Abstract
Objective
To determine the rate of remission of recently diagnosed (<1 year) type-2 diabetes mellitus (T2DM) in overweight/obese individuals, with a 6 month program of weight loss and exercise.
Subjects and Methods
Subjects (N=12) were overweight/obese (BMI 35.8±4.3 kg/m2), sedentary, and unfit (peak VO2 20.7±4.7 ml/kg/min) and recently (< 1 year) diagnosed with T2DM. They were willing to participate in a lifestyle program of behavioral weight loss counseling and supervised exercise located at a cardiac rehabilitation program prior to consideration of diabetes medications. Glycated homoglobin (HbA1c) before and after the study intervention was the primary study outcome along with secondary metabolic, fitness and body composition variables.
Results
Subjects had a baseline HbA1c of 6.5-8.0% (mean 6.8±0.2). Subjects lost 9.7±5.2kgs body weight (9%) and improved peak aerobic capacity by 18%. Two subjects withdrew for medical reasons unrelated to the lifestyle program. Eight of 10 completers (80%) went into partial T2DM remission with the mean HbA1c decreasing from 6.8±0.2 to 6.2±0.3% (P<0.001).
Conclusions
For individuals with recently diagnosed T2DM willing to undertake a formal lifestyle program, 80% of study completers and 67% of our total population achieved at least a partial T2DM remission at 6 months. Further study of this intervention at the time of diagnosis of T2DM with randomized controls and longer-term follow-up is warranted.
Introduction
The rate of remission of recently diagnosed (<1 year) T2DM with a program of weight loss and exercise alone is unknown. The American Diabetes Association recommends that, upon the diagnosis of type 2 diabetes mellitus (T2DM), metformin therapy should be administered for normalization of hyperglycemia (1). Whereas medical nutrition therapy is recommended as a primary approach for pre-diabetes or metabolic syndrome, it is described as adjunctive therapy to metformin in the setting of new T2DM. It is rarely applied in an intensive programmatic fashion and its value as a stand-alone therapy is unknown. Medicare, and most insurers, covers 3 sessions of medical nutrition therapy for new T2DM with a certified diabetes educator. In outpatient clinical practice, only 34% of individuals with T2DM ever see a registered dietician, much less undertake an intensive program of exercise and weight loss (2). Consequently, newly diagnosed patients with T2DM receive only minimal formal advice and rarely accomplish significant weight loss or an improvement in fitness. If lifestyle therapy alone could avoid or even postpone the cost and side effects of medical therapy while improving fitness, body composition and other cardiac risk factors, it might be considered as an alternate initial treatment option for motivated individuals. We, therefore, assessed the value of a 6-month program of intensive lifestyle changes on the rate of remission of T2DM in recently diagnosed (< 1 year) overweight/obese individuals who had not yet started a hypoglycemic medication. The lifestyle intervention of exercise and dietary counseling was delivered utilizing cardiac rehabilitation facilities and personnel.
In the LookAHEAD (Action for Health in Diabetes) study, which achieved a mean weight loss of 8.6% and a fitness increase of 21%, the remission rate (partial or complete) of T2DM at one year was 11.5% (3). Subjects randomized to the control condition, who were similarly motivated to undertake a lifestyle program, attained a mean weight loss of 0.7% of body weight at one year and an overall remission rate of 2%. This latter finding affirms that without a formal program of weight loss and exercise, even willing subjects do not, on average, lose much weight or achieve remission of hyperglycemia (3). In LookAHEAD, however, the mean duration since diagnosis was over 5 years and most patients (93%) were taking diabetic medications at study inception. The duration of time since diagnosis and the use of medications may have weighed against the achievement of even a partial remission. For individuals whose duration of T2DM was < 2 years, the remission rate in the intervention arm was 22%. The strongest correlates of remission were shorter duration of T2DM since diagnosis, a lower baseline HbA1c, no insulin therapy and greater weight loss.
