Abstract
Objectives
To study the extent to which otitis media in childhood is associated with adult hearing thresholds. Furthermore, to study if the effects of otitis media on adult hearing thresholds are moderated by age or noise exposure.
Design
Population-based cohort study of 32,786 participants who had their hearing tested by pure-tone audiometry in primary school and again at ages ranging from 20–56 years. 3066 children were diagnosed with hearing loss, the remaining sample had normal childhood hearing.
Results
Compared to participants with normal childhood hearing, those diagnosed with childhood hearing loss caused by otitis media with effusion (n=1255), chronic suppurative otitis media (n=108) or hearing loss after recurrent acute otitis media (n=613) had significantly reduced adult hearing thresholds in the whole frequency range (2 dB/17–20 dB/7–10 dB, respectively). The effects were adjusted for age, sex and noise exposure. Children diagnosed with hearing loss after recurrent acute otitis media had somewhat improved hearing thresholds as adults. The effects of chronic suppurative otitis media and hearing loss after recurrent acute otitis media on adult hearing thresholds were larger in participants tested in middle adulthood (ages 40 to 56 years) than in those tested in young adulthood (ages 20 to 40 years). Eardrum pathology added a marginally increased risk of adult hearing loss (1–3 dB) in children with otitis media with effusion or hearing loss after recurrent acute otitis media. Our study could not reveal significant differences in the effect of self-reported noise exposure on adult hearing thresholds between the groups with otitis media and the group with normal childhood hearing.
Conclusions
This cohort study indicates that chronic suppurative otitis media and recurrent acute otitis media in childhood are associated with adult hearing loss, underlining the importance of optimal treatment in these conditions. It appears that ears with a subsequent hearing loss after otitis media in childhood age at a faster rate than those without, however this should be confirmed by studies with several follow-up tests through adulthood.
Keywords: Hearing loss, noise, prognosis, sequelae, eardrum, acute otitis media, otitis media with effusion, chronic suppurative otitis media
INTRODUCTION
Otitis media (OM) is one of the most common childhood diseases. This complex disease occurs as related clinical subtypes: otitis media with effusion (OME; chronic inflammation of the middle ear with middle ear effusion, without signs and symptoms of acute infection), acute otitis media (AOM; acute inflammation of the middle ear with middle ear effusion, with signs and symptoms of acute infection) and chronic suppurative otitis media (CSOM; chronic infection of the middle ear and mastoid cells in which there is a chronic perforation of the eardrum and otorrhea) (Gates et al. 2002).
The hearing prognosis is normally very good, but damage of the middle or inner ear can cause hearing loss that persists after OM has resolved. Although many studies have shown hearing loss in children with a history of OM (Ahonen & McDermott 1984; Margolis et al. 1993; Hunter et al. 1996; Laitila et al. 1997; Yiengprugsawan et al. 2013), cohort studies with follow-up of hearing into adulthood are scarce (Augustsson & Engstrand 2006; de Beer et al. 2003; Jensen et al. 2013). Thus, whether the hearing loss gradually resolves, persists or even progresses in later life is not well described.
The association between OM and susceptibility to noise- or age-related hearing loss is also unclear. Increased susceptibility to noise is shown among young adults with recurrent childhood ear infections (Job et al. 2000) and the effect of childhood ear infections on adult hearing seems to increase with age (Tambs et al. 2004). Yet, these effects are reported from cross-sectional studies only.
Knowledge about the long-term hearing prognosis is important for hearing management, intervention planning and patient information. Therefore, we aim to clear out to which extent childhood OM is associated with adult hearing loss. Furthermore, we study if the effects of childhood OM on adult hearing thresholds are moderated by age or by self- reported noise exposure.
MATERIALS AND METHODS
Sample
This population-based cohort study investigates 32,786 participants who had their hearing tested by pure-tone audiometry in primary school and again at ages ranging from 20–56 years. We use baseline data from a hearing examination of primary school pupils in the Nord-Trøndelag county, Norway, hereafter called the Hearing Investigation of School Children in Nord-Trøndelag (HISCNT), and follow-up data from the Nord-Trøndelag Hearing Loss Study (NTHLS). Both studies are described in more detail elsewhere (Fabritius 1968; Tambs et al. 2004).
HISCNT was an audiometric screening of all 7, 10 and 13 years old school children in the entire Nord-Trøndelag county from 1954 to 1986, conducted by the Norwegian Ear, Nose and Throat (ENT) - specialist H. F. Fabritius and colleges. The HISCNT data only include information and identity of the children diagnosed with hearing loss, since negative findings were not recorded, but great effort was made to include all children in the screening. In this study we base our analyses on the approximation that all children born in Nord-Trøndelag between 1941 and 1977 took part, altogether 78,524 children. The children with positive audiometric screening were invited to a later ENT specialist examination. From 1954 to 1962, average attendance at the ENT examinations was 97% (Fabritius 1968) and we have no reason to believe that this high level of attendance changed later. Altogether, 10,269 children took part in the ENT examination.
