Abstract
Background and Purpose
Phosphorylation of δ opioid receptors (DOP receptors) by cyclin-dependent kinase 5 (CDK5) was shown to regulate the trafficking of this receptor. Therefore, we aimed to determine the role of CDK5 in regulating DOP receptors in rats treated with morphine or with complete Freund's adjuvant (CFA). As μ (MOP) and DOP receptors are known to be co-regulated, we also sought to determine if CDK5-mediated regulation of DOP receptors also affects MOP receptor functions.
Experimental Approach
The role of CDK5 in regulating opioid receptors in CFA- and morphine-treated rats was studied using roscovitine as a CDK inhibitor and a cell-penetrant peptide mimicking the second intracellular loop of DOP receptors (C11-DOPri2). Opioid receptor functions were assessed in vivo in a series of behavioural experiments and correlated by measuring ERK1/2 activity in dorsal root ganglia homogenates.
Key Results
Chronic roscovitine treatment reduced the antinociceptive and antihyperalgesic effects of deltorphin II (Dlt II) in morphine- and CFA-treated rats respectively. Repeated administrations of C11-DOPri2 also robustly decreased Dlt II-induced analgesia. Interestingly, DAMGO-induced analgesia was significantly increased by roscovitine and C11-DOPri2. Concomitantly, in roscovitine-treated rats the Dlt II-induced ERK1/2 activation was decreased, whereas the DAMGO-induced ERK1/2 activation was increased. An acute roscovitine treatment had no effect on Dlt II- or DAMGO-induced analgesia.
Conclusions and Implications
Together, our results demonstrate that CDK5 is a key player in the regulation of DOP receptors in morphine- and CFA-treated rats and that the regulation of DOP receptors by CDK5 is sufficient to modulate MOP receptor functions through an indirect process.
Tables of Links
| LIGANDS |
|---|
| DAMGO |
| Deltorphin II |
| Morphine |
| Piperidine |
| Roscovitine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,dAlexander et al., 2013a,b,c,d).
Introduction
Although their use is often limited by several unwanted effects, opioids remain the gold standard of care for the treatment of moderate to severe pain (McQuay, 1999). Clinically available opioids produce their effects mainly through the μ opioid receptor (MOP receptor). In addition to the MOP receptor subtype, the δ opioid receptor (DOP receptor) has shown a great potential for the development of novel analgesics as selective DOP receptor agonists were found to produce less adverse effects than commonly used opioids (Porreca et al., 1984; May et al., 1989; Dondio et al., 1997; Szeto et al., 1999; Brandt et al., 2001; Petrillo et al., 2003; Gallantine and Meert, 2005; Beaudry et al., 2009; Hudzik et al., 2014).
Admittedly, the analgesia mediated by the DOP receptor is modest in naïve animals. The limited efficacy of DOP receptor agonists is thought to be the consequence of a small proportion of receptors expressed at the plasma membrane in neurons throughout the pain pathway (Cahill et al., 2001; 2003; Morinville et al., 2003; Gendron et al., 2006; 2007a,b,). Over the last decade, we and others have shown that chronic inflammation and repeated morphine treatments significantly increase the DOP receptor-mediated analgesic effects as well as the density of DOP receptors expressed at the cell surface of spinal cord and dorsal root ganglia (DRG) neurons (Cahill et al., 2001; 2003; Morinville et al., 2003; Gendron et al., 2007a,b). Although the precise mechanisms mediating the regulation of DOP receptors is still unknown, it is worth noting that inflammation and morphine both regulate the trafficking and functions of DOP receptors through a MOP receptor-dependent mechanism (Morinville et al., 2004b; Gendron et al., 2007b), suggesting that these receptors may interact with each other, either directly or indirectly (for a review, see Gendron et al., 2015).
Among the potential mechanisms implicated in the regulation of DOP receptors, heterologous phosphorylation appears to play an important role. Indeed, intracellular loops and the carboxy-terminal tail of the DOP receptor possess a number of putative phosphorylation sites for various kinases. In addition to the GPCR kinases, phosphorylation sites regulating internalization and desensitization processes (Georgoussi et al., 2012), a consensus phosphorylation motif for cyclin-dependent kinases (CDK) is present in the second intracellular loop of the DOP receptor (T161PXK). Indeed, this site was previously reported to be directly phosphorylated by CDK5 (Xie et al., 2009).
CDK5 is a ubiquitous proline-directed serine/threonine kinase that is primarily active in the nervous system where its brain-specific activator p35 is also expressed. As opposed to other kinases of the CDK family, CDK5 is not involved in cell cycle progression. It rather plays important roles in processes such as neuronal activity, neuronal migration and neurite outgrowth. In the CNS, CDK5 was shown to be involved in the reward pathways (Arif, 2012). More recently, it was also shown that CDK5 can phosphorylate the serine 321 residue in the third intracellular loop of the dopamine D2 receptor, impeding its agonist-induced surface expression and G-protein coupling (Jeong et al., 2013). CDK5 is also involved in the regulation of the membrane expression of the glutamate receptors, NMDA and GluN2B (NR2B) (Zhang et al., 2008; Jeong et al., 2013). Therefore, the presence of a CDK5 phosphorylation motif in the second intracellular loop of the DOP receptor suggests that this kinase may also be involved in regulating the traffic and/or the activity of this receptor.
In this study, we thus assessed the role of CDK5 on the regulation of DOP and MOP receptors in the rat CFA model of inflammation and in rats treated with increasing doses of morphine over 48 h, two experimental conditions previously shown to induce a MOP receptor-mediated gain of function for DOP receptors.
Methods
Animals
Experiments were carried out in adult male Sprague-Dawley rats weighing 225–250 g (total of 254 rats; Charles River, St Constant, QC, Canada) maintained on a 12 h light/dark cycle (06:00–18:00 h). Laboratory chow and water were available ad libitum. Behavioural tests were conducted between 07:00 and 11:30 (light cycle). All experiments were approved by the animal care committee of the Université de Sherbrooke (Protocol #242-10B). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010), and conformed to the directives of the Canadian Council on Animal Care, and guidelines of the International Association for the Study of Pain. All animal experiments were designed to minimize the number of animals used and their suffering.
Induction of inflammation
Unilateral inflammation of the hind limb and development of hyperalgesia was induced by a single injection of 100 μL emulsified complete Freund's adjuvant (CFA) 50 μg·100 μL−1 (Calbiochem, San Diego, CA, USA) in the plantar surface of the left hind paw of rats under brief isoflurane anaesthesia (anaesthesia was induced with 5% isoflurane, 95% O2, then maintained with 2.5% isoflurane, 97.5% O2, 2 L·min−1). Inflammation was used to enhance cell surface availability of DOP receptors (Cahill et al., 2003; Morinville et al., 2004b; Gendron et al., 2006; 2007a,b,). Plantar tests were carried out 72 h after CFA injection as described later.
N-terminus ω-amino fatty acyl peptide synthesis ( [H2N-CH2-(CH2)9-CO-VKALDFRTPAKAKL-NH2] (DOPri2) and [H2N-CH2-(CH2)9-CO-RAAKVPKFLTLDKA-NH2] (DOPrscrambled))
Peptides C11-DOPri2 and C11-DOPrscrambled were synthesized using a solid phase continuous flow technique (Pioneer peptide synthesizer; Perkin Elmer, Guelph, ON, Canada) with 9-fluorenylmethoxycarbonyl (Fmoc) strategy. Fmoc-S-RAM TentaGel (Rapp Polymere, Tübingen, Germany) resin was used to start the peptide assembly. Fmoc-protected amino acids were coupled step-by-step on the solid phase. HATU [2-(1H—9-azabenzotriazole-1-yl)-1,1,3,3-tetramethyl-amonium hexa-fluorophosphate] was used as a coupling reagent in the presence of diisopropylethyl amine. At each coupling step, Fmoc was removed with 20% piperidine in dimethylformamide (DMF). Final acylation was performed manually on solid phase with twofold excess of Fmoc-HN-CH2-(CH2)9-COOH in dichloromethane using PyBOP (benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate) as a coupling agent in the presence of diisopropylethyl amine. The N-terminal Fmoc-protecting group was removed with 50% piperidine solution in DMF. Peptides were cleaved from the resin with trifluoroacetic acid (TFA) : H2O : triisopropyl silane = 95%:2.5%:2.5%, were filtered from resin and precipitated by dropping into anhydrous ethyl ether. Precipitated peptide TFA salts were centrifuged, dissolved in water and lyophilized. Finally, the products were chromatographically purified on reversed phase chromatography (C18) and their identity verified by MALDI mass spectrometry.
I.t. injections
I.t. injections were done under brief isoflurane anaesthesia as previously described (Mestre et al., 1994; Fairbanks, 2003). Briefly, a 30-G ½ needle mounted on a Luer tip Hamilton syringe (VWR, Montréal, QC, Canada) was inserted into the intervertebral space between the lumbar vertebrae L5–L6. Appropriate placement of the needle was confirmed by observing a slight flick of the tail.
