Abstract
In a preclinical model of natural reward devaluation by cocaine, taste cues elicit aversive taste reactivity when they predict impending but delayed cocaine self-administration. Here, we investigated this negative affective state as a function of cocaine dose. Male, Sprague-Dawley rats were given 45 brief intraoral infusions of a 0.15% saccharin solution prior to 2 h cocaine self-administration for 14 days. Rats were video recorded; taste reactivity and patterns of self-administration were quantified on the first and last days. On day 14, a significant decrease in appetitive taste reactivity and increase in aversive taste reactivity was observed (compared to day 1) that did not vary as a function of cocaine dose. In contrast, patterns of cocaine self-administration (i.e., the total number of lever presses and load-up behavior) varied as a function of dose across days. Further, load-up behavior was positively correlated with aversive taste reactivity (i.e., gapes) on day 14 across all doses tested. Collectively, these findings indicate that the emergence of negative affect in this preclinical model is not dependent on cocaine dose.
Keywords: behavior, rat, cocaine, aversion
Introduction
Cocaine addiction is characterized by cycles of drug use, abstinence, and resumption of drug taking (relapse). Embedded in the addiction cycle is the development of negative affective states (i.e. dysphoria, irritability, anhedonia) as well as the devaluation of natural rewards (Koob et al. 1998; Koob and Volkow 2010). During prolonged drug abstinence, cocaine addicts typically report increased feelings of negative affect, particularly when exposed to drug cues. This aversive condition often leads to craving and relapse, with the later believed to ‘correct’ for this negative emotional state (Risinger et al. 2005; Grigson 2008; Koob and Volkow, 2010; Nyland and Grigson 2013).
A preclinical model was developed in our laboratory to study natural reward devaluation by cocaine and the associated emergence of negative affective states in rats (Wheeler et al. 2008, 2011), building upon work by Grigson and colleagues (Grigson, 1997; Grigson and Twining, 2002). The model used here is based on the finding that rats exhibit stereotyped oromotor responses to palatable and unpalatable taste stimuli infused directly into the oral cavity that correspond to the hedonic valence of the stimulus. Importantly, oral facial responses, termed taste reactivity, reflect not only innate taste preferences but also conditioned changes in affect (Grill and Norgren 1978; Wheeler et al. 2008). Rats exhibit appetitive taste reactivity (i.e., licks, lateral tongue protrusion) during intraoral infusion of a sweet such as saccharin and aversive taste reactivity (i.e., gapes) during infusion of a bitter tastant (e.g., quinine). In this model, the sweet (saccharin) is delivered intraorally in discrete 3.5s intervals (once a minute over 45min, phase 1) and is immediately followed by access to cocaine self-administration for 2h (phase 2). We showed that the initially palatable saccharin solution became unpalatable as the tastant became associated with impending, but delayed, opportunity to self-administer cocaine. It was hypothesized that this “drug waiting” period, when the tastant was infused, allowed for a strong association to develop between the taste and delayed drug access, and enabled the emergence and expression of a negative affective state as measured by taste reactivity (Wheeler et al. 2008, 2011; Carelli and West 2014). Further, rats that exhibited the most aversive responses in phase 1 were the most motivated to consume cocaine in phase 2 once it was available. Thus, this negative affective state that is reflected by aversive taste activity in phase 1 appears to be ‘corrected’ by cocaine consumption during self-administration in phase 2.
Here, we build upon that work and examine the negative affective state and increased motivation to consume cocaine as a function of cocaine dose during self-administration. We hypothesized that the negative affective state that develops in this model is cocaine dose-dependent (i.e., the number of aversive gapes will increase with increasing dose of drug).
Methods
Subjects
Male, Sprague-Dawley rats (Harlan), aged 90–120 days and weighing approximately 275–350 grams were used (n=24). Animals were housed individually and maintained on a standard 12:12h light-dark cycle (lights on at 07:00h). During training and testing, animals were restricted to no less than 85% of their preoperative body weight by limiting water access (30 ml/day). Food was freely available. Animal procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (2011), and were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC).
Surgeries
Rats were anesthetized with a ketamine hydrochloride (100mg/kg) and xylazine (20mg/kg) mixture and surgically implanted with intra-oral cannulae and an intravenous catheter in a single surgery, as described previously (Roitman et al. 2008, Wheeler et al. 2008). Intravenous catheters for self-administration were purchased from a commercial source (Access Technologies, Skokie, IL) and inserted into the jugular vein using established procedures (Carelli and Deadwyler, 1996; Wheeler et al., 2008).