NTHLS took place from 1996 to1998 and was a part of the Nord-Trøndelag Health Study (HUNT 2), a large, general health screening study (Holmen et al. 2003). NTHLS included a pure-tone audiometry and questionnaires, and the total adult population (≥20 years) from 17 of the 23 municipalities in Nord-Trøndelag were invited. Valid audiometric data were collected from 50,723 participants. The participation rate was 67%, except in one municipality where the population was invited to the hearing examination after HUNT2 was finished (participation rate 41%). Among subjects aged 20–56 years 87% of the county population was invited with an overall participation rate of 59%.
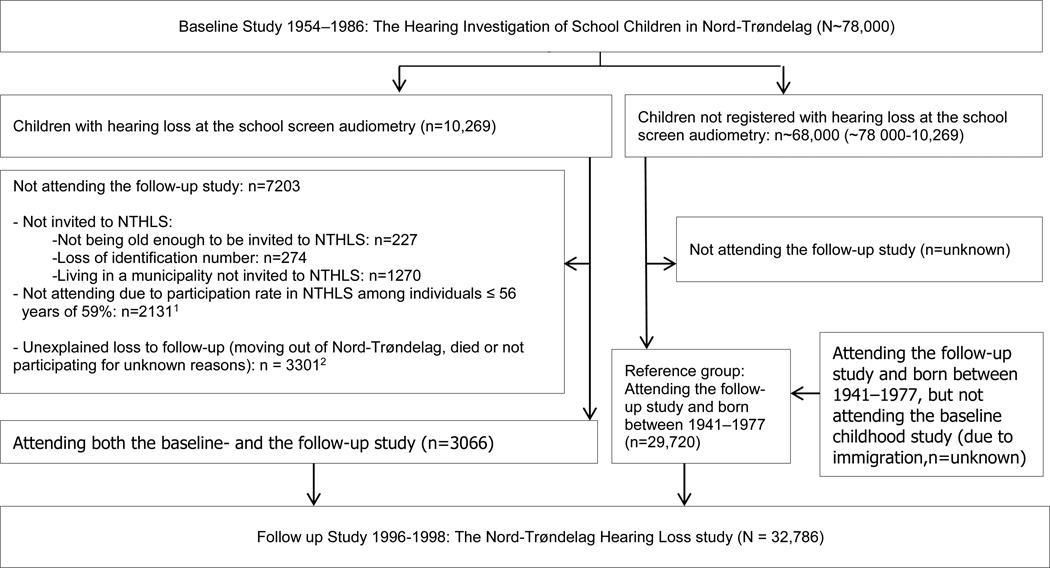
The present study included participants attending NTHLS (living in Nord-Trøndelag as adults) that were born between 1941 and 1977 (being in primary school age during HISCNT) and not registered with hearing loss in HISCNT as a reference group, representing normal childhood hearing (n=29,720). The participants reporting a history of recurrent OM on the questionnaire in NTHLS were excluded from the reference group (n=8213). In the case group (participants diagnosed with hearing loss in childhood), 3066 participants attended the follow-up study (Figure 1).
Figure 1.
1 Invited to NTHLS: 5197 (3066 = 0.59×), invited but not participating: 5197 × 0.41= 2131
2 Unexplained loss to follow-up: 7203 − (2131+227+274 +1270) = 3301
Measures
Childhood hearing loss
In HISCNT, the audiometric screening was performed by a trained hearing assistant or district health nurse in a quiet place at school, obtaining air-conduction thresholds by pure-tone audiometry at 0.25, 0.5, 1, 2, 4 and 8 kHz. Hearing loss was defined as at least 20 dB hearing threshold at 3 or more frequencies or a 30 dB hearing threshold at one or more frequencies. All children with positive audiometric screening were invited to a later ENT specialist examination at one of 14 different out- patient clinics in Nord-Trøndelag and answered a questionnaire concerning ear problems. The ENT examination included family and medical history, complete ENT examination and pure-tone audiometry with air- and bone-conduction thresholds. Depending on the underlying hearing disorder, the children had one or more control examinations. The ENT specialist recorded all the findings, treatment and presumed etiology (diagnosis) on the child`s health card. Some children were diagnosed with more than one type of etiology. In the present study, only the most severe etiology was registered according to the following hierarchy (definitions by Fabritius 1968): SNHL (air-conduction thresholds follow the bone-conduction thresholds) – anomalies of the outer or middle ear – otosclerosis – CSOM (eardrum perforation (minimum duration of perforation not defined) with intermittent secretion and conductive or mixed hearing loss. In this group, 4 participants had been operated with radical cholesteatoma removal, 13 participants with myringoplasty and 8 with a non specified ear operation. In the present study, 9 participants with dry eardrum perforations were included into this group - hearing loss associated with a history of recurrent (r) AOM (no middle ear effusion at the examination but a history of recurrent preschool ear infections, sometimes also occurring during school years, mostly including eardrum pathology and with conductive or mixed hearing loss) – OME (middle ear effusion, reduced mobility of the eardrum tested by Brüning`s magnifiyng glass, without sign or symptoms of acute infection) - AOM (middle ear effusion with signs and symptoms of acute infection)- otitis externa - foreign body - cerumen – other (intellectual disability, conductive hearing loss with unknown etiology, unknown etiology or no registered etiology).