Administration of drugs and peptides
Control vehicle was administered for each compound. Otherwise, saline was used as the control vehicle. Morphine sulfate (lots #BK8689 and CC0630; Sandoz, Montréal, QC, Canada and lot #43156/C; Medisca, Montréal, QC, Canada) was diluted in sterile saline solution (0,9 % NaCl) to concentrations of 5, 8, 10 and 15 mg·mL−1 and stored at room temperature protected from light. Morphine sulfate was injected s.c. every 12 h for 48 h (5, 8, 10 and 15 mg·kg−1) to enhance cell surface availability of the DOP receptor (Cahill et al., 2001; Morinville et al., 2003; 2004a,; Gendron et al., 2006; 2007a,). Tail immersion tests were carried out 12 h after the last morphine injection. Control rats received an equivalent volume of sterile saline.
Deltorphin II (Dlt II; lots #M08048T1 and W01124T1; American Peptide Company, Sunnyvale, CA, USA), a DOP receptor-selective agonist, was dissolved in a sterile saline solution at 1 mg·mL−1 and stored in aliquots at −20°C until used. For the experiments, Dlt II was diluted in sterile saline (1–30 μg in 30 μL).
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO; lot #29; Tocris Bioscience, Minneapolis MN, USA), a MOP receptor-selective agonist, was dissolved in a sterile saline solution at 10 mM and stored in aliquots at −20°C until used. For behavioural testing, DAMGO was diluted in sterile saline (30 or 100 ng in 30 μL).
Roscovitine, a selective inhibitor of CDK1 [IC50 0.65 μM (Meijer et al., 1997) ], CDK2 [IC50 0.1 μM (McClue et al., 2002) ] and CDK5 [IC50 0.16 μM (McClue et al., 2002) ] (lot #ARK-108; LC Laboratories, Woburn, MA, USA), was diluted in 100% DMSO at 200 μg·μL−1 and stored at −20°C until used. For administration, roscovitine was dissolved in sterile saline containing a final concentration of 50% DMSO. DMSO 50% in sterile saline was administered as a vehicle control.
C11-DOPri2, a peptide sequence mimicking the second intracellular loop of the DOP receptor fused to an 11-carbon aliphatic chain, was diluted in sterile saline at 205 mg·mL−1 and stored at −20°C until used. As a control, C11-DOPrscrambled, a peptide fused to an 11-carbon aliphatic chain containing the amino acids of DOPri2 in a random sequence, was diluted in sterile saline. C11-DOPri2 and C11-DOPrscrambled peptides were administered at a concentration of 6.15 μg (4 nmol·30 μL−1 in sterile saline.
For all experiments, roscovitine (1–30 μg in 30 μL), DMSO 50% (30 μL), C11-DOPri2 (6.15 μg), and C11-DOPrscrambled (6.15 μg) were injected either 30 min before each s.c. injection of morphine (total of four injections in 48 h) or 30 min before the CFA injection, and then twice a day (total of six injections in 72 h). Unless otherwise mentioned, the last injection of roscovitine, DMSO, C11-DOPri2 or C11-DOPrscrambled was performed 12 h before behavioural testing.
Behavioural testing
Plantar test
The response to a noxious heat stimulus was evaluated using the plantar test to measure the antihyperalgesic effects of drugs in rats treated with CFA. Animals were first habituated for 30 min in Plexiglas™ boxes positioned on a glass surface (IITC Life Science, Inc., Woodland Hills, CA, USA) 24 h before the baseline measurements. The following day (corresponding to the time point −72 h), the heat source was positioned under the plantar surface of the hind paw after a 15 min habituation period, and the latency for hind paw withdrawal in response to radiant heat was measured three times for each paw, in alternation. Subsequently, CFA was injected in the left hind paw as described earlier. Seventy-two hours after the injection of CFA, baseline withdrawal latencies (identified as 0 min) were measured for each hind paw twice in alternation. Afterwards, DAMGO or Dlt II were injected i.t. and the latencies to paw withdrawal were recorded every 15 min for 60 min. To prevent tissue damage, a cut-off time of 20 s was imposed. If an animal reached the cut-off, the light beam was automatically turned off and the animal was assigned the maximum score.
Tail flick test
Dlt II and DAMGO antinociception was assessed in morphine-treated rats (see earlier for details of the morphine treatment). Animals were acclimatized to the room and handling 2 days before the tail flick test. Twelve hours after the last morphine injection, tail flick latencies (tail immersion of 5 cm in a 52.0 ± 0.5°C water bath) following an i.t. challenge with either Dlt II (10 μg) or DAMGO (30 or 100 ng) were measured (in s) every 10 min for 60 min. To prevent tissue damage, a cut-off time of 10 s was imposed. If an animal reached the cut-off, the tail was immediately removed from the water and the animal was assigned the maximum score.
The antinociceptive effect of DAMGO and Dlt in the tail flick assay was expressed as a % of the ‘maximum possible effect’ (%MPE) calculated using the following formula:
Determination of ERK1/2 activity ex vivo
Rats were injected with CFA in the plantar surface of the hindpaw and received chronic roscovitine or vehicle (injected 30 min before CFA injection, and then twice a day). Seventy-two hours after CFA injection, rats received an i.t. injection of Dlt II (10 μg) or DAMGO (30 ng) for 20 min before being killed by decapitation under deep isoflurane anaesthesia (5% isoflurane, 95% O2, 2 L·min−1). L4–L6 DRGs (ipsilateral and contralateral) were collected and immediately frozen on dry ice. Proteins were extracted by triturating entire DRGs in 50 μL of lysis buffer (50 mM HEPES pH 7.8, 100 nM staurosporine, 1 mM Na3VO4, 1% Triton-X100, protease inhibitors). Samples were separated on 10% SDS-polyacrylamide gels. Proteins were transferred electrophoretically to PVDF membranes. Membranes were blocked with 1% gelatin, 0.05% Tween 20 in TBS buffer (pH 7.5). After being washed with TBS-Tween 20 (0.05%), membranes were incubated overnight at 4°C with anti-phosphorylated p42/p44MAPK (1:1000) or anti-p42/p44MAPK (1:1000), diluted in TBS-Tween 20 (0.05%). After the samples were washed with TBS-Tween 20, detection was accomplished using HRP-conjugated anti-rabbit IgG (1:2000) and an enhanced chemiluminescence detection system. Densitometry analysis was done using ImageJ software (Wayne Rasband, NIH, Bethesda, MD, USA).
Drug/molecular target nomenclature
The nomenclature of drug and molecular targets conforms to BJP's Concise Guide to Pharmacology (Alexander et al., 2013a,b), except for opioid receptors, which have been abbreviated in accordance with a more recent review published by members of the NC-IUPHAR Opioid Receptor Nomenclature Subcommittee (Cox et al., 2015).
Calculations and statistical analysis
Calculations were done with Excel (2010; Microsoft, Redmond, WA, USA), graphs with SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA), and statistical analysis with Prism GraphPad 6 (GraphPad Software Inc., La Jolla, CA, USA). Data are expressed as the mean ± SEM.
Results
Effect of roscovitine on DOP receptor-mediated analgesia
To assess the role of CDK5 in the regulation of DOP receptor-mediated analgesia, we administered different doses of roscovitine to morphine-treated rats via the i.t. route. As shown in Figure 1A, 20 min after the injection Dlt II had a weak antinociceptive effect in saline-treated rats (16.3 ± 4.5% MPE; no morphine pretreatment). Conversely, when rats were treated for 48 h with increasing doses of morphine (5, 8, 10, 15 mg·kg−1, every 12 h), the antinociceptive effect of Dlt II at 20 min was significantly increased (47.9 ± 5.4% MPE compared with 16.3 ± 4.5% MPE; for vehicle and no morphine-pretreatment, respectively, P < 0.05 using one-way anova with Tukey's multiple comparisons test). I.t. roscovitine administered 30 min before each morphine injection dose-dependently decreased the antinociceptive effect of Dlt II as compared with vehicle-treated rats and this effect reached statistical significance with 30 μg roscovitine (14.8 ± 8.7 % MPE compared with 47.9 ± 5.4 % MPE for 30 μg and vehicle respectively, P < 0.05 using one-way anova with Tukey's multiple comparisons test). In order to fully assess the effect of roscovitine on DOP receptor-mediated antinociception, a dose-response curve for Dlt II was performed in rats treated with 30 μg roscovitine or 30 μL of vehicle. There was a significant main effect for the dose of Delt II [F(2, 28) = 4.84, P < 0.05], but not for roscovitine treatment [F(1, 28) = 4.13, P > 0.05], and a significant interaction, [F(2, 28) = 4.99, P < 0.05]. As shown in Figure 1B, when chronic vehicle and chronic roscovitine groups were further compared using a Sidak's multiple comparisons test, a significant decrease in the analgesic effects for 10 μg Dlt II was observed in the animals chronically treated with roscovitine.