Experimental Design
Training and test sessions were conducted in a Plexiglas chamber (Med Associates, Inc., St Albans, VT) housed within a commercial sound-attenuated cubicle. Figure 1A shows the experimental design. Initially, mildly water-deprived (30ml/day) rats were trained to press a lever for water; they underwent surgical procedures as described above. One week later, rats received 14 daily conditioning sessions during which the behavioral task was conducted in two phases/day: 1) intra-oral tastant infusions and 2) cocaine self-administration. In phase 1, rats received discrete intraoral saccharin infusions, delivered in 3.5s intervals (0.15% saccharin solution, 200 μl/infusion). A total of 45 tastant infusions (trials; 1 trial/min) were delivered. Immediately thereafter, the intravenous catheter line was attached and phase 2 (cocaine self-administration) was initiated, as described previously (Wheeler et al., 2011). Briefly, rats were trained to self-administer cocaine on a fixed ratio 1 (FR1) schedule of reinforcement (2h). Each lever press resulted in intravenous infusion of cocaine (over 6s), termination of the cue light positioned above the lever, and onset of a tone (67db, 1 kHz) and house light (25W) stimulus. Lever presses during the 20s post response period had no programmed consequences. Three doses of cocaine were examined (0.16, 0.33 and 0.66 mg/inf) in a between-subjects design. The same flavor of 0.15% saccharin was used in phase 1 across all three cocaine doses (groups). Cocaine hydrochloride was obtained from the National Institute on Drug Abuse.
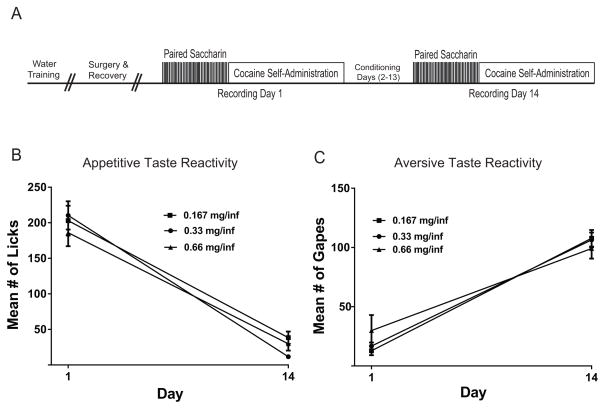
Figure 1.
(A) Schematic diagram of task. See text for details on task procedure. Mean number of licks (B) and gapes (C) during saccharin infusions (phase 1) on the first and last days of the taste-drug pairings as a function of cocaine dose. Note that the animals exhibited a significant decline in appetitive taste reactivity and a significant increase in aversive taste reactivity across days, which were not cocaine dose-dependent.
Taste Reactivity
Taste reactivity was analyzed in a frame by frame analysis using digital video recorded on days 1 and 14. Appetitive and aversive taste reactivity were counted using the procedure developed by Grill and Norgren (1978). Briefly, mouth movements expressed in the 6s following infusion onset that matched a “triangle” shape for a duration exceeding 90ms were counted as aversive. Instances in which the tongue protruded and crossed the midline were counted as appetitive.
Cocaine Self-Administration
Cocaine self-administration was examined across training days. On the last day of training (day 14), the average number of lever presses, latency to the first lever press, and average number of cocaine load-up presses were examined across cocaine doses using separate one-way ANOVAs. Load-up behavior was defined as rapid lever pressing in the beginning of the session in which the average inter-press interval (INT) during load-up was less than half the average INT during the remainder of the session.
Data Analysis
Changes in the total number of lever presses during self-administration across the 14 days of training were examined as a function of cocaine dose using a 2-way mixed design ANOVA. Changes in the average number of licks (appetitive taste reactivity) and average number of gapes (aversive taste reactivity) were compared on day 1 versus day 14 as a function of dose using separate 2-way mixed design ANOVAs. Pearson product-moment correlation coefficients examined relationships between aversive taste reactivity on day 14 to: 1) total number of load-up presses, 2) total number of lever presses, and 3) latency to the first lever press during self-administration. Statistical analyses of all behavioral data were performed using commercially available software (Statstica, Tulsa, OK).
