rats treated chronically with PCP. In contrast, F13714 disrupted performance when tested as mono-treatment and even tended to accentuate PCP-induced deficits when co-administrated (Depoortère et al., 2010). This supports the hypothesis that specific 5-HT1A receptor subpopulations need to be targeted rather that eliciting broad activation of all 5-HT1A receptors in different brain regions. Recent publications have pointed towards deficient pattern separation processes in patients with schizophrenia probably linked to DG dysfunction (Das et al., 2014; Schreiber and Newman-Tancredi, 2014). The ‘biased’ 5-HT1A receptor agonists described here, especially F15599, may have a favourable pharmacological profile as adjunctive therapy with atypical antipsychotics in schizophrenia. But this hypothesis has to be further examined in order to draw reliable conclusions.
Recent studies have implicated adult-born hippocampal neurons in the process of pattern separation (Kheirbek et al., 2012; Hunsaker and Kesner, 2013). The process of adult hippocampal neurogenesis entails the generation of functional neurons in particular in the subgranular zone of the DG (Frankland et al., 2013). Mechanisms by which hippocampal adult neurogenesis can be increased are therefore of therapeutic interest and a promising molecular target is the activation of 5–HT1A receptors because it has been shown that agonists at this site increase adult neuronal proliferation in the DG (Radley and Jacobs, 2002). Animal studies support this link between adult hippocampal neurogenesis and pattern separation. Animals in which neurogenesis was ablated showed specific impairment on performance related to pattern separation, but still showed intact hippocampal-dependent learning. This indicates the importance of the DG in pattern separation, and furthermore, that neurogenesis is important for the ability of the DG to perform this cognitive process adequately (Clelland et al., 2009). Although the acute treatment regimen in the present study probably affects transient effects including receptor activation, neurotransmitter release and synaptogenesis, it would be interesting to determine in future studies whether chronically administered ‘biased’ 5-HT1A receptor agonists can differentially regulate neurogenesis, as well as behavioural responses. Furthermore, investigating hippocampal involvement, as well as the role of neurogenesis, in the OPS task itself is also imperative in this respect (Van Hagen et al., 2014).
Taken together, our findings provide behavioural evidence that the activity of 5-HT1A receptor ‘biased’ agonists at distinct subpopulations of 5-HT1A receptors (raphe-located autoreceptors and/or post-synaptic heteroreceptors) can show divergent effects in a novel test for OPS. By improving pattern separation, the functional outcome of patients with memory deficits might improve significantly. Therefore, the extent to which ‘biased’ agonists that improve pattern separation are an appropriate add-on pharmacotherapy for such patients, remains to be investigated in future studies.
Acknowledgments
These studies were financed by Maastricht University.
Glossary
Abbreviations
- d2
discrimination index between objects for trial 2 = (b – a3)/e2
- DG
dentate gyrus
- F13714
3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylaminopyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone
- F15599
3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methylpyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone
- OLT
object location task
- OPS
object pattern separation
- ORT
object recognition task
- T1
trial 1
- T2
trial 2
- WAY-100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]- N-(2-pyridyl)cyclohexanecarboxamide
Author contributions
All authors hereby declare to have made substantial contributions to the design of the work/studies and interpretation of the data that followed from these studies. All authors have contributed significantly to the drafting and revising of this research paper. N. P. G. and J. P. participated in study design, conduction of experiments, data analysis and report preparation. R. S. initiated the present project and participated in study design, and report preparation. A. N.-T., and M. V. are employees from Neurolixis, Inc. and participated in study design, development of reagents and report preparation. All authors hereby approve the current version of the research paper for publication. Furthermore, all authors agree to be accountable for all aspects of the work presented in this research paper.
Conflicts of interest
Neurolixis, Inc. has a financial interest in F15599 and F13714, and provided these compounds but did not fund the present research. A. N.-T. and M. V. are employees from Neurolixis, Inc.
The authors have full control of all primary data and agree to allow the journal to review our data, if requested. No other circumstances can be perceived as a potential conflict of interest.