Figure 1.
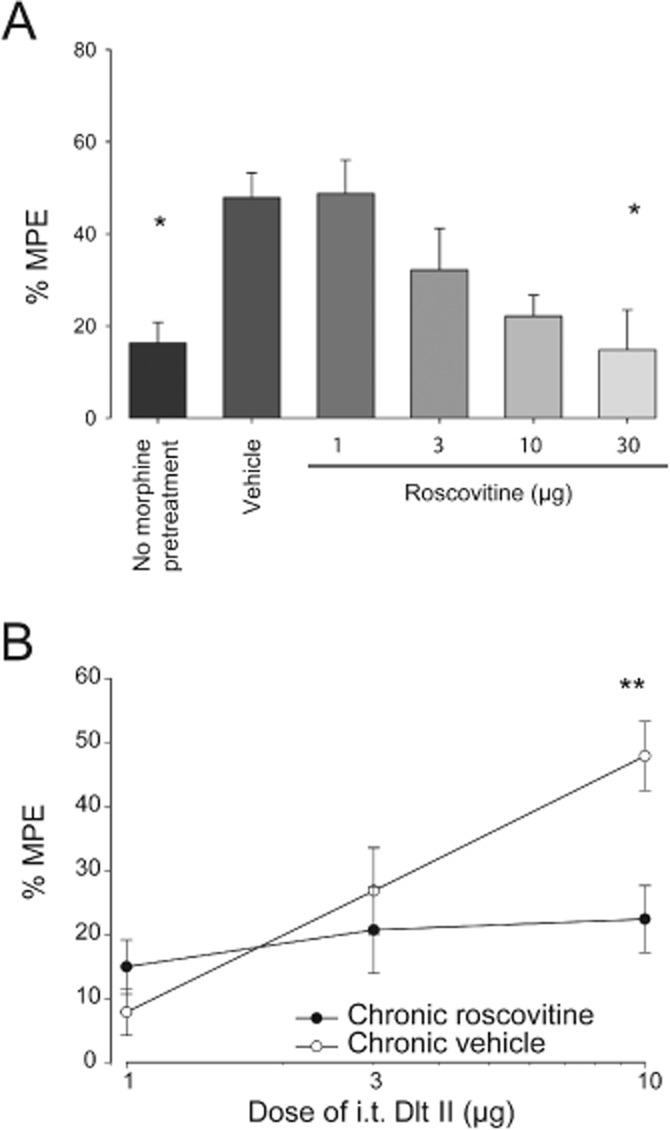
Determination of efficient roscovitine dose in morphine-treated rats. Sprague-Dawley rats were injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1). Twelve hours after the last morphine injection, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after Dlt II i.t. injection (10 μg) using the tail immersion test. Data at the 20 min time point, representing the peak effect of Delt II, were used for the calculation of the %MPE. (A) i.t. roscovitine (1, 3, 10, 30 μg) administered 30 min before each morphine injection produced a dose-dependent decrease in Dlt II-induced antinociception. *P < 0.05 (n= 6 animals per group). (B) The antinociceptive effect of increasing doses of i.t. Dlt II expressed as the %MPE (percentage of the MPE) is shown for animals pretreated with morphine over 48 h and with vehicle or roscovitine 30 μg. **P < 0.01 (n= 4–6 animals per group).
We then assessed the impact of inhibiting CDK5 with roscovitine (30 μg) on the Dlt II-induced antihyperalgesia in the CFA model of inflammation. As shown in Figure 2A, in vehicle-treated inflamed rats 10 μg of i.t. Dlt II induced a robust and transient antihyperalgesic effect. The paw withdrawal latency reached 9.89 ± 0.35 s 15 min after Dlt II injection and returned to baseline within 60 min. In roscovitine-pretreated rats, i.t. Dlt II produced a much lower antihyperalgesic effect than in vehicle-treated rats (Figure 2A; 6.67 ± 0.45 s compared with 9.89 ± 0.35 s 15 min after the injection of Dlt II). There was a significant main effect for roscovitine treatment [F(1, 198) = 22.35, P < 0.0001], and a significant interaction, [F(5, 198) = 5.25, P < 0.0001]. When chronic vehicle and chronic roscovitine groups were further compared using a Sidak's multiple comparisons test, a significant decrease in the analgesic effect of Delt II was found for the 15 and 30 min time points. When the area under curves (AUCs) were analysed and compared using an unpaired t-test, the antihyperalgesic effect of Dlt II was significantly reduced in roscovitine-pretreated rats (Figure 2C; AUC of 5.97 ± 1.24 compared with 11.09 ± 0.78 for roscovitine and vehicle groups respectively).
Figure 2.
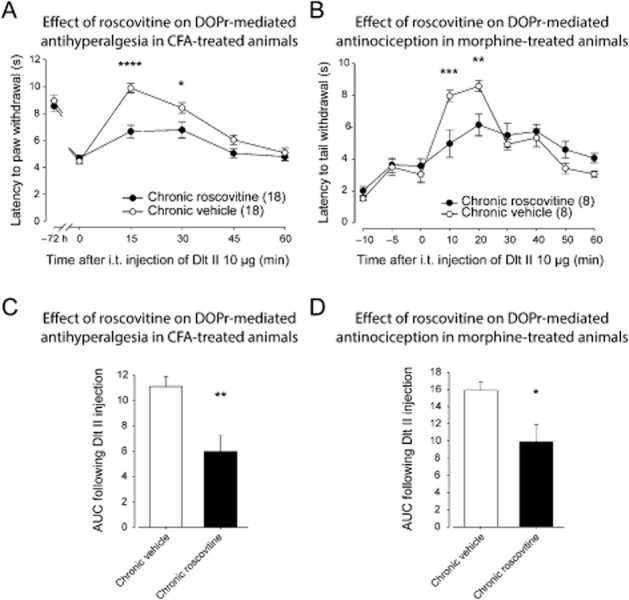
Effect of roscovitine on DOP receptor-mediated antihyperalgesia and antinociception. (A) Sprague-Dawley rats were injected with CFA in the plantar surface of the hindpaw. Thirty minutes before the CFA injection and every 12 h thereafter, rats were injected i.t. with roscovitine (30 μg) or vehicle (30 μL). Seventy-two hours after CFA injection, paw withdrawal latencies (in s) to noxious heat (plantar test) were recorded every 15 min for a period of 60 min following Dlt II administration (10 μg, i.t.). I.t.-administered roscovitine (30 μg) induced a significant decrease in DOP receptor-mediated antihyperalgesia. *P < 0.05 and ****P < 0.0001. (B) Sprague-Dawley rats injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1) received roscovitine (30 μg) or vehicle (30 μL) 30 min before each morphine injection. Twelve hours after the last morphine injection, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after Dlt II injection (10 μg, i.t.) using the tail immersion test. Roscovitine injection induced a significant decrease in DOP receptor-mediated antinociception. **P < 0.01 and ***P < 0.001. (C) Results presented in (A) are expressed as the AUC obtained between 0 and 60 min after Dlt II injection (the Y-axis baseline was set for each animal according to their latency to paw withdrawal after inflammation). **P < 0.01. (D) Results presented in (B) are expressed as the AUC obtained between 0 and 60 min after Dlt II injection (Y-axis baseline was set for each animal according to their latency to tail withdrawal at 0 min). *P < 0.05. Numbers given in parentheses represent the number of animals per group.
In the tail flick assay, i.t. Dlt II produced a transient increase in the time to tail withdrawal. The DOP receptor-mediated antinociceptive effect reached a maximum at 20 min (8.59 ± 0.34 s) and returned to baseline latencies by 50 min. There was no significant main effect for roscovitine treatment [F(1, 112) = 0.29, P > 0.05], and a significant interaction, [F(8, 126) = 4.80, P < 0.0001]. When the vehicle and roscovitine groups were further compared using a Sidak's multiple comparisons test, the antinociceptive effect of Dlt II was found to be significantly reduced 10 and 20 min after the injection in roscovitine-treated rats (Figure 2B). As it can be seen in Figure 2D, an unpaired t-test comparison for the AUC indicates that the antinociceptive effect of Dlt II was significantly reduced in roscovitine-pretreated rats (Figure 2D). Interestingly, roscovitine neither affected the level of hyperalgesia induced by the inflammation (Figure 2A) nor the baseline latency to tail withdrawal (Figure 2B).
