Abstract
Genetic variation at IGF1 has been linked to prostate cancer risk. However, the specific predisposing variants have not been identified. In this study, we fine-mapped the IGF1 locus for prostate cancer risk in African Americans.
We conducted targeted Roche GS-Junior 454 resequencing of a 156kb region of IGF1 in 80 African American aggressive prostate cancer cases. 334 IGF1 SNPs were examined for their association with prostate cancer risk in 1,000 African American prostate cancer cases and 991 controls. The top associated SNP in African Americans, rs148371593, was examined in an additional 3,465 prostate cancer cases and 3,425 controls of non-African American ancestry—European Americans, Japanese Americans, Latinos, and Native Hawaiians. The overall association of 334 IGF1 SNPs and prostate cancer risk was assessed using logistic kernel-machine methods. The association between each SNP and prostate cancer risk was evaluated through unconditional logistic regression. A false discovery rate threshold of q < 0.1 was used to determine statistical significance of associations.
We identified 8 novel IGF1 SNPs. The cumulative effect of the 334 IGF1 SNPs was not associated with prostate cancer risk (p=0.13) in African Americans. Twenty SNPs were nominally associated with prostate cancer at p<0.05. The top associated SNP among African Americans, rs148371593 (MAF=0.03; p=0.0014; q>0.1) did not reach our criterion of statistical significance. This polymorphism was rare in non-African Americans (MAF<0.003) and was not associated with prostate cancer risk (p=0.98).
Our findings do not support the role of IGF1 variants and prostate cancer risk among African Americans.
Introduction
Prostate cancer is the most common cancer in U.S. men. African Americans have the highest incidence rate of prostate cancer and at least twice the mortality rate of disease in comparison to other racial/ethnic groups (1). Insulin-like growth factor 1 (IGF1) is a potentially interesting candidate gene for prostate cancer as it stimulates cellular proliferation and inhibits apoptosis. Men with the higher levels of circulating IGF-I have been reported to have an increased risk of prostate cancer compared with men with the lowest levels of IGF-I (2–5). Past research and previous studies have linked genetic variation at IGF1 to prostate cancer risk (2, 3, 6), however, the specific predisposing IGF1 variants have not been identified. Detailed fine-mapping of the IGF1 locus may refine the genetic signal and aid in prioritizing risk variants for further follow-up and functional studies. Moreover, studying African Americans is an efficient means of localizing predisposing alleles given their high rates of prostate cancer and lower levels of linkage disequilibrium. These features provide for greater resolution in identifying IGF1 risk alleles and evaluating their effects among a population with the greatest burden of disease. In this study, we conducted a fine-mapping study of the IGF1 locus and prostate cancer risk among African Americans.
Materials and Methods
Study Subjects
The Multiethnic Cohort Study is a large population-based cohort study of more than 215,000 men and women from Hawaii and Los Angeles. The cohort is composed predominantly of individuals from five racial/ethnic groups: African Americans, Native Hawaiians, Japanese, Latinos, and Whites. Further methodological details of this study are provided elsewhere (7). Briefly, incident prostate cancer cases were identified by cohort linkage to population-based Surveillance, Epidemiology and End Results cancer registries covering Hawaii and California. Information on stage of disease and Gleason grade at the time of diagnosis were also collected from the cancer registries. Aggressive prostate cancer was defined as either regional, metastatic disease or localized disease with Gleason grade >8. Controls had no diagnosis of prostate cancer and were randomly selected from the control pool of participants that provided blood specimens for genetic analysis. Controls were frequency matched to cases by age (±5 years) and ethnicity. For this study, our African American and non-African American case-control studies of prostate cancer nested in the MEC included 1,098 cases and 1,081 controls and 3,480 cases and 3,447 controls, respectively. This study was approved by the Institutional Review Boards at the University of Hawaii, the University of Southern California, and the California Prevention Institute of California.
SNP Discovery and Selection
We used RainDance Technologies Custom Primer Library Design, and utilized Roche GS-Junior 454 next generation sequencing technology to target and resequence 156kb of IGF1 (including 50kb downstream and 25kb upstream, Chromosome 12: 102,741,896-102,898,083, human genome assembly 18) in pooled samples of 80 African American prostate cancer cases with aggressive disease (8 pools of 10 samples each). Variant analysis was performed with Roche Amplicon Variant Analysis (AVA) software. For the eight pools, a total of 395 SNPs were identified as high quality variants (maximum variant allele frequency (VAF) > 5% with max minor allele frequency (MAF) > 10% in regions of possible off-target reads). To increase our coverage of SNPs in this region, we selected 316 additional SNPs that had MAF > 1% in Yorubans (YRI) from the 1000 Genomes Project, resulting in a total of 711 SNPs. These 711 SNPs were scored for Illumina GoldenGate assay design; 259 SNPs did not meet assay criteria of a sufficient design score and/or located within >60 base pairs of a neighboring SNP, resulting in 452 SNPs. To reach 384 SNPs for the Illumina GoldenGate genotyping assay, an additional 68 SNPs were excluded based on lower design scores and proximity to neighboring SNP.