Methods
Patient inclusion criteria included a recent (< 1 year) diagnosis of T2DM with HbA1c between 6.5-8.0%, a body mass index (BMI) of 27-40kg/m2, waist circumference of >102 cm for men, >88 cm for women, never treated with a hypoglycemic agent and a willingness to embark on an intensive lifestyle program. The 6-month exercise and behavioral weight loss program included nearly daily sessions of longer distance walking that we have termed “high-caloric expenditure exercise” with 1-3, 45-60 minute sessions/week of supervised on-site exercise and home walking on other days for a total of 5-6 days per week of exercise (4). The exercise intensity is set somewhat lower than standard cardiac rehabilitation protocols (50-60% vs. 65-70% peak VO2) to allow for longer bouts of exercise. This type of exercise results in twice the weight loss compared with a thrice weekly program of higher intensity but shorter duration exercise over a 6-month period (4). The behavioral weight loss program included 24 weekly group counseling sessions coordinated by a R.D. and focused on self-monitoring and developing an action plan for diet and physical activity (5). The individual sessions each had a discussion topic such as “stimulus control” and “assertiveness training” and emphasized dietary records, itemization of food and caloric content. The daily caloric goal was 500 kcal less than predicted maintenance calories which was roughly calculated by multiplying body weight (lbs) by 12 (6). Study measures taken before and after the lifestyle intervention program included body composition by dual x-ray absorptiometry (General Electric Lunar Prodigy, Madison WI), assessment of peak aerobic capacity with expired gas analysis (Medgraphics, St. Paul, MN) and seated blood pressure (BP) by sphygmomanometry. Caloric intake was measured with 24-hour dietary records (Food Processor Nutrition Software, Salem, OR) and physical activity by accelerometer (Kenz Lifecorder-Plus Activity Monitor) in Kcal/day. Blood measures included HbA1c, fasting glucose, lipid profiles, high sensitivity C-reactive protein (CRP), insulin and the homeostatic model of insulin resistance ( HOMA-IR) (7). The protocol was approved by the University of Vermont Committee on Human Research and registered as a clinical trial (NCT02065544). Partial remission of T2DM was defined as HbA1c of 5.7% - 6.5% whereas a complete remission was defined as an HbA1c of < 5.7% (8). Statistical analyses included McNemar's test, Student's T-test and linear regression analysis.
Results
The study population consisted of 12 individuals, 8 female, with recently diagnosed T2DM (mean time since diagnosis 93 ± 115 days). Twenty-five individuals were initially screened but exclusions included four already taking a diabetes medication, three with BMI > 40kg/m2, three had T2DM > 1 year, two with baseline HbA1c of < 6.5% and one who was currently smoking. The mean age was 62+6 years and baseline weight was 103±14 kg with a BMI of 35.8±4.3 kg/m2. Use of non-diabetic medications was kept steady through the study and included 9 individuals on statins and aspirin, 5 on ACE-inhibitors and 4 on beta- and calcium blockers. Whereas 12 individuals started the protocol, 2 dropped out (both women); one for knee arthritis requiring elective knee replacement and another who was hospitalized with a flu-like syndrome and discharged on a hypoglycemic medication, eliminating her from the study. Data are presented for the remaining 10 subjects 3 of whom had an associated diagnosis of coronary heart disease.
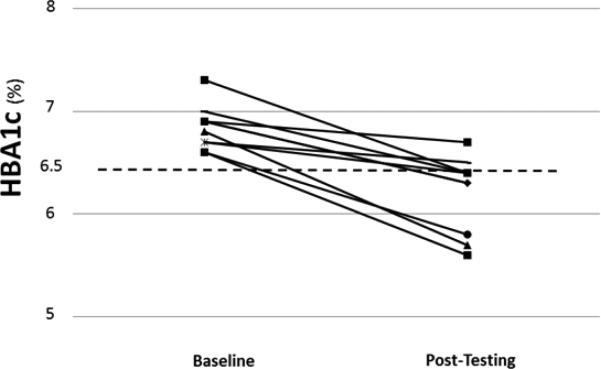
Mean weight loss was 9.7±5.2 kg (−9%) and peak aerobic capacity (ml/kg/min) increased by 18% (Table 1) accompanied by favorable body composition changes including a decrease in fat mass and waist circumference, but no significant change in lean mass (Table 1). This was accomplished by a mean increase of physical activity-related energy expenditure of 241±63 kcal/day (293±52 at baseline to 535±93 kcal/day) and a mean decrease of dietary caloric intake of 341±145 kcal/day (2163±475 at baseline to 1821±465 kcal/day). Eight of the 10 completers went into at least partial remission of their diabetes with the mean HbA1c dropping from 6.8±.2 to 6.2±.4 % (P<0.001) (Table 1) (Figure 1) with one individual accomplishing a “complete remission” to a HbA1c of 5.6%. Indeed, every single individual lowered her/his HbA1c (Figure 1). There was no difference in weight loss response or change in HbA1c by gender. Cardiac risk factors including fasting insulin levels, CRP, triglycerides, the Cholesterol/HDL-cholesterol atherogenic ratio and the HOMA-IR index all responded favorably and significantly to the study intervention (Table 1). Systolic BP was not lowered significantly whereas diastolic BP was lower after the intervention (Table 1).
Table 1.