Because roscovitine is a non-selective CDK inhibitor (it inhibits CDK1, CDK2 and CDK5 with similar potencies), but also because CDK5 has numerous putative targets in vivo, we used a peptide-based approach to confirm a specific role of CDK5 in regulating DOP receptor-mediated analgesia. The second intracellular loop of the DOP receptor (C11-DOPri2), containing the threonine 161 residue phosphorylated by CDK5, was thus used to prevent the direct phosphorylation of the DOP receptor by Cdk5. The DOPri2 mimicking peptide was fused to an 11-carbon chain to increase its membrane permeability (C11-DOPri2). As shown in Figure 3A, in inflamed rats treated with the scrambled peptide, 10 μg of i.t. Dlt II induced an antihyperalgesia with a maximal effect at 15 min. The effect returned to baseline latencies within 45 min. There was no significant main effect for C11-DOPri2 treatment [F(1, 94) = 3.16, P > 0.05], and a significant interaction, [F(5, 94) = 11.26, P < 0.0001]. However, when chronic C11-DOPri2 and chronic C11-DOPrscrambled groups were compared using a Sidak's multiple comparisons test, the C11-DOPri2 pretreatment was found to significantly reduce the antihyperalgesic effect of i.t. Dlt II at the 15 min time point (Figure 3A). An analysis of the AUC using an unpaired t-test also revealed a significant effect of C11-DOPri2 on Delt II-induced analgesia (Figure 3C). Note that C11-DOPri2 attenuated the DOP receptor-mediated antihyperalgesic effect without modifying the hyperalgesia induced by inflammation (Figure 3A; 4.89 ± 0.24 s compared with 4.78 ± 0.24 s at 0 min for chronic C11-DOPri2 and chronic C11-DOPrscrambled respectively). Similarly, C11-DOPri2 pretreatment did not modify the baseline tail flick latencies in morphine-pretreated rats (Figure 3B, compare times −10 to 0 min for chronic C11-DOPrscrambled and chronic C11-DOPri2). In C11-DOPrscrambled-treated rats, Dlt II induced a transient increase in tail flick latencies, reaching a maximal effect at 20 min. The effect returned to baseline latencies after 50 min. There was a significant main effect for C11-DOPri2 treatment [F(1, 90) = 6.66, P < 0.05], and a significant interaction, [F(8, 90) = 2.87, P < 0.01]. When chronic C11-DOPri2 and chronic C11-DOPrscrambled groups were further compared using a Sidak's multiple comparisons test, a significant decrease in the Dlt II-induced antinociception at 30 min was revealed in the C11-DOPri2-treated group (Figure 3B). As revealed by an unpaired t-test, this effect of C11-DOPri2 was further confirmed by a significant decrease in the AUC (Figure 3D).
Figure 3.
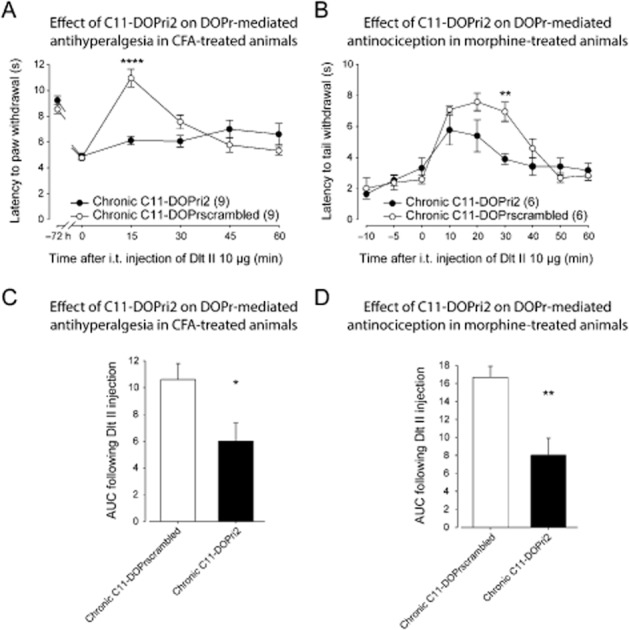
Effect of C11-DOPri2 on DOP receptor-mediated antihyperalgesia and antinociception. (A) Sprague-Dawley rats were injected with CFA in the plantar surface of the hindpaw. Thirty minutes before the CFA injection and every 12 h thereafter, rats were injected i.t. with C11-DOPri2 (6 and 15 μg) or C11-DOPrscrambled peptides (6 and 15 μg). Seventy-two hours after the CFA injection, paw withdrawal latencies (in s) to noxious heat (plantar test) were recorded every 15 min (from 0 to 60 min) following Dlt II administration (10 μg, i.t.). I.t.-administered C11-DOPri2 (6 and 15 μg) induced a significant decrease in DOP receptor-mediated antihyperalgesia. ****P < 0.0001. (B) Sprague-Dawley rats injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1) received i.t. injections of C11-DOPri2 (6 and 15 μg) or C11-DOPrscrambled (6 and 15 μg) 30 min before each morphine injection. Twelve hours after the last morphine injection, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after Dlt II injection (10 μg, i.t.) using the tail immersion test. C11-DOPri2 induced a significant decrease in DOP receptor-mediated antinociception. **P < 0.01. (C) Results presented in (A) are expressed as the AUC obtained between 0 and 60 min after Dlt II injection (the Y-axis baseline was set for each animal according to their latency to paw withdrawal after inflammation). *P < 0.05. (D) Results presented in (B) are expressed as the AUC obtained between 0 and 60 min after Dlt II injection (the Y-axis baseline was set for each animal according to their latency to tail withdrawal at 0 min). **P < 0.01. Numbers given in parentheses represent the number of animals per group.
Role of CDK5 in the MOP receptor-mediated antinociception and antihyperalgesia
We then sought to determine if CDK5 also influences the analgesic effect induced by the MOP receptor despite the fact that the consensus phosphorylation motif for CDK5 is not present in this receptor. As shown in Figure 4A, in vehicle-treated inflamed rats, 100 ng of i.t. DAMGO, a selective MOP receptor agonist, induced a robust and sustained increase in withdrawal latencies with maximum effect at 15 min. The effect returned to baseline values within 60 min. There was a significant main effect for roscovitine treatment [F(1, 126) = 24.19, P < 0.0001], and a significant interaction, [F(5, 126) = 3.30, P < 0.01]. A further analysis using a Sidak's multiple comparisons test revealed that the antihyperalgesic effect of DAMGO was significantly increased in roscovitine-pretreated rats as compared with vehicle-treated rats at 15, 30 and 45 min (Figure 4A). A comparison of the AUC for the roscovitine- and vehicle-treated groups with an unpaired t-test also revealed a significant increase in the analgesic effect of DAMGO in the roscovitine-treated rats (Figure 4C). These results suggest that the inhibition of CDK5 increases the MOP receptor-mediated antihyperalgesia in the CFA model of inflammation.
Figure 4.
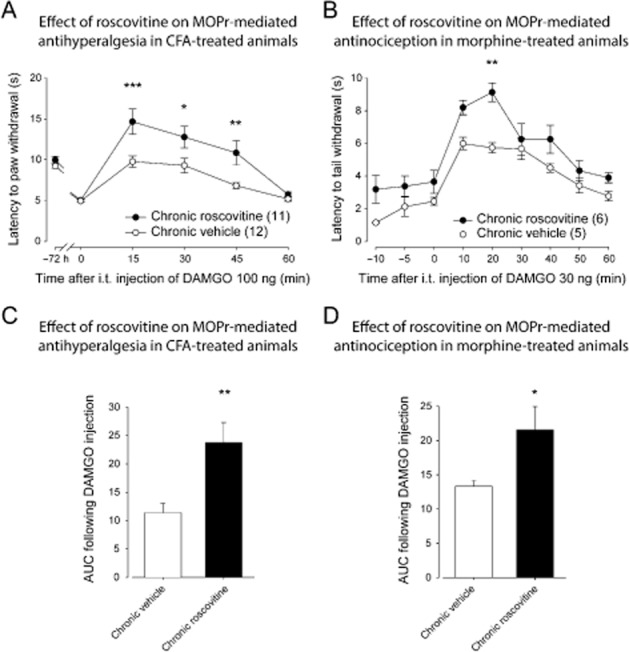
Effect of roscovitine on MOP receptor-mediated antihyperalgesia and antinociception. (A) Sprague-Dawley rats were injected with CFA in the plantar surface of the hindpaw. Thirty minutes before the CFA injection and every 12 h thereafter, rats were injected i.t. with roscovitine (30 μg) or vehicle (30 μL). Seventy-two hours after the CFA injection, paw withdrawal latencies (in s) to noxious heat (plantar test) were recorded every 15 min (from 0 to 60 min) following DAMGO administration (100 ng, i.t.). I.t.-administered roscovitine (30 μg) induced a significant increase in MOP receptor-mediated antihyperalgesia. *P < 0.05, **P < 0.01 and ***P < 0.001. (B) Sprague-Dawley rats injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1) received a roscovitine (30 μg) or a vehicle (30 μL) i.t. injection 30 min before each morphine injection. Twelve hours after the last morphine injection, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after DAMGO injection (30 ng, i.t.) using the tail immersion test. Roscovitine induced a significant increase in MOP receptor-mediated antinociception. **P < 0.01. (C) Results presented in (A) are expressed as the AUC obtained between 0 and 60 min after DAMGO injection (the Y-axis baseline was set for each animal according to their latency to paw withdrawal after inflammation). **P < 0.01. (D) Results presented in (B) are expressed as the AUC obtained between 0 and 60 min after DAMGO II injection (the Y-axis baseline was set for each animal according to their latency to tail withdrawal at 0 min). *P < 0.05. Numbers given in parentheses represent the number of animals per group.
