Abstract
Objective
This study aims to understand whether the enhanced dPMI, seen in writer’s cramp patients previously, extends to other populations of focal dystonia patients (e.g. cervical dystonia) as an endophenotypic marker.
Methods
We studied 9 healthy subjects and 9 patients with CD. dPMI was tested by applying conditioning transcranial magnetic stimulation to the left dorsal premotor cortex and then a test pulse to the ipsilateral motor cortex at an interval of 6 ms. We also looked at the duration of the cortical silent period (CSP)—a measure of cortical excitability.
Results
CD patients had enhanced dPMI at rest (mean 57.0%, SD 16.2) in contrast to healthy volunteers (mean 124.1%, SD 35.7) (p<0.001). CSP latencies (in ms) in CD patients (mean 108.0, SD 33.1) were significantly shorter than in healthy volunteers (mean 159.1, SD 55.2) (p<0.05).
Conclusions
CD patients showed enhanced dPMI in a hand muscle—distant from their affected body part—similar to writer’s cramp patients. This enhanced inhibition was independent of disease severity and neck posture. This suggests that enhanced dPMI may be an endophenotypic marker of dystonia.
Significance
The abnormal dorsal premotor-motor connection in cervical dystonia is a potential novel and important avenue for therapeutic targeting.
Keywords: TMS, dystonia, motor cortex
INTRODUCTION
Dystonia is a neurological disorder characterized by abnormal posturing due to sustained muscle contractions, which interferes with the performance of motor tasks (Breakefield et al., 2008). Cervical dystonia (CD) emerges during middle to late adulthood and is characterized by dystonic turning and tilting of the neck (Brashear, 2009). This abnormal posture not only often leads to pain but also often causes significant disability in activities of daily living such as driving and reading (Brashear, 2009). In addition, CD can affect a patient’s ability to work. In a recent retrospective cross-sectional analysis, more than half (55%) of working age patients with CD were not employed secondary to their dystonia (Martikainen et al., 2010).
The neurophysiology of focal dystonia is characterized by loss of inhibition (Breakefield et al., 2008). This decreased inhibition is reflected in multiple levels from abnormal patterns of muscle activity, loss of spinal and brainstem reflexes and impaired inhibition at the motor cortical level (Cohen and Hallett, 1988; Chen et al., 1995; Kanovsky et al., 2003). In contrast to this literature, we have shown in previous studies that patients with writer’s cramp or focal hand dystonia consistently show enhanced dorsal premotor-motor cortical inhibition (dPMI) (Beck et al., 2009; Pirio Richardson et al., 2014). This enhanced inhibition is hypothesized to be due to compensatory networks that play a role in reducing unwanted motor output. This is supported by studies using low-frequency repetitive transcranial magnetic stimulation (rTMS) over the premotor cortex that have modulated symptoms in FHD and in secondary dystonia with significant improvement in painful spasms (Allam et al., 2007; Lefaucheur et al., 2004; Murase et al., 2005).
This study aims to understand whether this enhanced dPMI seen in writer’s cramp patients extends to other populations of focal dystonia patients (e.g. cervical dystonia) as an endophenotypic marker or whether it is an abnormality solely seen in the setting of task-specific dystonia. Further, if this enhanced dPMI is a reflection of compensatory brain mechanisms to reduce abnormal movements, it is important to evaluate the presence or absence of dPMI in areas that have no abnormal muscle contractions (e.g. the hand muscles in the setting of cervical dystonia).
METHODS
Participants
We studied 9 CD patients (4 men) and 9 age-matched healthy volunteers (2 men) with mean ages 54 years (SD 15) and 53 years (SD 6.2), respectively. The CD patients were studied at least 3 months after the last botulinum toxin treatment and off any medications that could affect cortical excitability (e.g. benzodiazepines). All CD patients completed the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) at the time of the experimental visit. The CD patients had a mean TWSTRS total score of 32.0 (SD 13.3). All subjects met all inclusion criteria and signed an informed consent. The study was approved by the University of New Mexico Health Sciences Center Institutional Review Board.
Study Design
dPMI
Surface gold electromyography (EMG) electrodes were placed on the bilateral sternocleidomastoid (SCM) muscles and the right first dorsal interosseous muscle (FDI) in bipolar montages. The EMG signal was amplified using a conventional EMG machine (Nicolet Viking) with bandpass between 10 and 2000 Hz. The signal was digitized at a frequency of 5 kHz and fed into a computer for off-line analysis. The resting motor threshold (RMT) was determined over the primary motor cortex corresponding to the right (dominant) FDI. The coil over the dorsal premotor cortex (dPM) was placed 2 cm anterior and 1 cm medial to the “hotspot” for FDI, ipsilaterally. With both coils placed, RMT and active motor threshold (AMT) during a 10% maximum voluntary contraction of FDI as measured by force transducer, were determined to the nearest 1% of stimulator output.