In this paper we investigated the children diagnosed by the ENT specialist with hearing loss together with ongoing OM or associated with a history of OM. Ongoing OM is characterized by special anamnestic and otoscopic findings and mostly causes conductive hearing loss (impaired air-conduction- and normal bone-conduction thresholds). OM may also cause SNHL resulting in mixed hearing loss (impaired air- and bone-conduction thresholds but with an air-bone gap). Eardrum pathology included myringosclerosis, segmental or total atrophy, retraction and adhesion.
Noise exposure
We used questionnaire data from NTHLS for the assessment of noise exposure. Occupational noise exposure was measured by questionnaire items about loud noise at work in general (scored 0–3) and specific noise from the following: staple gun/hammering, metal hammering/riveting, circular saw/machine planing, chainsaw operation, trac-tor/construction machines, sledge hammer operation, blasting, and machine-room and other factory noise (these items were scored using “yes” or “no”). Non-occupational noise exposure was measured by questionnaire items about impulse noise (ie, explosions, shootings); playing in a band or going to discotheques, rock concerts, or similar loud events (these items were scored using “no” = 0, “perhaps or I do not know” = 1, and “yes” = 2). A general index based on all the noise scores was generated in order to estimate the overall effect of noise, similar as in Tambs et al. (2006). The noise index was calculated separately for each frequency range. The scores for each separate item were weighted by its respective regression in initial regression analyses and summed. Non-significant negative values were replaced with zero.
Outcome
NTHLS included a pure-tone audiometry: air conduction (AC) thresholds from 0.25 to 8 kHz were determined in accordance with ISO 8253–1 (1989). We defined 3 outcome variables: adult hearing level, pure tone average (PTA) of (1) 0.25-0.5 kHz, (2) 1–2 kHz and (3) 3–8 kHz. Hearing was evaluated in the ear observed as the worse hearing ear at the last baseline examination, defined by the highest PTA at 0.25–8 kHz. For the most part, hearing thresholds < 20 dB were not registered in the baseline audiograms, meaning that the values for many single frequencies were missing. These missing values were replaced by the mean value of the values < 20 dB registered in the total material (n=10,269), for each frequency, respectively. In cases of bilateral symmetrical baseline hearing, adult hearing threshold was evaluated in one random ear. In the reference group, the adult hearing threshold was evaluated in one random ear.
Statistical Methods
Descriptive statistics at baseline compared participants that did and did not attend the follow-up study using chi-square tests for categorical variables (sex and etiology) and independent T-test for the continuous variable (childhood hearing threshold) with significance level of 0.05.
We performed a multiple regression analysis with significance level of 0.05 (SPSS version 20) to estimate the differences in adult hearing threshold between each OM group and the reference group, adjusting for age, sex and noise exposure. As the regression model showed clear signs of heteroscedasticity (the variance of the residuals varied systematically across the distribution), the standard errors were estimated with bootstrapping (random sampling with replacement). The predictor variables were: (1) diagnostic group entered as a number of dichotomous dummy variables, one for each OM group (CSOM, OME and sequeale AOM) and with normal childhood hearing as the reference category; (2) age (continuously scored 20–56 years); (3) sex, and (4) noise exposure (continuously scored). The analyses were repeated re-classifying all the diagnostic groups into OME with and without eardrum pathology and sequelae AOM with and without eardrum pathology.
Secondly, we tested if the effects of OME, CSOM or sequelae AOM on adult hearing thresholds were moderated by age or noise exposure (6 interaction terms). The outcome variable was defined as adult hearing threshold in the high frequency range (PTA 3–8 kHz), since these are the frequencies most sensitive to hearing loss caused by age and noise exposure. First, each interaction term was tested separately by adding it to the initially described regression model with the main effects of diagnostic group, age, sex and noise exposure. Then, the interaction terms that were significant were tested altogether in the same regression model. The interaction terms that remained significant were kept in the final regression model. If significant interaction effects were found, separate analyses of the effects of OM stratified by the categories of the moderator variables were performed.
A correlation between birth year and childhood hearing threshold might have confounded the results from the interaction analyses, thus we also tested for such a correlation.
RESULTS
Descriptive statistics
A flow-chart is shown in Figure 1. Baseline childhood characteristics of the participants that did and did not attend the follow-up adult study are presented in Table 1. Average age at the last hearing examination in the baseline childhood study was 10 years. There were no statistically significant differences in childhood hearing thresholds (in any frequency range) between participants that did (n=3066) or did not (n=7203) participate in the follow-up adult study. Also, there were no statistically significant differences in the etiologies, except for a slightly smaller prevalence of acute otitis media and a slightly smaller prevalence of unknown etiology among participants than among non-participants in the follow up adult study. There were slightly more women (49%) among participants than among non-participants (45%).
Table 1.