In morphine-pretreated rats, 30 ng of i.t. DAMGO induced an increase in the tail flick latencies, reaching a maximal effect at 10 min and returning to baseline latencies after 60 min. There was a significant main effect for roscovitine treatment [F(1, 81) = 32.63, P < 0.0001], but the interaction was not significant, [F(8, 81) = 1.03, p > 0.05]. When the chronic vehicle and the chronic roscovitine groups were further compared using a Sidak's multiple comparisons test, roscovitine was found to significantly increased the DAMGO-induced antinociception at 20 min (Figure 4B). An unpaired t-test analysis of the AUC further supports that roscovitine increased the antinociceptive effect of DAMGO (Figure 4D). Noteworthy, a 48 h morphine-pretreatment did not modify the antinociceptive effect of DAMGO when compared with untreated rats (not shown).
To see whether the regulation of MOP receptor by CDK5 is dependent on the phosphorylation of the DOP receptor at the Thr161 residue, we used C11-DOPri2 pretreatment prior to measuring the antihyperalgesic and antinociceptive effects of DAMGO. As shown in Figure 5A, in C11-DOPrscrambled-treated inflamed rats, 30 ng of i.t. DAMGO induced an increase in withdrawal latency with maximal effect at 30 min. The effect returned to baseline latencies after 45 min. There was a significant main effect for C11-DOPri2 treatment [F(1, 60) = 46.80, P < 0.0001], and a significant interaction, [F(5, 60) = 13.73, P < 0.0001). When the chronic C11-DOPrscrambled and the chronic C11-DOPri2 groups were further compared using a Sidak's multiple comparisons test, the C11-DOPri2 pretreatment was found to significantly increase the antihyperalgesic effect of i.t. DAMGO at 15 and 30 min (Figure 5A). An unpaired t-test analysis of the AUC further supports that C11-DOPri2 increased the antihyperalgesic effect of DAMGO (Figure 5C). As observed following roscovitine-pretreatment, injection of C11-DOPri2 did not modify the inflammation-induced hyperalgesia at 0 min (Figure 5A). In a similar way, C11-DOPri2 pretreatment did not modify baseline tail flick latencies when compared with C11-DOPrscrambled in morphine-pretreated rats (Figure 5B, from −10 to 0 min for chronic C11-DOPrscrambled compared with chronic C11-DOPri2). In C11-DOPrscrambled-treated rats, DAMGO induced a transient increase in tail flick latencies reaching maximal effect at 10 min and returning to baseline latencies after 60 min. There was a significant main effect for C11-DOPri2 treatment [F(1, 90) = 24.76, P < 0.0001], but no significant interaction, [F(8, 90) = 1.09, P > 0.05]. When the chronic C11-DOPrscrambled and the chronic C11-DOPri2 groups were further compared using a Sidak's multiple comparisons test, a significant increase in DAMGO-induced antinociception at 10 min was found for the C11-DOPri2-treated group (Figure 5B). An analysis of the AUC using an unpaired t-test further supports a significant effect of C11-DOPri2 on the DAMGO-induced antinociception (Figure 5D).
Figure 5.
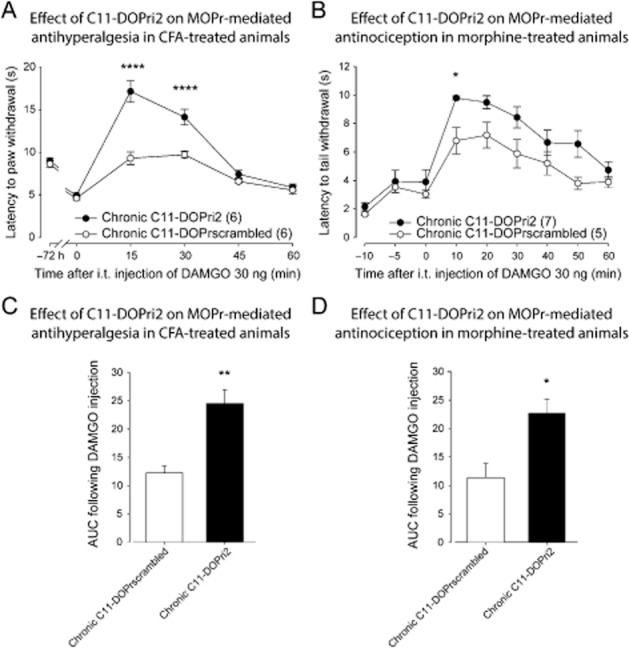
Effect of C11-DOPri2 on MOP receptor-mediated antihyperalgesia and antinociception. (A) Sprague-Dawley rats were injected with CFA in the plantar surface of the hindpaw. Thirty minutes before the CFA injection and every 12 h thereafter, rats were injected i.t. with C11-DOPri2 (6 and 15 μg) or C11-DOPrscrambled peptides (6 and 15 μg). Seventy-two hours after the CFA injection, paw withdrawal latencies (in s) to noxious heat (plantar test) were recorded every 15 min (from 0 to 60 min) following DAMGO administration (30 ng, i.t.). I.t.-administered C11-DOPri2 induced a significant increase in MOP receptor-mediated antihyperalgesia. ****P < 0.0001. (B) Sprague-Dawley rats injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1) received i.t. injections of C11-DOPri2 (6 and 15 μg) or C11-DOPrscrambled peptides (6 and 15 μg) 30 min before each morphine injection. Twelve hours after the last morphine injection, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after DAMGO injection (30 ng, i.t.) using the tail immersion test. C11-DOPri2 induced a significant increase in MOP receptor-mediated antinociception. *P < 0.05. (C) Results presented in (A) are expressed as the AUC obtained between 0 and 60 min after DAMGO injection (the Y-axis baseline was set for each animal according to their latency to paw withdrawal after inflammation). **P < 0.01. (D) Results presented in (B) are expressed as the AUC obtained between 0 and 60 min after DAMGO II injection (the Y-axis baseline was set for each animal according to their latency to tail withdrawal at 0 min). *P < 0.05. Numbers given in parentheses represent the number of animals per group.
Effect of acute roscovitine on the DOP and MOP receptor-mediated analgesia
One possible explanation of a modification of DOP and MOP receptor-induced analgesia following chronic CDK5 inhibition is a direct effect of CDK5 on opioid receptors functions and/or signalling cascades. To test this hypothesis, we measured the analgesic effects of Dlt II and DAMGO in inflamed and in morphine pretreated rats 30 min after a single injection of vehicle or roscovitine. As shown in Figure 6, an acute pretreatment with roscovitine (30 μg) did not modify the DOP receptor-mediated antihyperalgesia (Figure 6A). There was no significant main effect for acute roscovitine treatment [F(1, 60) = 0.33, P > 0.05], and no significant interaction [F(5, 60) = 1.03, P > 0.05]. In addition, acute roscovitine pretreatment did not modify Dlt II antinociceptive effect (Figure 6B). There was no significant main effect for acute roscovitine treatment [F(1, 98) = 0.03, P > 0.05], and no significant interaction [F(8, 98) = 0.36, P > 0.05]. Similarly, roscovitine pretreatment had no effect on DAMGO-induced analgesia (Figure 6C and D). There was no significant main effect for acute roscovitine treatment on DAMGO antihyperalgesic effect [F(1, 58) = 0.02, P > 0.05], and no significant interaction [F(5, 58) = 0.05, P > 0.05]. Similarly, there was no significant main effect for acute roscovitine treatment on DAMGO-induced antinociceptive effect [F(1, 89) = 0.05, P > 0.05], and no significant interaction [F(8, 89) = 1.23, P > 0.05].
Figure 6.