Results
Phase 1: Taste Reactivity
A two-way mixed design ANOVA on appetitive taste reactivity across cocaine doses revealed a significant main effect of day (F1,21=207.08, p<0.001), but not of dose (F2,21=0.37, NS) and no significant day X dose interaction (F2,21=1.25, NS). The results indicate that appetitive taste reactivity was lower on day 14 compared to day 1 across all rats independent of cocaine dose (Figure 1B). A two-way mixed design ANOVA on aversive taste reactivity revealed a significant main effect of day (F1,21=135.42, p<0.001), but not of dose (F2,21=1.90, NS) and no significant day X dose interaction (F2,21=0.0682, p>0.05). The results indicate that aversive taste reactivity increased for all rats across training days (on day 14 compared to day 1), independent of cocaine dose (Figure 1C).
Phase 2: Cocaine self-administration
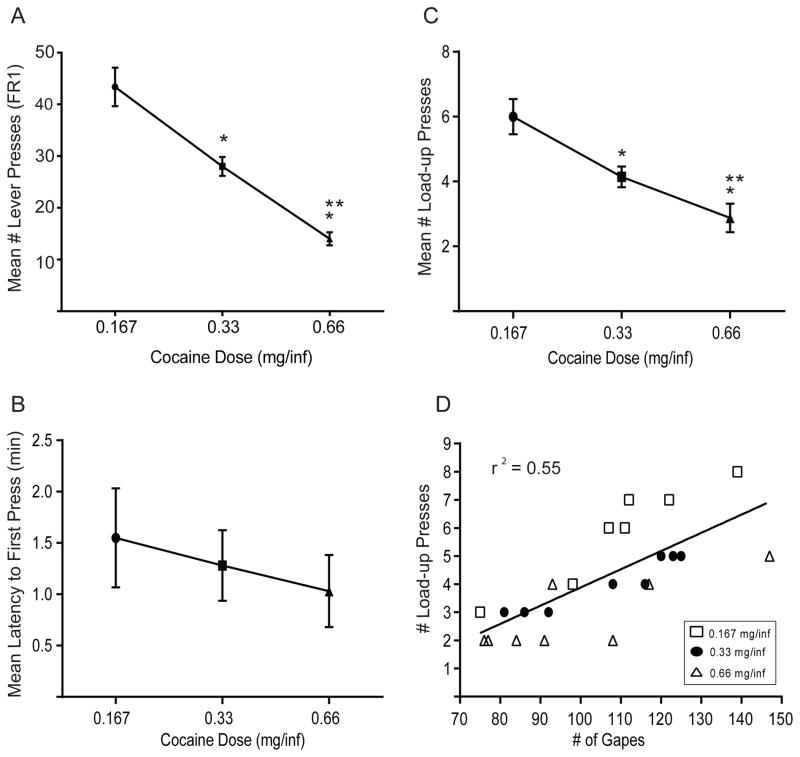
All rats acquired self-administration across the 14 training days. A two-way mixed design ANOVA on cocaine self-administration revealed significant main effects of day (F13,20=2.62, p<0.01) and dose (F2,20=48.21, p<0.001) but no significant dose X day interaction (F26,260=1.00, NS). Next, we examined aspects of self-administration behavior on day 14 across the three cocaine doses. First, we determined if the total number of lever presses during the self-administration phase varied as a function of cocaine dose. A one-way ANOVA revealed a significant main effect of cocaine dose (F2,21= 21.62 p<0.001) on lever pressing for cocaine. Post hoc tukey tests revealed that rats pressed the lever significantly less as the dose of cocaine increased (Figure 2A). Second, a one-way ANOVA revealed a non-significant trend toward a decrease in latency to the first press as a function of cocaine dose (F2,21= 1.50, p > 0.05, Figure 2B). Finally, a one-way ANOVA revealed a significant main effect of cocaine dose (F2,21 = 8.29, p<0.01) on load-up behavior (Figure 2C). Post hoc tukey tests revealed that as cocaine dose increased, the number of load-up presses decreased.
Figure 2.
Mean number of lever presses (A), mean latency to the first press (B) and mean number of load-up presses (C) as a function of cocaine dose on day 14. (D) Mean number of load-up presses is significantly correlated with aversive taste reactivity (gapes) across all cocaine doses (r2 = 0.74) and for all individual doses (see text for details). *p< 0.05 compared to 0.167mg/inf; **p< 0.05 compared to 0.167 and 0.33mg/inf.