Diagnoses by sex and hearing thresholds in the worse ear (WE) for low, mid, and high frequencies (from last visit) among the cohort of Nord Trøndelag school children screened for hearing loss who did and did not participate in the follow-up study, NTHLS.
| Diagnosis | Did not attend the Follow-up Adult Study (n=7,203) |
Attended the Follow-up Adult Study (n=3,066) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | Sex (male / female) |
Hearing thresholds1 WE frequencies: Low / Mid / High |
No. | % | Sex (male / female) |
Hearing thresholds1 WE frequencies: Low / Mid / High |
|
| Total | 7,203 | 100.0 | 54.9 | 18 19 22 | 3,066 | 100.0 | 51.0 | 18 19 22 |
| Sensorineural hearing loss | 1,027 | 14.3 | 64.3 | 23 28 37 | 462 | 15.1 | 64.1 | 24 29 39 |
| Outer/middle ear anomalies | 10 | 0.1 | 50.0 | 40 42 44 | 7 | 0.2 | 57.1 | 42 44 44 |
| Otosclerosis | 5 | 0.1 | 20.0 | 40 39 26 | 5 | 0.2 | 0.0 | 29 31 20 |
| Otitis Media (OM), Total | 4,688 | 65.1 | 51.1 | 17 17 19 | 2,061 | 67.2 | 49.1 | 16 17 19 |
| Chronic suppurative OM | 213 | 3.0 | 54.9 | 28 27 31 | 108 | 3.5 | 43.5 | 23 24 28 |
| Hearing loss after rAOM | 1,403 | 19.5 | 52.3 | 18 19 22 | 613 | 20.0 | 51.4 | 18 19 22 |
| Otitis Media with Effusion | 2,811 | 39.0 | 50.2 | 16 16 17 | 1,255 | 40.9 | 48.7 | 15 16 16 |
| Acute Otitis Media | 261 | 3.6 | 51.7 | 16 17 19 | 85 | 2.8 | 44.7 | 16 17 20 |
| Otitis externa | 32 | 0.4 | 59.4 | 15 18 23 | 13 | 0.4 | 30.8 | 16 17 20 |
| Foreign body | 18 | 0.2 | 61.1 | 18 19 19 | 7 | 0.2 | 42.9 | 20 21 21 |
| Cerumen | 370 | 5.1 | 49.2 | 18 19 22 | 162 | 5.3 | 45.1 | 17 18 21 |
| Unknown / other diagnosis | 1,053 | 14.6 | 50.6 | 16 17 18 | 349 | 11.4 | 49.9 | 16 16 18 |
Hearing thresholds are defined as pure tone averages of the low (0.25-0.5 kHz), middle (1–2 kHz), and high (3–8 kHz) frequency ranges in the worse hearing ear (WE) at the last audiometric test of the school investigation.
Main results
The differences in adult hearing thresholds between each OM group and the reference group are presented in Table 2. The effects are adjusted for age, sex and noise-exposure (the effects of the covariates were all statistically significant). All OM groups showed statistically significant reduced adult hearing thresholds compared to the reference group.
Table 2.
Differences in mean adult hearing tresholds (dB) between participants diagnosed with childhood hearing loss caused by various types of otitis media and participants not diagnosed with childhood hearing loss and a negative history of recurrent otitis media.
| OTITIS MEDIA SUBGROUPS | Mean differences (95% CI)* | ||
|---|---|---|---|
| 0.25-0.5 kHz | 1–2 kHz | 3–8 kHz | |
| Main subtypes of otitis media | |||
| Otitis Media with Effusion (n=1,255) | 2 (1 – 2) | 2 (1 – 2) | 2 (1 – 2) |
| Hearing loss after reccurent Acute Otitis Media (n=613) | 7 (6 – 9) | 8 (7 – 9) | 10 (9 – 12) |
| CronicSuppurative Otitis Media (n=108) | 17 (14 – 21) | 17 (14 – 21) | 20 (15 – 24) |
| Eardrum pathology | |||
| Otitis Media with Effusion: normal eardrum (n=868) | 1 (0 – 2) | 1 (1 – 2) | 1 (0 – 2) |
| Otitis Media with Effusion: eardrum pathology (n=386) | 3 (2 – 4) | 3 (2 – 5) | 3 (2 – 4) |
| Hearing loss after recurrent AOM: normal eardrum (n=171) | 5 (3 – 7) | 7 (5 – 9) | 10 (7 – 12) |
| Hearing loss after recurrent AOM: eardrum pathology (n=442) | 8 (7 – 9) | 9 (7 – 10) | 11 (9 – 12) |
The effects are adjusted for age, sex and noise exposure and all p-values are < 0.05
The interaction analyses revealed a significant interaction between age and CSOM with an interaction effect (unstandardized coefficient) of 0.5 dB (95% confidence interval (CI) 0.0 – 0.9, p =0.031). In other words, the effect of CSOM on adult hearing thresholds increased by 0.5 dB per year between 20 and 56 years of age. Stratified analyses by diagnostic group (CSOM or reference group) showed that the effect of age on adult hearing thresholds was 0.6 dB (95% CI 0.6–0.6, p<0.001) per year in the reference group and 1.1 dB (95% CI 0.7–1.6, p<0.001) per year in the CSOM group. The average age in the CSOM group was 42 years, thus we performed stratified analyses for the participants under and over this age. The adjusted mean difference in adult hearing threshold between the CSOM group and the reference group was larger in participants tested in middle adulthood (ages 20 to 42 years), 24 dB (95% CI 17–31, p=0.001), than in participants tested in young adulthood (ages 43 to 56 years), 16 dB (95% CI 12–21, p=0.001).