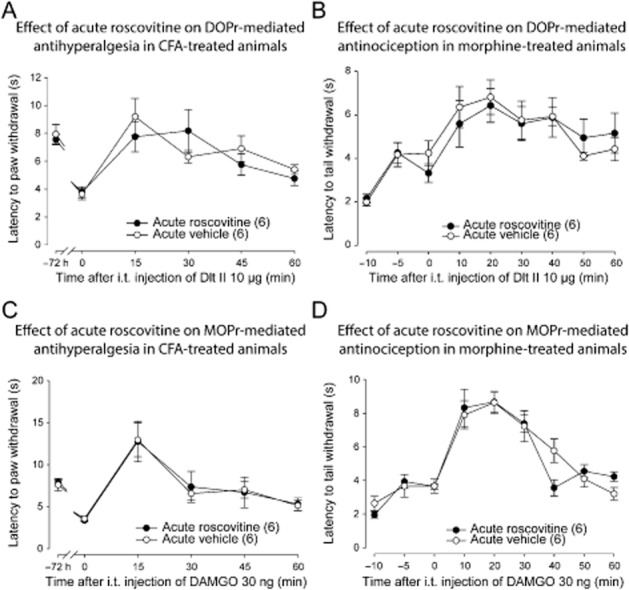
Effect of acute roscovitine on DOP and MOP receptor-mediated analgesia. (A) Sprague-Dawley rats were injected with CFA in the plantar surface of the hindpaw. Seventy-two hours after the CFA injection, rats received an i.t. injection of roscovitine (30 μg) or vehicle (30 μL). Thirty minutes after the injection of roscovitine or vehicle, the paw withdrawal latencies (in s) to noxious heat (plantar test) were recorded every 15 min for 60 min following Dlt II administration (10 μg, i.t.). I.t.-administered roscovitine (30 μg) did not modify DOP receptor-mediated antihyperalgesia. (B) Sprague-Dawley rats were injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1). Twelve hours after the last morphine injection they received an i.t. injection of roscovitine (30 μg) or vehicle (30 μL). 30 min thereafter, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after Dlt II injection (10 μg, i.t.) using the tail immersion test. Roscovitine injection did not modify DOP receptor-mediated antinociception. (C) Rats were injected with CFA in the plantar surface of the hindpaw. Seventy-two hours after CFA injection, they received an i.t. injection of roscovitine (30 μg) or vehicle (30 μL). Thirty minutes after the injection, paw withdrawal latencies (in s) to noxious heat (plantar test) were recorded every 15 min for 60 min following DAMGO administration (30 ng, i.t.). I.t.-administered roscovitine (30 μg) did not modify MOP receptor-mediated antihyperalgesia. (D) Sprague-Dawley rats were injected s.c. once every 12 h with escalating doses of morphine (5, 8, 10 and 15 mg·kg−1). Twelve hours after the last morphine injection they received an i.t. injection of roscovitine (30 μg) or vehicle (30 μL). Thirty minutes after the injection, tail flick latencies (in s) were measured every 10 min (from 0 to 60 min) after DAMGO injection (30 ng, i.t.) using the tail immersion test. Roscovitine injection did not modify MOP receptor-mediated antinociception. Numbers given in parentheses represent the number of animals per group.
Effect of roscovitine on DOP and MOP receptor-mediated activation of ERK1/2
DOP and MOP receptors are known to signal through the MAPK (ERK1/2) pathway (Eisinger and Schulz, 2005; Williams et al., 2013). We therefore measured the activation of ERK1/2 in lumbar DRGs of rats following administration of Dlt II and DAMGO. In ipsilateral L4-L6 DRGs isolated from inflamed rats, 10 μg i.t. Dlt II induced a 127.38 ± 5.66 % increase of phosphorylation of ERK1/2 as compared with saline (Figure 7A and B; P < 0.05 using one-way anova followed by Tukey's multiple comparisons test). Interestingly, in roscovitine-pretreated rats, DOP receptor-mediated ERK1/2 activation was completely abolished (Figure 7A and B; Rosco + Dlt II compared with Dlt II; P < 0.01, using one-way anova followed by Tukey's multiple comparisons test). In rats injected with 30 ng of i.t. DAMGO, ERK1/2 phosphorylation was slightly increased, although it did not reach statistical significance when compared with saline-injected rats. Remarkably, chronic roscovitine pretreatment induced a robust increase in MOP receptor-mediated ERK1/2 activation (Figure 7A and B; Rosco + DAMGO compared with DAMGO; P < 0.01, using one-way anova followed by Tukey's multiple comparisons test).
Figure 7.
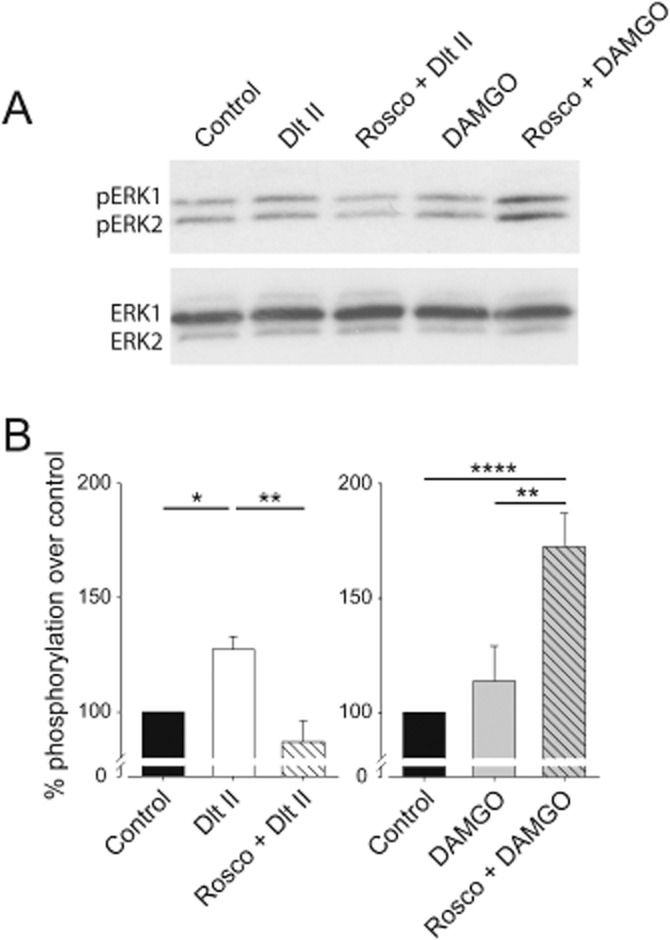
Roscovitine modifies the activity of DOP and MOP receptors ex vivo. Sprague-Dawley rats were injected with CFA in the plantar surface of the hindpaw. Thirty minutes before the CFA injection and every 12 h thereafter, rats were injected i.t. with vehicle or roscovitine (30 μg, i.t.). Seventy two hours after the CFA injection, rats received a single i.t. injection of Dlt II (10 μg) or DAMGO (30 ng) for 20 min, afterwards the DRGs were quickly collected and frozen in dry ice. Western blot analysis of phosphorylated ERK1/2 (pERK1 and pERK2) was performed as described in the Methods section. (A) Representative autoradiogram of ERK1/2 activation by Dlt II or DAMGO. The lower panel shows total ERK1/2. (B) Densitometric analysis of p42/p44MAPK activation. In CFA-inflamed rats, Dlt II and DAMGO increased ERK1/2 phosphorylation in vehicle-treated rats. In roscovitine-treated rats, Dlt II-induced ERK1/2 phosphorylation was reduced whereas DAMGO-induced ERK1/2 phosphorylation was increased. *P < 0.05, **P < 0.01 and ***P < 0.001. (n = 3–5 animals per group).
Discussion and conclusions
We have previously shown that chronic morphine and inflammation induced by CFA increase the density of δ opioid receptors (DOP receptors) at the plasma membrane of spinal cord and DRG neurons in rodents (Cahill et al., 2001; 2003; Morinville et al., 2003; Gendron et al., 2006; 2007a,b,). Among other mechanisms, receptor phosphorylation represents an important step in the regulation of receptor trafficking and activity. Interestingly, a consensus phosphorylation motif for CDKs is present in the second intracellular loop of DOP receptor and its phosphorylation by CDK5 was shown to be involved in regulating the trafficking of this receptor. The present study therefore investigated the role of CDK5 in regulating the activity of DOP receptor. We found that roscovitine dose-dependently (1–30 μg, i.t.) reduced the antinoniceptive effect of Dlt II in morphine-treated rats. Similarly, roscovitine induced a robust decrease in Dlt II-induced antihyperalgesia in the rat model of CFA-induced inflammation. Administration of the C11-DOPi2-mimicking peptide also produced a robust decrease in Dlt II-mediated antinociceptive and antihyperalgesic effects. Interestingly, although the MOP receptor does not contain the consensus recognition motif for CDK5, we observed that roscovitine significantly increased the antinociception and antihyperalgesia induced by DAMGO. Blockade of DOP receptor phosphorylation with C11-DOPi2 also significantly increased MOP receptor-mediated antinociceptive and antihyperalgesic effects. No effect of acute CDK5 inhibition on DOP and MOP receptor-mediated analgesia was found.