The Magstim 2002 (Magstim Co., Whitland, Dyfed, UK), connected to two custom figure 8 coils with an inner loop diameter of 35 mm, was used. The motor coil corresponding to the FDI “hotspot” was placed tangential to the scalp with handle pointing backward and laterally at a 45-degree angle away from the midline. The dPM coil was oriented to produce current in an anterior-to-posterior direction (see Beck et al., 2009). The coils overlap slightly with the dPM coil contacting the scalp and the M1 coil elevated. At least 24 motor evoked potentials (MEPs) (12 test pulses and 12 conditioning + test pulses delivered randomly) were collected from the right FDI at rest. The interstimulus interval (ISI) between the conditioning dPM TMS pulse and the test motor TMS pulse was 6 ms (Pirio Richardson et al., 2014). The intensity of the conditioning pulse was at 90% of active motor threshold and the test intensity was 120% of resting motor threshold (Pirio Richardson et al., 2014).
We then identified the hotspot for SCM over the primary motor cortex. Based on the location of SCM hotspot in Thompson et al., 1997, we placed the same custom figure 8 coil as above over the vertex (Cz). The subjects were fitted and measured for caps with premarked locations for typical electroencephalography recordings for reference. We set the Bistim2 output to 100% of stimulator output. We moved the coil in 1 cm increments along the trajectory from Cz toward C3. In real time, we evaluated for the presence or absence of MEPs elicited in the contralateral SCM. If no MEP was elicited along this line, we moved 1 cm anterior and repeated the mapping and 1 cm posterior to the vertex and repeated the mapping. Once the hotspot for SCM was located, we determined the RMT as described above and attempted to repeat the dPMI protocol as described for the hotspot for FDI.
Statistics
The results of the experimental tests were entered into a computerized database. Outcome measures (i.e., peak-to-peak amplitude change) were compared using a two-sample t-test in R version 3.0.3 (R Core Team, 2014). P values less than 0.05 were considered significant. To test the hypothesis that there is enhanced dPMI at rest in CD patients, normalized MEPS (conditioning plus test/test alone) recorded from FDI were compared between the CD group and the healthy volunteers. Although the FDI muscle was tested at rest, patients with CD had variable activation and posturing in the neck during the experiment. To investigate the role, if any, of neck muscle activation on dPMI, we adjusted for the degree of SCM activation in an ANCOVA. We also adjusted for any effect of disease severity using the total TWSTRS score in a separate ANCOVA.
Cortical Silent Period (CSP)
Using the same paired pulse (conditioning pulse over the dPM and test pulse over the hotspot for FDI) paradigm as described above, at least 24 MEPs (12 test pulses and 12 conditioning + test pulses delivered randomly) were collected from the right FDI at 10% maximum voluntary contraction. Off-line, the cortical silent period (CSP) latencies were determined from the onset of the TMS test pulse artifact through the MEP to the resumption of EMG activity.
Statistics
The results of the experimental tests were entered into a computerized database. Repeated measures for test and premotor-conditioned plus test CSP latencies were modeled using a linear mixed model (SAS PROC MIXED) in SAS version 9.3 (Cary, NC, USA). P values less than 0.05 will be considered as significant.
RESULTS
dPMI
The peak-to-peak MEP amplitude from FDI at rest was compared in CD patients and healthy volunteers and presented in Table 1. The results are presented as the ratio of dPM conditioned MEP amplitude to unconditioned (test) MEP amplitude (a ratio less than 1 indicates inhibition from the conditioning stimuli). CD patients had enhanced dPMI at rest (mean 57.0%, SD 16.2) in contrast to healthy volunteers (mean 124.1%, SD 35.7) (p<0.001) (See Figure 1). To ensure that the abnormal neck posture was not accounting for the differences seen between patients and healthy volunteers, we adjusted for the degree of SCM activity present during the experiment in patients and volunteers using an ANCOVA. Adjusting for the root mean square (RMS) of EMG activity in the most active SCM showed that SCM EMG activity was not a significant predictor with the group difference in dPMI persisting significantly at p<0.001. Adjusting for total TWSTRS score showed that disease severity as measured by TWSTRS was not a significant predictor with the group difference still significant at p<0.05. In addition, we looked at the effect of TMS stimulator intensity on the group difference between CD patients and healthy volunteers. Adjusting separately for RMT and AMT, motor thresholds in both cases were not significant predictors in the ANCOVA and the group difference was still significant at p<0.001.