Case-Control Genotyping
All assays were undertaken by laboratory personnel blinded to prostate case-control status. For African Americans, the Illumina GoldenGate assay was used to genotype 384 IGF1 variants in 1,098 African American prostate cancer cases and 1,081 controls. We excluded subjects with a call rate <95% (n=43) and those missing genetic ancestry estimates (n=145), resulting in a final analysis set of 1,000 African American cases and 991 African American controls. Of the 384 variants selected for Illumina genotyping, 38 SNPs failed genotyping (call rate <95%) or were not in Hardy Weinberg equilibrium (p<0.0001). Of the 38 SNPs that failed Illumina genotyping, 32 SNPs were prioritized for genotyping by the Taqman assay on the OpenArray platform. Between the Illumina GoldenGate and Taqman OpenArray genotyping, a total of 384 variants were genotyped in African American cases and controls, of which 6 SNP assays failed, one SNP was not in Hardy-Weinberg equilibrium (p<0.0001), 6 SNPs were excluded due to a low call rate (<95%), and 41 were monomorphic. For the 13 SNPs that did not pass the QC, data were abstracted for 4 SNPs from a previous genome-wide association study (GWAS) of prostate cancer among these subjects (8). This resulted in a final analysis set of 334 SNPs with an average genotyping success rate of 99.83% and average genotype concordance rate for QC duplicates (~2% of all samples) of 98.5%.
For replication in non-African American samples, the Taqman assay was used to genotype the top associated SNP (rs148371593) in 3,480 prostate cases and 3,447 controls of European, Hawaiian, Japanese, and Latino ancestry. We excluded subjects (n=37) that had no genotyping call, resulting in a total of 3,465 prostate cases and 3,425 controls. For non-African Americans, the average genotyping success rate was 99.5% and the genotype concordance rate for QC duplicates (~2% of all samples) was 99.3%.
Ancestry estimation
For African Americans, in order to correct for genetic ancestry, we included in the regression analysis the first two eigenvectors from principal component analysis of previously collected GWAS data (genomic inflation factor λ=1.08) (8). For non-African Americans, 93 ancestry informative markers that capture the major continental genetic diversity (9) were previously genotyped (10), principal components of ancestry were estimated by EIGENSTRAT (11), and the first four eigenvectors were included in the regression analysis.
Association testing
For African Americans, to test for the overall effect of 334 IGF1 SNPs together, we performed a logistic kernel-machine test, which is better powered than the omnibus test in the presence of correlation between SNPs (6). To examine single SNP association with prostate cancer, we conducted unconditional logistic regression, adjusting for age, family history and genetic ancestry. The p-values were estimated by a 1-degree of freedom Wald test for trend. To correct for multiple testing, we applied the false discovery rate (FDR) method and used a threshold of q < 0.1 to determine statistical significance (5). All analyses were run using the R software (7) with the packages lme4 for the regression models, GenABEL to compute the genomic inflation factor λ, and SKAT for the kernel-machine test (12). Manhattan and correlation plots were also done using the R software.
Results
The average ages for African American prostate cancer cases and controls were 69.3 and 69.8, respectively (Supplemental Table 1). Among all cases, 89.6% presented with localized disease and the remaining had regional/distant disease. Study characteristics for the non-African American subjects are presented in Supplemental Table 1.
Of the 334 IGF1 SNPs identified by sequencing African Americans, 8 SNPs were novel and not found in the 1000 Genomes (13) database (one was found in cases only with MAF 0.001, the remaining 7 SNPs had a MAF in controls ranging from 0.0005 – 0.021; Supplemental Tables 2 and 3) and 74 SNPs were rare with a MAF < 0.01 (Supplementary Tables S1 and S2). In a kernel-machine test, there was no overall cumulative effect of the 334 IGF1 SNPs on prostate cancer risk among African Americans (p-value=0.13). In single SNP analysis, 20 SNPs were nominally associated with prostate cancer risk at p-values< 0.05 (Figure 1 and Table 1). However, none were statistically significant (q<0.1) after performing FDR correction for multiple testing (5). The top association was with SNP rs148371593, located in the 3′ region of IGF1, for which the minor A allele (MAF=0.027) was found to have an inverse association with prostate cancer risk in African Americans (OR=0.83, 95% CI= 0.75,0.93; p=0.0014). SNP rs148371593 was weakly or not correlated (r2<0.45) with other SNPs in our set (Supplemental Figure 1). In a non-African American sample set of 3,465 prostate cases and 3,425 controls, only 7 heterozygotes for rs148371593 were observed—2 Hawaiian (one case and one control), and 5 of Latino ancestry (2 cases and 3 controls). The minor A allele was absent in European and Japanese ancestry populations and it was extremely rare in Latino and Native Hawaiian populations (MAF of 0.003 and 0.001, respectively). Combining Latinos and Native Hawaiians, no significant association was observed (OR=0.98; 95% CI=0.53,1.82; p=0.95; data not shown).