In the present study, pharmacological as well as peptidergic approaches were used to investigate the role of CDK5 on the regulation of DOP and MOP receptor-mediated analgesia. A limitation to the use of the pharmacological inhibitor roscovitine to identify the CDK isoform involved in the observed effects is its limited selectivity towards CDK5 versus CDK1 and CDK2 (Meijer et al., 1997; McClue et al., 2002). Because all CDKs are able to phosphorylate the consensus site S/T-PX-K/H/R (Beaudette et al., 1993; Songyang et al., 1996), one could argue that the effects reported in this study could therefore be attributed to CDK1 and/or CDK2 rather than to CDK5. For many reasons, a role for CDK1 and/or CDK2 in regulating the activity of neuronal MOP and DOP receptors is, however, unlikely. Firstly, in the adult nervous system, the expression of CDK1 and CDK2 was shown to be very limited (Hellmich et al., 1992; Miyajima et al., 1995; Negis et al., 2011; Wu et al., 2011). By contrast, CDK5 is known to be expressed at high levels in post-mitotic neurons (Lee et al., 1996) where it has a cytoplasmic location (Smith and Tsai, 2002; Jeong et al., 2013). One should also note that among the CDK members, CDK5 is the only isoform known to modulate the activity and the insertion of receptors into the plasma membrane. Indeed, CDK5 was shown to associate with the 5-HT receptor 5-HT6 and to mediate its role in neuronal differentiation (Duhr et al., 2014) and migration (Jacobshagen et al., 2014). In addition to the D2 dopamine receptor and NMDA (GluN2) glutamate receptors (Zhang et al., 2008; Jeong et al., 2013), CDK5 was also found to mediate TRPV1 insertion into plasma membrane and to increase its activity (Xing et al., 2012). Furthermore, carrageenan (Pareek et al., 2006) and CFA (Yang et al., 2007) have been shown to increase the activity of CDK5 in DRG neurons. Similarly, fentanyl, a MOP receptor agonist, has also been shown to increase the activity of CDK5 in the striatum and in the cerebral cortex of rats (Ramos-Miguel and Garcia-Sevilla, 2012). Although this was not directly addressed in our study, these numerous evidences led us to conclude that among the roscovitine-sensitive CDKs, CDK5 is likely to be the isoform involved in the phosphorylation and in the regulation of DOP receptor and its activity. This hypothesis is further supported by previous findings from others showing that the expression of a dominant negative form of CDK5 in primary cultures of DRG neurons reduced the level of phosphorylation of DOP receptor on its threonine 161 (Xie et al., 2009).
In rodents, it has been shown that the inhibition of CDK5 reduces DOP receptor-mediated analgesia as well as DOP receptor insertion into the plasma membrane (Xie et al., 2009). However, the specific involvement of CDK5 in mediating the regulation of DOP receptor activity by inflammation or chronic morphine treatment is still unknown. In the present study, we revealed a role for CDK5 in the regulation of DOP receptor-mediated analgesia in inflamed- and in morphine-treated rats. We found that the inhibition of CDK5 increases the analgesic effect of DAMGO in CFA- and morphine-treated rats. The fact that roscovitine and the C11-DOPi2-mimicking peptide were found to modify the analgesic effects of Dlt II (negatively) and DAMGO (positively) suggests that the phosphorylation of DOP receptor by CDK5 plays an important role in the regulation of these receptors during chronic morphine treatment and under chronic inflammatory pain conditions. As we found that acute CDK5 inhibition did not interfere with DOP receptor- and MOP receptor-mediated analgesia, our results exclude a direct effect of CDK5 inhibition on the signalling cascades of either receptors. For a yet undetermined reason, our results, however, differ from others who described that the acute inhibition of CDK5 with roscovitine inhibits the DOP receptor-mediated calcium influx in cultured DRG neurons (Xie et al., 2009) as well as the analgesic effects of Dlt (Xie et al., 2009; Chen et al., 2012).
Together with the present study, these results suggest that DOP receptor plays a role in the regulation of MOP receptor in inflamed- and in morphine-treated rats, but not in naïve animals. Different levels of interactions between the MOP and DOP receptor could therefore be responsible for these observations. Indeed, chronic morphine exposure was found to promote the MOP receptor/DOP receptor heteromer formation in many brain regions and in DRGs (Gupta et al., 2010). Interestingly, increasing the interaction between MOP and DOP receptors was previously found to decrease the analgesic efficacy of morphine by inducing the co-degradation of MOP receptor following the activation of DOP receptor (He et al., 2011). Although we cannot rule out an indirect regulation of the MOP and DOP receptor through their signalling cascades or within a neuronal network, in morphine-treated rats, and so possibly in inflamed rats, the fact that the inhibition of CDK5 increases MOP receptor functions is probably due to a loss of interaction with the DOP receptor, preventing MOP receptor degradation.
CDK5 has been shown to be involved in CFA-induced hyperalgesia (Yang et al., 2007; Zhang et al., 2012). However, in the present study the CFA baseline latencies were similar among all groups. This discrepancy could be due to the different doses of roscovitine used here and by others. In our experimental design, we determined that the lowest dose of i.t. roscovitine able to inhibit the CFA-induced increase in DOP receptor function was 30 μg. By contrast, previous studies used as much as 100 μg of roscovitine. Admittedly, higher doses of roscovitine may produce a more robust effect on CFA-induced hyperalgesia. However, 100 μg of roscovitine may also have the potential to inhibit other CDK isoforms (McClue et al., 2002). Indeed, in their studies Yang et al. and Zhang et al. found that 100 μg of roscovitine reduced the hyperalgesia induced by inflammation between days 1 and 5 (Yang et al., 2007; Zhang et al., 2012). In the same study, this effect was not seen when a dose of 10 μg of roscovitine was used. Another possible explanation is the time frame at which the behavioural experiments were performed. Yang and collaborators indeed demonstrated a reduction of CFA-induced hyperalgesia in rats treated with 10 μg roscovitine during the first hours following CFA injection, but the effect was reversed after 3 days (Yang et al., 2007). Our current study was designed to assess the mechanisms regulating DOP receptor in an inflammatory model. The 72 h treatment was selected based on our previous findings suggesting that the inflammation-induced up-regulation of DOP receptor in DRG neurons takes place 72 h after CFA injection, but not after 48 h (Gendron et al., 2006). Taken together, these results indicate that the effect of CDK5 on the inflammation-induced hyperalgesia may occur during a short time frame after CFA injection or with high doses of roscovitine and potentially explain why we did not see any difference in the CFA-induced hyperalgesia in our experiments.
Opioid receptors are known to activate p42/p44MAPK (ERK1/2) in vitro (for review, see Law et al., 2000), but to our knowledge, we are the first to show opioid-mediated ERK1/2 activation in freshly isolated DRGs. In the present study, we used this approach as a functional assay to correlate our behavioural findings. As expected, we found that the inhibition of CDK5 decreases the DOP receptor-mediated phosphorylation of ERK1/2 while it increased the effect of DAMGO. These results support our behavioural observations and reveal that CDK5 plays opposite roles in the regulation of DOP and MOP receptor functions.
Although the current study was not specifically designed to investigate the localization of MOP and DOP receptors, our observations strongly support the existence of an interaction between MOP and DOP receptors. Admittedly, we cannot entirely rule out the possibility that DOP and MOP receptor are located in different neurons of a network and that decreasing DOP receptor function is sufficient to modify the input of this neuron on a MOP receptor-positive neuron to regulate its functions. Although this assumption can easily be made for spinal cord neurons, such a network is difficult to reconcile with the pseudo-unipolar features of DRG neurons. Indeed, we showed that CDK5 inhibition had opposite effects on DOP and MOP receptor-mediated ERK1/2 activation in DRGs. One could still argue that CDK5 may be acting directly on the MOP receptor. However, the MOP receptor does not contain a consensus CDK phosphorylation motif in any of its intracellular loops (i.e. S/T-PX-K/H/R (Beaudette et al., 1993; Songyang et al., 1996). Moreover, it is worth noting that the effect of roscovitine on MOP receptors has been mimicked by the DOPri2 peptide in vivo. As the action of this mimicking peptide on MOP receptor could only be indirect, that is by decreasing the CDK5-mediated phosphorylation of DOP receptors, our results strongly suggest that the effects principally occur in spinal and DRG neurons co-expressing DOP and MOP receptors, where they may interact to regulate each other's functions. Although the existence of MOP and DOP receptors in the same neurons has been challenged by others (Scherrer et al., 2009), we have shown that these receptors both inhibit substance P release from primary afferents and play similar roles in alleviating various types of pain (Beaudry et al., 2011; Normandin et al., 2013). Others have also demonstrated that MOP and DOP receptors are co-expressed in DRG neurons (Minami et al., 1995; Gupta et al., 2010; Wang et al., 2010). Indeed, using engineered mice expressing the fluorescent version of both MOP and DOP receptors (double knock-in mice), Erbs and collaborators found that approximately 30% of DRG neurons co-express these receptors (Erbs et al., 2014). Therefore, our results provide additional evidence for MOP and DOP receptor coexpression in neurons.