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, Gijselaers HJ, et al. Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res. 2012a;232:335–347. doi: 10.1016/j.bbr.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Akkerman S, Prickaerts J, Steinbusch HWM, Blokland A. Object recognition testing: statistical considerations. Behav Brain Res. 2012b;232:317–322. doi: 10.1016/j.bbr.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Albert PR, Zhou Q-Y, Van Tol H, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–5832. [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié M-B, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br J Pharmacol. 2006;149:170–178. doi: 10.1038/sj.bjp.0706859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié M-B, Bardin L, Auclair AL, Carilla-Durand E, Depoortere R, Koek W, et al. F15599, a highly selective post-synaptic 5-HT1A receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol. 2010;13:1285–1298. doi: 10.1017/S1461145709991222. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adut cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry. 2001;62(Suppl. 15):12–17. [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Bruno O, Fedele E, Prickaerts J, Parker L, Canepa E, Brullo C, et al. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol. 2011;164:2054–2063. doi: 10.1111/j.1476-5381.2011.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland C, Choi M, Romberg C, Clemenson G, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Cour CM, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol. 2006;70:1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- Das T, Ivleva EL, Wagner AD, Stark CEL, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014;159:193–197. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin NMWJ, Prickaerts J, van Loevezijn A, Venhorst J, de Groote L, Houba P, et al. Two novel 5-HT6 receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats. Neurobiol Learn Mem. 2011;96:392–402. doi: 10.1016/j.nlm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Melon C, Dalmus M, Jentzsch KR. 5-HT1A receptors are differentially involved in the anxiolytic-and antidepressant-like effects of 8-OH-DPAT and fluoxetine in the rat. Eur Neuropsychopharmacol. 2004;14:487–495. doi: 10.1016/j.euroneuro.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Auclair AL, Bardin L, Colpaert FC, Vacher B, Newman-Tancredi A. F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur Neuropsychopharmacol. 2010;20:641–654. doi: 10.1016/j.euroneuro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. The genomic clone G-21 which resembles a β-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988;335:358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Köhler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends Neurosci. 2013;36:497–503. doi: 10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Green A. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol. 2006;147:S145–S152. doi: 10.1038/sj.bjp.0706427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 2003;72:1665–1682. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a, b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Huot P, Fox SH, Newman-Tancredi A, Brotchie JM. Anatomically selective serotonergic type 1A and serotonergic type 2A therapies for Parkinson's disease: an approach to reducing dyskinesia without exacerbating Parkinsonism. J Pharmacol Exp Ther. 2011;339:2–8. doi: 10.1124/jpet.111.184093. [DOI] [PubMed] [Google Scholar]
- Jones C, Johnston L, Jackson MJ, Smith LA, van Scharrenburg G, Rose S, et al. An in vivo pharmacological evaluation of pardoprunox (SLV308) – a novel combined dopamine D2/D3 receptor partial agonist and 5-HT1A receptor agonist with efficacy in experimental models of Parkinson's disease. Eur Neuropsychopharmacol. 2010;20:582–593. doi: 10.1016/j.euroneuro.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Cosquer B, Cassel J-C. Activation of septal 5-HT1A receptors alters spatial memory encoding, interferes with consolidation, but does not affect retrieval in rats subjected to a water-maze task. Hippocampus. 2008;18:99–118. doi: 10.1002/hipo.20368. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Chronic administration of the 5-HT1A receptor agonist 8-OH-DPAT differentially desensitizes 5-HT1A autoreceptors of the dorsal and median raphe nuclei. Synapse. 1997;25:107–116. doi: 10.1002/(SICI)1098-2396(199702)25:2<107::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Newman-Tancredi A, et al. [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imag. 2010;37:594–605. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Becker G, Vacher B, Billard T, Lancelot S, Newman-Tancredi A, et al. Radiosynthesis and preclinical evaluation of 18F-F13714 as a fluorinated 5-HT1A receptor agonist radioligand for PET neuroimaging. J Nucl Med. 2012;53:969–976. doi: 10.2967/jnumed.111.101212. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans Roy Soc Lond B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Sumiyoshi T. Does stimulation of 5-HT1A receptors improve cognition in schizophrenia? Behav Brain Res. 2008;195:98–102. doi: 10.1016/j.bbr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A. Biased agonism at serotonin 5-HT1A receptors: preferential postsynaptic activity for improved therapy of CNS disorders. Neuropsychiatry. 2011;1:149–164. [Google Scholar]
- Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT1 and 5-HT2, receptor subtypes. J Pharmacol Exp Ther. 2002;303:815–822. doi: 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Martel J-C, Assié M-B, Buritova J, Lauressergues E, Cosi C, et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol. 2009;156:338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ögren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, et al. The role of 5-HT1A receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, Steinbusch HW, Smits JF, de Vente J. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337:125–136. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, et al. EVP-6124, a novel and selective α7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of α7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–1110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Newman-Tancredi A. Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT1A receptor activation. Neurobiol Learn Mem. 2014;110:72–80. doi: 10.1016/j.nlm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Sommer B, Allers KA. Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8:15–27. doi: 10.1111/j.1743-6109.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- Şik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Valdizán EM, Castro E, Pazos A. Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int J Neuropsychopharmacol. 2010;13:835–843. doi: 10.1017/S1461145709990940. [DOI] [PubMed] [Google Scholar]
- Van Hagen BTJ, van Goethem NP, Lagatta DC, Prickaerts J. The object pattern separation (OPS) task; a behavioral paradigm derived from the object recognition task. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.10.041. In press. [DOI] [PubMed] [Google Scholar]
- Vanmierlo T, Rutten K, Dederen J, Bloks VW, van Vark-van der Zee LC, Kuipers F, et al. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol Aging. 2011;32:1262–1272. doi: 10.1016/j.neurobiolaging.2009.07.005. [DOI] [PubMed] [Google Scholar]