In the sequelae AOM group there were many individuals with no or minimal hearing loss at the last audiometric test in HISCNT, thus the interaction analyses were only conducted for a subgroup with PTA ≥20 dB in the high frequency range (n=356). The interaction effect was 0.3 dB (95% CI 0.1 – 0.6, p=0.014). In other words, the effect of sequelae AOM on adult hearing thresholds increased by 0.3 dB per year after 20 years of age. Stratified analyses by diagnostic group (sequeale AOM or reference group) showed that the effect of age on adult hearing thresholds was 0.9 dB (95% CI 0.2–1.7, p<0.001) per year in the sequelae AOM group, compared to 0.6 dB per year in the reference group. The average age in the sequeale AOM group was 40 years, thus we performed stratified analyses for the sample under and over this age. The effect of sequelae AOM on adult hearing thresholds in participants tested in young adulthood (ages 20 to 40 years) was 13 dB (95% CI 10–15, p=0.001), whereas the effect of sequelae AOM in participants tested in middle adulthood (ages 41 to 56 years) was 17 dB (95% CI 14–20, p=0.001).
Birth year was not significantly correlated with childhood hearing thresholds, and thus cannot have confounded the results.
The effects of OM on adult hearing thresholds were not significantly moderated by noise exposure.
DISCUSSION
Principal Findings
This cohort study examines the relation between childhood OM and adulthood hearing thresholds. Compared to participants with normal childhood hearing, those diagnosed with childhood hearing loss caused by OME, CSOM or hearing loss after rAOM had significantly reduced adult hearing thresholds in the whole frequency range (1 dB/17–19 dB/7–10 dB, respectively). The effects were adjusted for age, sex and noise exposure. Children diagnosed with hearing loss after rAOM had somewhat improved hearing thresholds as adults. The effects of CSOM and high frequency hearing loss after rAOM on adult hearing thresholds were larger in participants tested in middle adulthood than in those tested in young adulthood. Eardrum pathology added a marginally increased risk of adult hearing loss (1–3 dB). Our study could not reveal significant differences in the effect of self-reported noise exposure on adult hearing thresholds between the groups with OM and the group with normal childhood hearing.
Strengths and Weaknesses of the Study
Loss to follow-up
Among the 10,269 children with hearing loss at the school screen audiometry, only 3066 (29.9%) attended the follow-up adult study (Figure 1). Many participants were not invited to the follow-up adult study. NTHLS included only 17 of 23 municipalities, whereas HISCNT included the whole county, so about 13% of the participants in HISCNT were living in a municipality not invited to NTHLS. A few were lost due to not being old enough to be invited to NTHLS or loss of identification number. The participation rate at NTHLS among individuals 56 years of age or younger (the population of this study) was 59%. The remaining loss to follow-up (n~3300) is difficult to explain, but most likely a substantial number moved out of Nord-Trøndelag after the baseline childhood study, to larger cities with better job and educational or other opportunities. Also, a few died (about 2%; information provided by Statistics Norway). Nevertheless, we do not think these factors introduced a selection bias. Statistical analyses revealed no significant differences in the distribution of hearing thresholds at the baseline childhood study between participants that did and did not participate in the follow-up adult study; also, the distribution of etiologies and sex were about the same (Table 1). Furthermore, the follow-up adult study was not only a hearing investigation but a part of a very large general health screening examination (the HUNT study). We do not expect the adults’ hearing status affected the likelihood of participation in the convenient, centrally located study-intake centers in each Nord-Trøndelag municipality.
Data from 1954–1986
Although HISCNT took place between 1954 and 1986, we expect that the diagnostics and classification of OM were not very different from today: The OM diagnoses were set through an ENT examination, including testing of the eardrum mobility and audiometry, which are not very different from today. The classifications of the different OM subtypes at that time (Fabritius 1968) correspond well with the classifications used today (Gates et al. 2002). Considering the course and treatment of OM at that time, antibiotics and ear drops were prescribed, and middle ear surgery, as myringotomy, tympanostomy, adenotomy, tympanoplastics and cholesteatoma surgery, were performed at the Namsos hospital. Yet, it is likely that the socioeconomic status and the treatment of OM were somewhat poorer than today. We do not have information on whether potential ototoxic eardrops were commonly used in this period. If some of the large effects of OM on adult hearing thresholds reflect a poorer course and treatment at that time, this even underlines the importance of optimal treatment in these conditions.