Body Composition, Fitness and Metabolic Indices
| Pre-Intervention | Post-Intervention | P Valuea | |
|---|---|---|---|
| Body weight, kg | 102.6 ± 15.8 | 92.9 ± 15.3 | .0002 |
| Body mass index, kg/m2 | 34.8 ± 3.9 | 31.6 ± 4.1 | .0001 |
| Fat mass, kg | 40.0 ± 16.1 | 32.7 ± 14.4 | .0003 |
| Lean mass, kg | 54.9 ± 16.0 | 52.0 ± 14.1 | .08 |
| Body fat, % | 41.3 ± 10.3 | 37.8 ± 11.4 | .001 |
| Waist circumference, cm | 111 ± 11 | 104 ± 10 | .008 |
| HbA1c, % | 6.8 ± 0.2 | 6.2 ± 0.4 | .0002 |
| HbA1c, mmol/mol | 51 ± 2 | 44 ± 3 | .0002 |
| Fasting glucose (mg/dL) | 136 ± 22 | 105 ± 9 | .002 |
| Insulin, μU/ml | 18 ± 10 | 10 ± 6 | .002 |
| HOMA-IR, mass units | 5.8 ± 3.2 | 2.5 ± 1.6 | .001 |
| Peak V̇O2, mL·kg−1·min−1 | 20.7 ± 4.7 | 24.4 ± 6.9 | .02 |
| hs-CRP, ng/mL | 5.5 ± 4.3 | 3.6 ± 4.3 | .006 |
| Total cholesterol, mg/dL | 172 ± 36 | 162 ± 43 | .18 |
| Triglycerides, mg/dL | 188 ± 114 | 117 ± 67 | .03 |
| HDL-C, mg/dL | 45 ± 15 | 46 ± 14 | .23 |
| LDL-C, mg/dL | 93 ± 8 | 95 ± 8 | .48 |
| Cholesterol/HDL-C | 4.2 ± 1.4 | 3.7 ± 1.1 | .048 |
| Systolic BP, mmHg | 138 ± 9 | 130 ± 13 | .11 |
| Diastolic BP, mmHg | 78 ± 6 | 72 ± 2 | .02 |
Abbreviations: BP, blood pressure; HbA1c, glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high sensitivity C-reactive protein; LDL-C, low density lipoprotein cholesterol; V̇O2, oxygen uptake.
Pre- and post-intervention data analyzed using paired t-tests.
Figure 1.
Hemoglobin A1c Measures Before and After the Lifestyle Intervention
Discussion
The novel finding of this study is that a clear majority (8/10) of overweight individuals with recently diagnosed T2DM who completed an intensive lifestyle program went into at least partial remission defined as an HbA1C of < 6.5% thereby delaying the need for hypoglycemic medications. Moreover, the program brought about favorable effects on body composition, aerobic fitness and metabolic markers of cardiovascular risk such as CRP, insulin levels, insulin resistance, lipid parameters and blood pressure. No medication utilized in this clinical situation provides similar broad reaching preventive effects. While the study was limited by its small size and a lack of randomized controls, it nonetheless highlights the potential of an intensive lifestyle program, begun early after a diagnosis of T2DM, to postpone the need for hypoglycemic medications along with favorable effects on numerous parameters of cardiovascular risk.
An advantage of the present approach is that it could be accomplished in the cardiac rehabilitation setting which includes over 2,000 programs nationwide (9). Cardiac Rehabilitation programs have been shown effective in accomplishing weight loss alongside improvements in exercise capacity and insulin resistance (4, 10,11), but this intervention has not been combined with behavioral weight loss counseling in the setting of newly diagnosed T2DM. It is noted that in 2012 the prevalence of diabetes in the U.S. approached 26 million individuals with an incidence of over 2 million new cases (12). In that exercise alone has important effects on metabolic parameters in insulin resistant individuals (13), the ability to deliver a supervised exercise program is an important consideration. It is extremely unlikely that similar subjects would achieve such positive results without the support and guidance of the lifestyle program as demonstrated by the control group of Look AHEAD who lost less than1% body weight (3). Whether these favorable effects on glucose control also postpones the occurrence of diabetes-related complications remains unknown along with the duration of the partial remission of diagnosed T2DM. As in the case of lifestyle treatment for the prevention of T2DM, effective weight loss maintenance is likely to be a key determinant of the duration of the partial remission (14,15).
Prior lifestyle studies on remission of T2DM were limited either by institution of the lifestyle program at a much later date after the diagnosis of T2DM such as in the LookAHEAD study (3) or by the utilization of a less intensive physical activity program (16). In LookAHEAD, where the median duration of T2DM since diagnosis was 5 years and most patients were already taking hypogycemic medications, the time since diagnosis of T2DM was an independent predictor of remission of T2DM, as were the baseline HbA1c and the amount of weight loss. For individuals with a diagnosis of T2DM of less than 2 years, the partial remission rate was 22%. Whereas we did not have long-term follow up, in LookAHEAD, of those who had a remission, about one-third returned to clinical diabetes each year. In the present study, the intervention was instituted earlier in the disease process thus the natural history may be different.