In conclusion, the current study reveals that the up-regulation of DOP receptor functions either by inflammation or morphine, in vivo, shares a common mechanism involving CDK5. We also demonstrated that the regulation of DOP receptors by CDK5 negatively regulates the functions of MOP receptors. Although the exact mechanisms involved in the activation of CDK5 under the experimental conditions used in this study remain unknown, our results improve our understanding of the regulation of opioid receptors. A better understanding of such mechanisms is mandatory to improving the therapeutic profile of opioids, most importantly those acting on DOP receptors.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research grant to C. L., J.-L. P. and L. G. (MOP123399), and by internal funds from the Faculty of Medicine and Health Sciences of the Université de Sherbrooke and the Centre de Recherche du CHUS to L. G. who is also the recipient of a FRQ-S Junior 2 salary support. H. B. was the recipient of the Alexander Graham Bell Canada Graduate Scholarships Program awarded by the Natural Sciences and Engineering Research Council (NSERC) of Canada and of a doctoral scholarship from the FRQ-S. A.-A. M.-B. was the recipient of a summer training fellowship from the NSERC.
Glossary
Abbreviations
- AUC
area under the curve
- CDK
cyclin-dependent kinase
- CFA
complete Freund's adjuvant
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- Dlt II
deltorphin II
- DOP receptor
δ opioid receptor
- DRG
dorsal root ganglia
- MOP receptor
μ opioid receptor
- MPE
maximum possible effect
List of author contribution
H. B., C. L., J.-L. P. W. N. and L. G. participated in research design. H. B., A.-A. M.-B., C. D. and W. N. conducted the experiments. H. B., W. N. and L. G. wrote the paper.
Conflicts of interest
The authors declare no conflict of interest.
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013d;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif A. Extraneuronal activities and regulatory mechanisms of the atypical cyclin-dependent kinase Cdk5. Biochem Pharmacol. 2012;84:985–993. doi: 10.1016/j.bcp.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Beaudette KN, Lew J, Wang JH. Substrate specificity characterization of a cdc2-like protein kinase purified from bovine brain. J Biol Chem. 1993;268:20825–20830. [PubMed] [Google Scholar]
- Beaudry H, Proteau-Gagne A, Li S, Dory Y, Chavkin C, Gendron L. Differential noxious and motor tolerance of chronic delta opioid receptor agonists in rodents. Neuroscience. 2009;161:381–391. doi: 10.1016/j.neuroscience.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci. 2011;31:13068–13077. doi: 10.1523/JNEUROSCI.1817-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. Studies of tolerance and dependence with the delta-opioid agonist SNC80 in rhesus monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther. 2001;299:629–637. [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Xie WY, Hu F, Zhang Y, Wang J, Wang Y. Disruption of delta-opioid receptor phosphorylation at threonine 161 attenuates morphine tolerance in rats with CFA-induced inflammatory hypersensitivity. Neurosci Bull. 2012;28:182–192. doi: 10.1007/s12264-012-1216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. Challenges for opioid receptor nomenclature: IUPHAR review 9. Br J Pharmacol. 2015;172:317–323. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondio G, Ronzoni S, Eggleston DS, Artico M, Petrillo P, Petrone G, et al. Discovery of a novel class of substituted pyrrolooctahydroisoquinolines as potent and selective delta opioid agonists, based on an extension of the message-address concept. J Med Chem. 1997;40:3192–3198. doi: 10.1021/jm9608218. [DOI] [PubMed] [Google Scholar]
- Duhr F, Deleris P, Raynaud F, Seveno M, Morisset-Lopez S, Mannoury la Cour C, et al. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol. 2014;10:590–597. doi: 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Schulz R. Mechanism and consequences of delta-opioid receptor internalization. Crit Rev Neurobiol. 2005;17:1–26. doi: 10.1615/critrevneurobiol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2014;222:677–702. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, et al. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O'Donnell D, Vincent JP, et al. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Mittal N, Beaudry H, Walwyn W. Recent advances on the δ opioid receptor: from trafficking to function. Br J Pharmacol. 2015;172:403–419. doi: 10.1111/bph.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgoussi Z, Georganta EM, Milligan G. The other side of opioid receptor signalling: regulation by protein-protein interaction. Curr Drug Targets. 2012;13:80–102. doi: 10.2174/138945012798868470. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudzik TJ, Pietras MR, Caccese R, Bui KH, Yocca F, Paronis CA, et al. Effects of the delta opioid agonist AZD2327 upon operant behaviors and assessment of its potential for abuse. Pharmacol Biochem Behav. 2014;124:48–57. doi: 10.1016/j.pbb.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Jacobshagen M, Niquille M, Chaumont-Dubel S, Marin P, Dayer A. The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development. 2014;141:3370–3377. doi: 10.1242/dev.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Park YU, Kim DK, Lee S, Kwak Y, Lee SA, et al. Cdk5 phosphorylates dopamine D2 receptor and attenuates downstream signaling. PLoS ONE. 2013;8:e84482. doi: 10.1371/journal.pone.0084482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Lee MH, Nikolic M, Baptista CA, Lai E, Tsai LH, Massague J. The brain-specific activator p35 allows Cdk5 to escape inhibition by p27Kip1 in neurons. Proc Natl Acad Sci U S A. 1996;93:3259–3263. doi: 10.1073/pnas.93.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CN, Dashwood MR, Whitehead CJ, Mathias CJ. Differential cardiovascular and respiratory responses to central administration of selective opioid agonists in conscious rabbits: correlation with receptor distribution. Br J Pharmacol. 1989;98:903–913. doi: 10.1111/j.1476-5381.1989.tb14620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClue SJ, Blake D, Clarke R, Cowan A, Cummings L, Fischer PM, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine) Int J Cancer. 2002;102:463–468. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuay H. Opioids in pain management. Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of mu-, delta- and kappa-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Brain Res Mol Brain Res. 1995;30:203–210. doi: 10.1016/0169-328x(94)00290-u. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Nornes HO, Neuman T. Cyclin E is expressed in neurons and forms complexes with cdk5. Neuroreport. 1995;6:1130–1132. doi: 10.1097/00001756-199505300-00014. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, et al. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, et al. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109:266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Negis Y, Unal AY, Korulu S, Karabay A. Cell cycle markers have different expression and localization patterns in neuron-like PC12 cells and primary hippocampal neurons. Neurosci Lett. 2011;496:135–140. doi: 10.1016/j.neulet.2011.03.100. [DOI] [PubMed] [Google Scholar]
- Normandin A, Luccarini P, Molat JL, Gendron L, Dallel R. Spinal mu and delta opioids inhibit both thermal and mechanical pain in rats. J Neurosci. 2013;33:11703–11714. doi: 10.1523/JNEUROSCI.1631-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, et al. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci U S A. 2006;103:791–796. doi: 10.1073/pnas.0510405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo P, Angelici O, Bingham S, Ficalora G, Garnier M, Zaratin PF, et al. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J Pharmacol Exp Ther. 2003;307:1079–1089. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther. 1984;230:341–348. [PubMed] [Google Scholar]
- Ramos-Miguel A, Garcia-Sevilla JA. Crosstalk between cdk5 and MEK-ERK signalling upon opioid receptor stimulation leads to upregulation of activator p25 and MEK1 inhibition in rat brain. Neuroscience. 2012;215:17–30. doi: 10.1016/j.neuroscience.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Tsai LH. Cdk5 behind the wheel: a role in trafficking and transport? Trends Cell Biol. 2002;12:28–36. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH, Soong Y, Wu D, Olariu N, Kett A, Kim H, et al. Respiratory depression after intravenous administration of delta-selective opioid peptide analogs. Peptides. 1999;20:101–105. doi: 10.1016/s0196-9781(98)00141-7. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Cao J, Peng C, Yang H, Cui Z, Zhao J, et al. Temporal and spatial expression of cyclin H in rat spinal cord injury. Neuromolecular Med. 2011;13:187–196. doi: 10.1007/s12017-011-8150-1. [DOI] [PubMed] [Google Scholar]
- Xie WY, He Y, Yang YR, Li YF, Kang K, Xing BM, et al. Disruption of Cdk5-associated phosphorylation of residue threonine-161 of the delta-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing BM, Yang YR, Du JX, Chen HJ, Qi C, Huang ZH, et al. Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J Neurosci. 2012;32:14709–14721. doi: 10.1523/JNEUROSCI.1634-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YR, He Y, Zhang Y, Li Y, Han Y, Zhu H, et al. Activation of cyclin-dependent kinase 5 (Cdk5) in primary sensory and dorsal horn neurons by peripheral inflammation contributes to heat hyperalgesia. Pain. 2007;127:109–120. doi: 10.1016/j.pain.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Zhang XQ, Wang WY, Xue QS, Lu H, Huang JL, et al. Increased synaptophysin is involved in inflammation-induced heat hyperalgesia mediated by cyclin-dependent kinase 5 in rats. PLoS ONE. 2012;7:e46666. doi: 10.1371/journal.pone.0046666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28:415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