Lack of baseline documentation for the reference group
In the reference group, we assumed that all persons born between 1941 and 1977 and living in Nord-Trøndelag as adults, attended HISCNT. This is at best an approximation. Some may have had undetected childhood hearing loss because they did not take part in HISCNT. This could be due to immigration to Nord-Trøndelag after HISCNT was finished. The effect of migration is probably minor, as the population of Nord-Trøndelag has had low immigration (Holmen et al. 2003). Considering the large number in this group the impact of this misclassification would most likely be a marginal underestimation of the results. If the reference group attended HISCNT, the vast majority of hearing loss is probably detected, since there were three separate hearing examinations. Thus, we expect few participants with CSOM or hearing loss after rAOM in the reference group. On the other hand, the reference group could of course have had temporary childhood hearing disorders like OME outside the screening dates. We expect this also to give a marginal underestimation of the results.
Unregistered childhood hearing thresholds < 20 dB were replaced by the average of the registered childhood hearing thresholds < 20 dB at each frequency. This may have caused some inaccuracy in the childhood hearing level.
Diagnostic accuracy
We assume that the diagnostic accuracy was high because the diagnoses were set by an ENT specialist after an ENT examination and audiometry with both air- and bone-conduction thresholds. In children with more than one etiology, the present study only registered the one considered to be most severe. Although SNHL (no audiometric air-bone gap) is a possible outcome after OM, we presume a higher level of diagnostic inaccuracy in these few cases, so the children diagnosed with both OM and SNHL were (as previously described) only registered with SNHL. In CSOM, a better documentation of cholesteatoma had been desirable, even if the ear surgeries were registered.
Comparisons of the Results with Other Studies
OM can damage both the middle and the inner ear, causing a hearing loss that persists after OM has resolved. Many studies have described histopathological cochlear changes associated with OM (Paparella et al. 1972, Joglekar et al., 2010), and a high or extended high frequency hearing loss, compatible with increased damage at the base of the cochlea, has been shown in children with a history of OM (Ahonen & McDermott 1984; Margolis et al. 1993, Hunter et al. 1996, Laitila et al. 1997). Furthermore, ongoing CSOM has repeatedly been associated with impaired bone-conduction thresholds (Paparella 1972, Redaelli et al. 2005; Yoshida et al. 2014; Luntz et al. 2013). A recent study reported that SNHL related to AOM in adults improved after adequate treatment (Park et al. 2014). Considering long-term middle ear impairments, cohort studies of children with OM and a positive or negative history of tympanostomy have reported that eardrum pathology may persist for years, yet associated with minor increased hearing thresholds (Stenstrom et al. 2005; Khodaverdi et al. 2013), that is in line with our results.
To our knowledge, there are few cohort studies with follow-up of adult hearing after OM (Augustsson & Engstrand 2006; de Beer et al 2003; Jensen et al. 2013), so this study adds valuable knowledge confirming that CSOM and rAOM in childhood can be associated with a clinically relevant adult hearing loss. Our follow-up study did not include bone-conduction thresholds, so we cannot conclude whether the effects on adult hearing thresholds are due to middle- or inner ear pathology. However, this study showed that eardrum pathology added only a marginal increased risk of adult hearing loss in children with OME or hearing loss after rAOM. As expected, the very common condition childhood OME had a minimal effect on adult hearing thresholds. All these small effects (1–3 dB) should have no clinical relevance, and are within the measurement uncertainty of the audiometric test. Previously, a study of extremely long-lasting OME has found permanent effects on hearing (Ryding et al. 2005), and also an association between OME and SNHL has been described (Luo & Feng 2001).
Furthermore, the present study showed that children with hearing loss after rAOM had somewhat improved hearing thresholds as adults. It might be that the hearing thresholds of normal hearing children are elevated in childhood and therefore also improves. The progression of the reference group is however unknown, as we only know that their childhood hearing thresholds were better than 20 dB. A recent study of age-dependent hearing ability in German children indicated slightly elevated thresholds among children with the following mean hearing thresholds between 9 and 12 years of age (n=106): 8 dB for 0.25-0.5 kHz, 5 dB for 1–2 kHz and 3 dB for 3–8 kHz (Muller & Schneider, 2014). Even if we use these childhood hearing thresholds to represent the childhood hearing of our reference group, the differences in hearing thresholds between the sequeale AOM group and the reference group were larger in childhood (10/14/19 dB in the low/mid/high frequency range) than in adulthood (7/8/10 dB in the low/mid/high frequency range).
Interaction with age
As previously described, OM has repeatedly been associated with cochlear affection. It could be that an already damaged hair cell is more susceptible to further damage by other factors, like age or noise-exposure, resulting in a synergistic accumulative effect. A study of self-reported OM in the NTHLS data material found that the effects of childhood OM were stronger among older adults than among younger adults (Tambs et al. 2004). In this cross sectional study, it was unclear whether the stronger effects in the oldest subcohort was in fact a cohort effect, in which more severe and poorer treated childhood disease in the oldest participants resulted in more severe hearing loss. Our cohort study showed that the effects of CSOM and hearing loss after rAOM on adult hearing thresholds were larger in participants tested in middle adulthood than in participants tested in young adulthood. Our data provided childhood hearing thresholds, and there was no significant correlation between childhood hearing thresholds and birth-year in the OM groups. Thus, the finding suggests that ears with subsequent hearing loss after childhood OM age at a faster rate than those without. This finding is supported by a recent study of a Dutch cohort (aged 24–81 years, N=1,721) observed for an average of 12 years with repeated audiometries, describing a strong association between poorer baseline hearing threshold and faster deterioration of hearing (Linssen et al. 2014). However, the present study had only one single follow-up test, so the participants tested in young adulthood were not the same as those tested in middle adulthood. An evaluation of the biological course requires repeated hearing tests during the follow up, so this finding should be further investigated in such a cohort study. Furthermore, we did not have data assuring curation of OM at the time of follow-up. Some CSOM cases can heal and years later re-rupture due to a new infection leading to a new period of prolonged otorrhea, which could again deteriorate the hearing. Considering the long observation-time (mean 31 years) and the access to middle ear surgery and antibiotic treatment at that time, we expect curation to be likely.