Our prior studies showed that animals that exhibited the most aversive taste reactivity on the last day of training displayed the highest levels of load-up presses for cocaine, once available (Wheeler et al., 2008, 2011; Wheeler and Carelli, 2009). To determine if this finding is cocaine dose-dependent, Pearson correlation coefficients were conducted. The results revealed that the number of load-up presses was correlated with the number of aversive responses (gapes) when all doses were combined (r2=0.55, p<0.05, Figure 2D) and for each individual cocaine dose (0.167 mg/inf: r2=0.88, p<0.05; 0.33 mg/inf: r2= 0.91, p<0.05; 0.66 mg/inf: r2= 0.63, p<0.05). There were no significant correlations between aversive responses and mean number of lever presses (all doses combined, r2=0.07, NS; 0.167 mg/inf r2=0.11; 0.33 mg/inf r2=0.028; 0.66 mg/inf r2=0.0006), or latency to first press (all doses combined r2=0.10, NS; 0.167 mg/inf r2=0.12; 0.33 mg/inf r2=0.000035; 0.66 mg/inf r2=0.31).
Discussion
The main objective of the present study was to determine if the negative affective state that develops in the preclinical model of natural reward devaluation by cocaine is dose-dependent. The current findings replicate earlier work by showing that rats exhibited a shift from appetitive to aversive taste reactivity as the tastant came to predict impending but delayed cocaine availability (Wheeler et al., 2008, 2011; Wheeler and Carelli, 2009). Here, we extend those findings and show that once this aversive state develops (day 14), it does not vary as a function of cocaine dose during the self-administration phase, consistent with Cason and Grigson (2013).
An important feature of this preclinical model is that rats exhibiting the most aversive responses are also the most motivated to consume cocaine once available (Wheeler et al., 2008, 2011). That is, we previously reported that rats that exhibited the most gapes during phase 1 showed the greatest number of load-up presses and were fastest to press the lever for cocaine once self-administration was available in phase 2. It is well known that load-up behavior during the start of cocaine self-administration sessions is cocaine dose-dependent (Carelli and Deadwyler 1996). However, here we extend those findings and show that in this preclinical model positive correlations exist between load-up presses and aversive taste reactivity across all doses tested. This finding supports the view that across all doses tested, rats are more motivated to consume cocaine when they experience a negative aversive state. A possible explanation for this finding may be that the rapid rate of responding at the start of the self-administration phase in phase 2 (i.e., load-up behavior) reflects the animals attempt to achieve an optimal level of drug in their system to overcome the aversive state that develops in phase 1. Indeed, prior studies have shown that the rate of self-administration responding is linked to achievement and maintenance of an optimal level of drug (Pettit and Justice, 1989, 1991), perhaps reflective of a hedonic set point (Koob and Caine, 1999; Koob and Volkow, 2010). Although the emergence of negative affect in this preclinical model is not cocaine dose-dependent, the present findings also show that load-up behavior may reflect a correction of this aversive state that is observed across cocaine dose.
Drug users identify negative affect as a main reason for continued drug use and relapse (Sinha et al., 2000; Baker et al., 2004). Indeed, the emergence of negative affect and natural reward devaluation related to repeated drug use is considered a key aspect in models of drug addiction (Koob et al., 1998; Grigson and Twining, 2002; Grigson, 2008; Koob and Volkow, 2010). As such, the current preclinical model may be a useful tool to examine possible behavioral interventions to reduce the emergence of this negative affective state, as well as neurobiological mechanisms that may underlie it.
Acknowledgments
This research was supported by DA014339 to RMC and T32-DA07244 to JG.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interests to declare.
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Carelli RM, West EA. When a good taste turns bad: Neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology. 2014;76:360–369. doi: 10.1016/j.neuropharm.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Ther. 1996;277(1):385–393. [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physio Behev. 2013;112–113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behav Neurosci. 1997;111:129–136. [PubMed] [Google Scholar]
- Grigson PS. Reward Comparison: The Achilles’ heel and hope for addiction. Drug Discov Today Dis Models. 2008;5(4):227–233. doi: 10.1016/j.ddmod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116(2):321–333. [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; 2011. [PubMed] [Google Scholar]
- Koob GF, Caine SB. Cocaine addiction therapy--are we partially there? Nat Med. 1999;5(9):993–995. doi: 10.1038/12429. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Nyland JE, Grigson PS. A drug-paired taste cue elicits withdrawal and predicts cocaine self-administration. Behav Brain Res. 2013;240:87–90. doi: 10.1016/j.bbr.2012.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34(4):899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539(1):94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11(12):1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56(Suppl 1):149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69(11):1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]