Table 1.
Means and standard deviation (in parentheses) shown for MEP peak-to-peak amplitude with test and test plus conditioning stimuli (mV), dPMI (as a percentage of conditioned+test MEP/ test MEP alone), RMT (percent stimulator output) and AMT (percent stimulator output) for CD patients and healthy volunteers.
| MEP | dPMI * | RMT | AMT ** | ||
|---|---|---|---|---|---|
| Test | Conditioning + Test | ||||
|
|
|||||
|
Healthy
Volunteers |
0.741 (0.068) | 0.823 (0.056) | 124.1 (35.7) | 77.2 (9.1) | 71.3 (11.6) |
| CD Patients | 0.927 (0.042) | 0.536 (0.036) | 57.0 (16.2) | 76.7 (10.8) | 64.2 (9.6) |
p<0.001,
p<0.0508
Figure 1.
Boxplots of group and individual data showing enhanced dPMI percentage in CD patient group as measured in the hand. Values less than 100% indicate an inhibitory conditioning effect of the dorsal premotor cortex on the motor cortex.
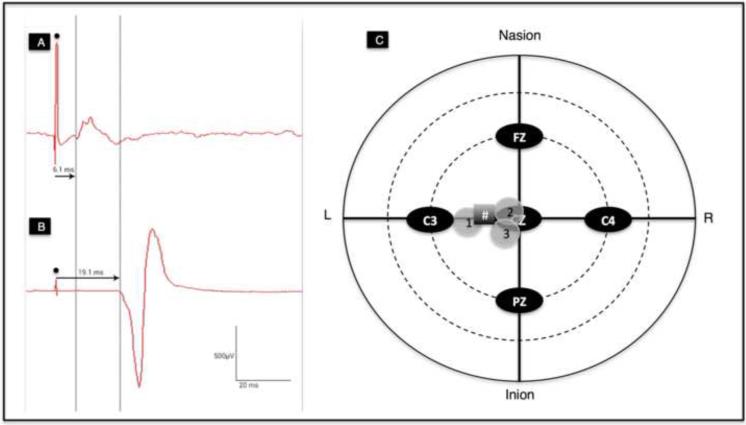
We were able to record MEPs over the SCM successfully in 3 subjects using the smaller custom coils. We were able to verify the localization of SCM representation as in Thompson et al., 1997 (See Figure 2). At even maximal stimulator output with the small custom coils, the evoked MEPs were of small amplitude <300 □V so that we did not proceed with dPMI given the concern of ceiling affects.
Figure 2.
A) Tracing of MEP over the right SCM at a latency of 6.1 ms (• artifact from test stimulus); B) Tracing of MEP over the right FDI at latency of 19.1 ms recorded simultaneously when stimulation applied over SCM hotspot (• artifact from test stimulus); C) Plot of SCM hotspots identified from healthy subjects 1, 2 and 3, respectively. # symbol denotes the approximate location of SCM hotspot identified in Thompson et al., 1997.
Cortical Silent Period (CSP)
CSP latencies (in ms) in CD patients (mean 108.0, SD 33.1) were significantly shorter than in healthy volunteers (mean 159.1, SD 55.2) (p<0.05) (See Figure 3). Interestingly, the premotor conditioning stimulation decreased latencies in both groups by almost 10 milliseconds (CD patients: mean 99.4 SD 34.0, healthy volunteers: mean 150.4, SD 61.4) (p<0.05) (Figure 4).
Figure 3.
Boxplots of group data showing significantly shorter cortical silent period in the CD patient group compared to healthy volunteers. Please see Figure 4 for the individual subject results represented by individual data points.
Figure 4.
CSP individual and mean group data shown in a spaghetti plot. The shorter CSP in the patient group is again seen as well as the decreased latency in both the patient and healthy volunteer group with the premotor conditioning stimulation (p<0.05).