Interaction with noise exposure
Increased susceptibility to noise has been shown among young adults with self-reported recurrent childhood ear infections (Job et al. 2000). Our study could not reveal significant differences in the effect of self-reported noise exposure on adult hearing thresholds between the OM groups and the group with normal childhood hearing. This negative finding could also be due to information bias regarding self-reported noise exposure or lack of power to detect a significant interaction effect. The questionnaire in NTHLS contained detailed data about both non-occupational and occupational noise exposure, which showed a significant effect on adult hearing thresholds. Also, we evaluated a large number of participants with CSOM or hearing loss after rAOM, subtypes in which a cochlear affection (increasing the susceptibility to further hair-cell damage by noise exposure) could be expected. Our follow-up study did not include bone-conduction thresholds, so we do not know if the hearing loss is due to conductive hearing loss or SNHL. However, we can think of no reason why a possible difference in adult hearing thresholds between the OM participants with and without noise-exposure should be due to differences in conductive hearing loss and not due to the effects of noise exposure. Thus, our study suggests that there are no significant differences in the effect of noise exposure on adult hearing thresholds between individuals with and without childhood OM. Yet, this should be further investigated by studies with a prospective documentation of noise exposure and repeated follow-up tests.
CONCLUSIONS
This cohort study indicates that CSOM and rAOM in childhood are associated with adult hearing loss, underlining the importance of optimal treatment in these conditions. It appears that ears with a subsequent hearing loss after OM in childhood age at a faster rate than those without, however this should be confirmed by studies with several follow-up tests through adulthood.
Acknowledgement
We are grateful to the late Dr. H. M. Fabritius and to Namsos Hospital and Dr. Eskil Bjørgan for making the HISCNT data available to us. The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre, Faculty of Medicine, Norwegian University of Science and Technology (NTNU), Verdal, Norwegian Institute of Public Health, and Nord-Trøndelag County Council. The NT Hearing Loss Study, which is a part of HUNT, was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD), NIH, research contract No. N01-DC-6–2104. The NT County Health Officer and the Community Health Officers in Levanger and other municipalities provided organizational and other practical support. We also thank the NTHLS team for their diligence. The present study was supported by a grant from The Extra Foundation: Health and Rehabilitation through the member organisation The National Association of Hard of Hearing. The NT Hearing Loss Study was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD), NIH, research contract No. N01-DC-6–2104. All funding follows the guidelines on good publication practis. There was independence between researchers and funders/sponsors.
Footnotes
conflict of interest
There was no conflicts of interest.
References
- Ahonen JE, McDermott JC. Extended high-frequency hearing loss in children with cleft palate. Audiology. 1984;23(5):467–476. doi: 10.3109/00206098409070086. [DOI] [PubMed] [Google Scholar]
- Augustsson I, Engstrand I. Hearing loss as a sequel of secretory and acute otitis media as reflected by audiometric screening of Swedish conscripts. Int J Pediatr Otorhinolaryngol. 2006;70(4):703–710. doi: 10.1016/j.ijporl.2005.09.004. [DOI] [PubMed] [Google Scholar]
- De Beer BA, Graamans K, Snik AF, et al. Hearing deficits in young adults who had a history of otitis media in childhood: use of personal stereos had no effect on hearing. Pediatrics. 2003;111(4 Pt 1):e304–e308. doi: 10.1542/peds.111.4.e304. [DOI] [PubMed] [Google Scholar]
- Fabritius HF. Hearing investigations of school children in North Trondelag County. J Oslo City Hosp. 1968;18(1):5–44. [PubMed] [Google Scholar]
- Gates GA, Klein JO, Lim DJ, Mogi G, Ogra PL, Pararella MM, Tos M. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Ann Otol Rhinol Laryngol Suppl. 2002;188:8–18. doi: 10.1177/00034894021110s304. [DOI] [PubMed] [Google Scholar]
- Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, Lund-Larsen PG. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Norsk epidemiologi. 2003;13(1):19–32. [Google Scholar]