In summary, upon diagnosis of T2DM in an overweight individual, in addition to the usual clinical options of starting metformin and visits with a diabetes educator a third option of a referring motivated individuals to a well-established intensive behavioral weight loss program with an important exercise component, should be seriously considered (17). The national network of over 2,000 cardiac rehabilitation/secondary prevention programs is poised to accept these patients in treatment.
Condensed Abstract.
We studied the effect of a 6-month program of exercise and weight loss on remission of type 2 diabetes (T2DM) in individuals recently diagnosed with T2DM. Eight of ten individuals who completed the program went into partial remission of their T2DM.
Acknowledgements
Supported by the Vermont Center on Behavior and Health (NIH/NIGMS P20GM103644-01) and by the Schwendler Trust Award for Cardiovascular Clinical Research, University of Vermont General Clinical Research Center.
Abbreviations
- T2DM
Type 2 Diabetes Mellitus
- HbA1c
Glycated Hemoglobin
- BMI
Body Mass Index
- CRP
High Sensitivity C-Reactive Protein
- HOMA-IR
Homeostatic Model of Insulin Resistance
Footnotes
The authors have no conflicts of interest to report.
References
- 1.American Diabetes Association Standards of Medical Care in Diabetes 2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy AG1, MacLean CD, Littenberg B, Ades PA, Pinckney RG. The challenge of achieving national cholesterol goals in patients with diabetes. Diabetes Care. 2005;2:1029–34. doi: 10.2337/diacare.28.5.1029. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, et al. and the Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–96. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ades PA, Savage PD, Toth MJ, Harvey-Berino J, Schneider DL, Bunn JY, et al. High-Caloric Expenditure Exercise: A New Approach to Cardiac Rehabilitation for Overweight Coronary Patients. Circulation. 2009;119:2671–8. doi: 10.1161/CIRCULATIONAHA.108.834184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownell K. The LEARN program for weight management. 10th Edition American Health Publishing Company; Dallas TX.: 2004. [Google Scholar]
- 6.Harvey-Berino J. Weight loss in the clinical setting: application for cardiac rehabilitation. Coron Artery Dis. 1998;9:795–798. doi: 10.1097/00019501-199809120-00003. [DOI] [PubMed] [Google Scholar]
- 7.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–5. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curnier D, Savage PD, Ades PA. Geographic Distribution of Cardiac Rehabilitation Programs in the U.S. J Cardiopulm Rehabil. 2005;25:80–84. doi: 10.1097/00008483-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Milani RV. Cardiac Rehabilitation and Exercise Training in Metabolic Syndrome and Diabetes. J Cardiopulm Rehabil. 2005;25:59–66. doi: 10.1097/00008483-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Rubenfire M, Mollo L, Krishnan S, Finkel S, Weintraub M, Gracik T, Kohn D, Oral EA. The metabolic fitness program: lifestyle modification for the metabolic syndrome using the resources of cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2011;31:282–9. doi: 10.1097/HCR.0b013e318220a7eb. [DOI] [PubMed] [Google Scholar]
- 12.Geiss LS, Wang J, Cheng YL, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and Incidence Trends for Diagnosed Diabetes Among Adults Aged 20 to 79 Years, United States, 1980-2012. JAMA. 2014;312(12):1218–1226. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 13.Earnest CP1, Johannsen NM, Swift DL, Gillison FB, Mikus CR, Lucia A, Kramer K, Lavie CJ, Church TS. Aerobic and Strength Training in Concomitant Metabolic Syndrome and Type 2 Diabetes. Med Sci Sports Exerc. 2014 Jan 1; doi: 10.1249/MSS.0000000000000242. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014;37:912–921. doi: 10.2337/dc13-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, Toledo FG, Jakicic JM. Surgical vs. Medical treatments for Type 2 Diabetes mellitus: A randomized clinical trial. JAMA Surg. 2012 Jun 4; doi: 10.1001/jamasurg.2014.467. Doi: 10.1001/jamasurg.2014.467. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ades PA, Savage PD, Harvey-Berino J. The treatment of obesity in cardiac rehabilitation. J Cardiopulm Rehabil and Prev. 2010;30:289–98. doi: 10.1097/HCR.0b013e3181d6f9a8. [DOI] [PMC free article] [PubMed] [Google Scholar]