DISCUSSION
CD patients showed enhanced dPMI in a hand muscle—distant from their affected body part—similar to writer’s cramp patients (Pirio Richardson et al., 2014; Beck et al., 2009). This enhanced inhibition was independent of disease severity as measured by TWSTRS total score and independent of neck posture during the experiment. This is suggestive that enhanced dPMI may be an endophenotypic marker of dystonia as it is now seen in two focal dystonia populations—both in affected and unaffected body parts. This however, is in contrast to much of the known physiology of focal dystonia that suggests a loss of inhibition (Quartarone and Hallett, 2013). Indeed, we showed shorter CSP latencies in our CD patients compared to healthy volunteers suggesting that our CD patients do have an overall reduction in inhibitory tone and are behaving in a physiologic manner consistent with the literature—yet they still show enhanced inhibition in a particular network (Samargia et al., 2014).
Although our main outcome measure was to evaluate the conditioning effect of dPM in a patient population, it is worth commenting on the results seen in healthy volunteers. In our study, healthy volunteers showed slight facilitation from dPM to motor cortex (mean 124.1%, SD 35.7). This finding is in contrast to the results of Civardi et al. (2001), where they demonstrated a short latency inhibitory effect from a frontal site—suggested to be premotor cortex—on motor output. We derived our interstimulus interval (6 ms) and our conditioning intensity (0.9AMT) from the peak inhibitory effect seen in healthy people in their study (Beck et al., 2009 and Pirio Richardson et al., 2014). However, we found that when using neuronavigation-aided delivery of TMS, the scalp landmarks used in the Civardi et al., 2001 paper were more anterior than where we identified the premotor cortex on MRI (Pirio Richardson et al., 2014). As a result, we cannot directly compare our results to those of Civardi in the healthy population, but have replicated the finding that with the technique used in this study, healthy volunteers show slight facilitation in response to the dPM conditioning stimulation (Beck et al., 2009 and Pirio Richardson et al., 2014). Given that we applied the conditioning stimuli at a specific interstimulus interval (6 ms) rather than a range of intervals, it is possible that we may have missed subtle changes in premotor inhibitory and excitatory influences on the motor cortex in both healthy and dystonic subjects. Further examination of the dorsal premotor to motor cortex using our methodology by varying the interstimulus intervals might reveal further differences (or similarities) in physiology between patients and healthy volunteers. This need for further study limits our ability to extend our conclusion beyond the enhanced inhibition seen at this specific interval.
Previously we have suggested that the enhanced dPMI is a reflection of compensatory mechanisms—supported by rTMS trials aimed at enhancing inhibition over the premotor cortex demonstrating symptom improvement (Allam et al., 2007; Lefaucheur et al., 2004; Murase et al., 2005). Although we were unable to directly measure dPMI over the SCM as described above, we have indirect evidence that dPMI is compensatory in nature. The degree of dPMI enhancement in CD patients over the hand (an unaffected body part) showed greater enhancement (57%) compared to writer’s cramp patients (88.5%) measured in the same muscle but in this case—their affected body part (Pirio Richardson et al., 2014). The data from the writer’s cramp patients was obtained in a separate study using the same methodology as described above--yet we cannot exclude the possibility that the differences in dPMI enhancement were due to different experimental conditions rather than due to underlying differential compensation of affected and unaffected body parts. However, both populations show enhanced inhibition suggestive of a general compensatory response—although this conclusion is clearly speculative and needs direct experimental confirmation.
Our current study supports the growing evidence that the left dPM and its connectivity to M1 is abnormal in patients with focal dystonia—although the exact nature of the connectivity change differs in the literature depending on the neurophysiologic or neuroimaging technique employed. In a study using premotor theta burst stimulation (cTBS) to assess changes in dPM and M1 connectivity in writer’s cramp patients, they showed that after 5 days of cTBS, the expected suppression in MEP amplitude is achieved but not present after the first day of treatment (Huang et al., 2012). They conclude that this is consistent with a reduced dPM-M1 interaction (Huang et al., 2012). While difficult to compare directly to our results given the different methodology to measure dPM-M1 connections, we certainly see in both their data and ours that dystonia patients are moving in a direction opposite to what is expected in the healthy state—and that dPM is a key common area of abnormality.
Further, this impaired dPM-M1 connectivity is seen in imaging studies as well such as in writer’s cramp (Castrop et al., 2012). And, more recently in CD patients where the investigators also looked specifically at premotor connectivity using resting state fMRI and showed reduced connectivity between premotor and primary motor networks (amongst other regions) (Delnooz et al., 2013). This abnormality partially normalizes with botulinum toxin administration—yielding further support for the importance of restoring normal dPM-M1 connectivity, no matter how it is measured.