- Hunter LL, Margolis RH, Rykken JR, et al. High frequency hearing loss associated with otitis media. Ear Hear. 1996;17(1):1–11. doi: 10.1097/00003446-199602000-00001. [DOI] [PubMed] [Google Scholar]
- Jensen RG, Koch A, Homoe P. The risk of hearing loss in a population with high prevalence of chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2013;77(9):1530–1535. doi: 10.1016/j.ijporl.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Job A, Raynal M, Tricoire A, et al. Hearing status of French youth aged from 18 to 24 years in 1997: a cross-sectional epidemiological study in the selection centres of the army in Vincennes and Lyon. Rev Epidemiol Sante Publique. 2000;48(3):227–237. [PubMed] [Google Scholar]
- Joglekar S, Morita N, Cureoglu S, et al. Cochlear pathology in human temporal bones with otitis media. Acta Otolaryngol. 2010;130(4):472–476. doi: 10.3109/00016480903311252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaverdi M, Jorgensen G, Lange T, et al. Hearing 25 years after surgical treatment of otitis media with effusion in early childhood. Int J Pediatr Otorhinolaryngol. 2013;77(2):241–247. doi: 10.1016/j.ijporl.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Laitila P, Karma P, Sipila M, et al. Extended high frequency hearing and history of acute otitis media in 14-year-old children in Finland. Acta Otolaryngol Suppl. 1997;529:27–29. doi: 10.3109/00016489709124072. [DOI] [PubMed] [Google Scholar]
- Linssen AM, van Boxtel MP, Joore MA, Anteunis LJ. Predictors of Hearing Acuity: Cross-sectional and Longitudinal Analysis. J Gerontol A Biol Sci Med Sci. 2014;69(6):759–765. doi: 10.1093/gerona/glt172. [DOI] [PubMed] [Google Scholar]
- Luntz M, Yehudai N, Haifler M, et al. Risk factors for sensorineural hearing loss in chronic otitis media. Acta Otolaryngol. 2013;133(11):1173–1180. doi: 10.3109/00016489.2013.814154. [DOI] [PubMed] [Google Scholar]
- Luo H, Feng T. Clinical observation on sensorineural hearing loss secondary to secretory otitis media. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2001;36(4):295–297. [PubMed] [Google Scholar]
- Margolis RH, Hunter LL, Rykken JR, Giebink GS. Effects of otitis media on extended high-frequency hearing in children. Ann Otol Rhinol Laryngol. 1993;102:1–5. doi: 10.1177/000348949310200101. [DOI] [PubMed] [Google Scholar]
- Muller R, Schneider J. Age-Dependent Improvement of Hearing Ability in Children: An International Approach. Ear Hear. 2014;35(4):468–475. doi: 10.1097/AUD.0000000000000037. [DOI] [PubMed] [Google Scholar]
- Paparella MM, Oda M, Hiraide F, et al. Pathology of sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol. 1972;81(5):632–647. doi: 10.1177/000348947208100503. [DOI] [PubMed] [Google Scholar]
- Park JH, Park SJ, Kim YH, et al. Sensorineural hearing loss: a complication of acute otitis media in adults. Eur Arch Otorhinolaryngol. 2014;271(7):1879–1884. doi: 10.1007/s00405-013-2675-x. [DOI] [PubMed] [Google Scholar]
- Redaelli De Zinis LO, Campovecchi C, Parrinello G, Antonelli AR. Predisposing factors for inner ear hearing loss association with chronic otitis media. Int J Audiol. 2005;44:593–598. doi: 10.1080/14992020500243737. [DOI] [PubMed] [Google Scholar]
- Ryding M, Konradsson K, White P, et al. Hearing loss after "refractory" secretory otitis media. Acta Otolaryngol. 2005;125(3):250–255. doi: 10.1080/00016480510003183. [DOI] [PubMed] [Google Scholar]
- Stenstrom R, Pless IB, Bernard P. Hearing thresholds and tympanic membrane sequelae in children managed medically or surgically for otitis media with effusion. Arch Pediatr Adolesc Med. 2005;159(12):1151–1156. doi: 10.1001/archpedi.159.12.1151. [DOI] [PubMed] [Google Scholar]
- Tambs K, Hoffman HJ, Engdahl B, et al. Hearing loss associated with ear infections in Nord-Trondelag, Norway. Ear Hear. 2004;25(4):388–396. doi: 10.1097/01.aud.0000134554.71093.5e. [DOI] [PubMed] [Google Scholar]
- Tambs K, Hoffman HJ, Borchgrevink HM, Holmen J, Engdahl B. Hearing loss induced by occupational and impulse noise: results on threshold shifts by frequencies, age and gender from the Nord-Trondelag Hearing Loss Study. Int J Audiol. 2006;45(5):309–317. doi: 10.1080/14992020600582166. [DOI] [PubMed] [Google Scholar]
- Yiengprugsawan V, Hogan A, Strazdins L. Longitudinal analysis of ear infection and hearing impairment: findings from 6-year prospective cohorts of Australian children. BMC Pediatr. 2013;13:28. doi: 10.1186/1471-2431-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Miyamoto I, Takahashi H. Relationship between CT findings and sensorineural hearing loss in chronic otitis media. Auris Nasus Larynx. 2014;41(3):259–263. doi: 10.1016/j.anl.2013.12.001. [DOI] [PubMed] [Google Scholar]