Our finding of shortened CSP latencies in CD patients is suggestive of overall reduced inhibition in the motor system compared to healthy volunteers as suggested above and is consistent with previous studies (Filipovic et al., 1997). The result showing CSP latencies further shortening with dPM conditioning stimulation in both patients and healthy volunteers is a novel finding. In contrast to our dPMI data showing enhanced inhibition with dPM conditioning stimulation, patients and healthy volunteers both show shortened CSP latencies indicating a potentially facilitatory effect due to dPM conditioning stimulation. The equal response in both healthy volunteers and dystonia patients may reflect the underlying anatomic connections between dPM-M1 rather than the nature of dysfunctional connectivity in the dystonic disease state as assessed by dPMI. There is evidence from non-human primate studies that direct conditioning stimulation of the premotor cortex had a facilitatory influence on evoking motor responses in M1 (Maier et al., 2013). In human TMS studies, a strong correlation between MEP latency and functional connectivity between M1 and premotor areas was found in healthy volunteers (Volz et al., 2014). There are also direct connections from premotor cortex to the spinal cord, which may play a role in conditioning motor response; however, these connections are sparse (Zinger et al., 2013) and in healthy volunteers, we saw no change in spinal excitability (measured by H-reflexes) with dPM conditioning (Pirio Richardson et al., 2014). Finally, the neurophysiologic processes that generate the MEP and the CSP likely differ and the neurofunctional rather than structural nature of dystonia might explain differential effects in CSP vs. MEP/dPMI results (Kallioniemi et al., 2014).
One of the challenges in understanding the causal relationship between physiologic abnormalities seen in focal dystonia is that although the disease manifests in a focal manner, there are often widespread brain abnormalities detected such as abnormal sensorimotor plasticity and altered tactile discrimination thresholds (Quartarone et al., 2008; Kimmich et al., 2014). Physiologic abnormalities have even been found in subjects merely at risk for disease such as nonmanifesting DYT1 carriers (Edwards et al., 2006). In this study, we have shown that enhanced dPMI was found at a site distant to the affected body part—again supporting a widespread brain abnormality present in focal dystonia patients. An interpretation of our current findings would be that enhanced dPMI is more consistent with an endophenotypic change widespread in the brain and more in line with previously demonstrated reduced reciprocal inhibition and abnormal brain plasticity in focal dystonia patients and subjects at risk for disease (Chen et al., 1995; Edwards et al., 2006). Even with this explanation, the dPM would still be a potential target for therapeutic intervention.
It is clear that patients with CD often show evidence of physiologic compensation through the sensory trick—which temporarily restores normal posture. Although the underlying physiology behind this phenomenon is not known, a recent study has shown that during performance of a sensory trick, there was reduction in motor cortex excitability—e.g. enhanced inhibition (Amadio et al., 2014). This data combined with our findings suggest that exploiting underlying compensatory networks is a novel and important avenue for therapeutic targeting.
Highlights.
In cervical dystonia, where the chief neurophysiologic abnormality is typically excitatory, we show enhanced inhibition in a dorsal premotor to motor cortical network.
Further, this enhanced inhibition was seen in a muscle not involved in the dystonic posture.
This inhibition may represent a compensatory network that could be exploited for therapeutic purposes.
Acknowledgement
I would like to thank Meg Radigan for her efforts as coordinator for this study and the patients who kindly volunteered to participate. I would like to thank Ronald Schrader, PhD for his statistical expertise and Colleen Frangos for her help with the figures.
Funding
The project was supported (in part or in full) by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number KL2 TR000089 and Grant Number UL1 TR000041 through a Pilot Project Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Pirio Richardson reports no disclosures.
REFERENCES
- Allam N, Brasil-Neto JP, Brandao P, Weiler F, Barros Filho J, Tomaz C. Relief of primary cervical dystonia symptoms by low frequency transcranial magnetic stimulation of the premotor cortex: case report. Arq Neuropsiquiatr. 2007;65:697–699. doi: 10.1590/s0004-282x2007000400030. [DOI] [PubMed] [Google Scholar]
- Amadio S, Houdayer E, Bianchi F, Tesfaghebriel Tekle H, Urban IP, Butera C, Guerriero R, Cursi M, Leocani L, Comi G, Del Carro U. Sensory tricks and brain excitability in cervical dystonia: a transcranial magnetic study. Mov Disord. 2014 doi: 10.1002/mds.25888. doi: 10.1002/mds.25888. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Beck S, Houdayer EP, Pirio Richardson S, Hallett M. The role of inhibition from the left dorsal premotor cortex in right-sided focal hand dystonia. Brain Stimul. 2009;2:208–14. doi: 10.1016/j.brs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear A. Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics. 2009;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Castrop F, Dresel C, Hennenlotter A, Zimmer C, Haslinger B. Basal ganglia-premotor dysfunction during movement imagination in writer’s cramp. Mov Disord. 2012;27:1432–1439. doi: 10.1002/mds.24944. [DOI] [PubMed] [Google Scholar]
- Chen RS, Tsai CH, Lu CS. Reciprocal inhibition in writer’s cramp. Mov Disord. 1995;10:556–561. doi: 10.1002/mds.870100505. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Hallett M. Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology. 1988;88:1005–1012. doi: 10.1212/wnl.38.7.1005. [DOI] [PubMed] [Google Scholar]
- Delnooz CCS, Pasman JW, Beckmann CF, van de Warrenburg BPC. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One. 2013;8:e62877. doi: 10.1371/journal.pone.0062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–2186. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Ljubisavljevic M, Svetel M, Milanovic S, Kacar A, Kostic VS. Impairment of cortical inhibition in writer’s cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett. 1997;222:167–70. doi: 10.1016/s0304-3940(97)13370-5. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Lu CS, Rothwell JC, Lo CC, Chuang WL, Weng YH, Lai SC, Chen RS. Modulation of the disturbed motor network in dystonia by multisession suppression of premotor cortex. PLoS One. 2012;7:e47574. doi: 10.1371/journal.pone.0047574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi E, Saisanen L, Kononen M, Zwiszus F, Julkunen P. On the estimation of silent period thresholds in transcranial magnetic stimulation. Clin Neurophysiol. 2014 Mar 22; doi: 10.1016/j.clinph.2014.03.012. Pii:S1388-2457(14)00162-X. doi: 10.1016/j.clinph.2014.03.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kanovský P, Bares M, Streitová H, Klajblová H, Daniel P, Rektor I. Abnormalities of cortical excitability and cortical inhibition in cervical dystonia. Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J Neurol. 2003;250:42–50. doi: 10.1007/s00415-003-0942-2. [DOI] [PubMed] [Google Scholar]
- Kimmich O, Molloy A, Whelan R, Williams L, Bradley D, Balsters J, Molloy F, Lynch T, Healy DG, Walsh C, O’Riordan S, Reilly RB, Hutchinson M. Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates. Mov Disord. 2014;29:804–811. doi: 10.1002/mds.25822. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Fenelon G, Menard-Lefaucheur I, Wedling S, Nguyen JP. Low-frequency repetitive TMS of premotor cortex can reduce painful axial spasms in generalized secondary dystonia: a pilot study of three patients. Neurophysiol Clin. 2004;34:141–145. doi: 10.1016/j.neucli.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Maier MA, Kirkwood PA, Brochier T, Lemon RN. Responses of single corticospinal neurons to intracortical stimulation of primary motor and premotor cortex in the anesthetized macaque monkey. J Neurophysiol. 2013;109:2982–2998. doi: 10.1152/jn.01080.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen KK, Luukkaala TH, Marttila RJ. Working capacity and cervical dystonia. Parkinsonism Relat Disord. 2010;16:215–217. doi: 10.1016/j.parkreldis.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, Igasaki R, Sakata-Igasaki M, Mima T, Ikeda A, Shibasaki H. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain. 2005;128:104–115. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- Pirio Richardson S, Beck S, Bliem B, Hallett M. Abnormal dorsal premotor-motor inhibition in writer’s cramp. Mov Disord. 2014;29:797–803. doi: 10.1002/mds.25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant’angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013;28:958–967. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014 URL http://www.R-project.org/ [Google Scholar]
- Samargia S, Schmidt R, Kimberley TJ. Shortened cortical silent period in adductor spasmodic dysphonia: evidence for widespread cortical excitability. Neurosci Lett. 2014;560:12–5. doi: 10.1016/j.neulet.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ML, Thickbroom GW, Mastaglia FL. Corticomotor representation of the sternocleidomastoid muscle. Brain. 1997;120:245–255. doi: 10.1093/brain/120.2.245. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Hamada M, Rothwell JC, Grefkes C. What makes the muscle twitch: motor system connectivity and TMS-induced activity. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu032. doi: 10.1093/cercor/bhu032. [DOI] [PubMed] [Google Scholar]
- Zinger N, Harel R, Gabler S, Israel Z, Prut Y. Functional organization of information flow in the corticospinal pathway. J Neurosci. 2013;33:1190–1197. doi: 10.1523/JNEUROSCI.2403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]