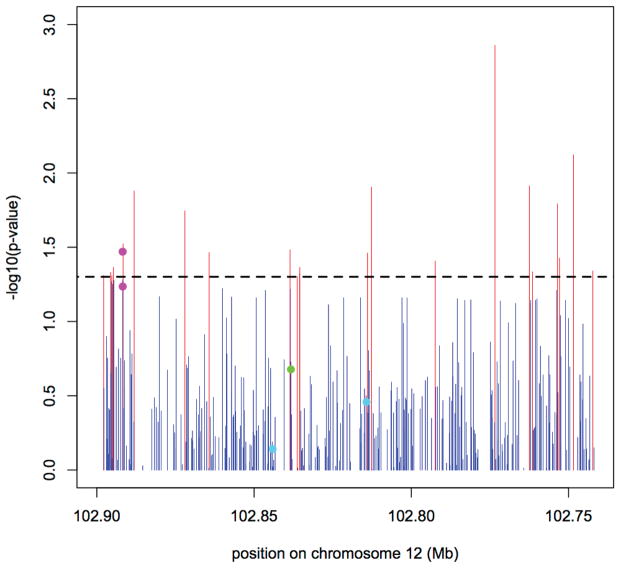
Figure 1. Association between IGF1 and prostate cancer risk among African Americans.
P-values below 0.05 are plotted in red. The two pink dots mark rs7978742 and rs7965399, respectively, the two variants originally reported by the MEC to be associated with prostate cancer in (2). The green dot marks variant rs7136446, which was found to be associated with prostate cancer risk and higher circulating levels of IGF-I (6, 14), and the two aquamarine dots mark variants, rs1520220 and 10735380, which were found to be associated with a higher IGF-I circulating levels and higher prostate cancer risk (16, 17).
Table 1.
Associations for 20 IGF1 SNPs and prostate cancer risk among African Americans at P<0.05
| SNP | positiona | MAb | MAFc | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| rs148371593 | 102773400 | A/G | 0.027 | 0.83 | (0.75,0.93) | 0.001 |
| chr12_102748461 | 102748461 | A/G | 0.001 | 1.43 | (1.10,1.90) | 0.008 |
| rs78360701 | 102762488 | A/G | 0.001 | 1.42 | (1.10,1.90) | 0.012 |
| rs5742687 | 102812676 | G/A | 0.001 | 1.42 | (1.10,1.90) | 0.012 |
| rs140149239 | 102888147 | A/C | 0.004 | 1.27 | (1.10,1.50) | 0.013 |
| rs140231439 | 102753563 | G/A | 0.009 | 1.18 | (1.00,1.40) | 0.016 |
| rs138668850 | 102871977 | A/C | 0.025 | 0.87 | (0.78,0.98) | 0.018 |
| rs7965399 | 102891686 | G/A | 0.267 | 1.04 | (1.00,1.10) | 0.030 |
| rs76845058 | 102838572 | G/C | 0.009 | 0.81 | (0.67,0.98) | 0.033 |
| rs146567847 | 102864270 | A/G | 0.009 | 1.17 | (1.00,1.30) | 0.034 |
| rs5742681 | 102813915 | G/C | 0.004 | 0.69 | (0.49,0.97) | 0.035 |
| rs73187920 | 102752878 | A/C | 0.046 | 0.92 | (0.84,0.99) | 0.037 |
| rs60741636 | 102792368 | C/G | 0.008 | 0.81 | (0.65,0.99) | 0.039 |
| rs77458341 | 102894716 | G/A | 0.272 | 1.04 | (1.00,1.10) | 0.043 |
| rs17878342 | 102835435 | G/A | 0.024 | 0.89 | (0.79,1.00) | 0.043 |
| rs35638230 | 102742243 | A/C | 0.012 | 1.14 | (1.00,1.30) | 0.046 |
| rs11111256 | 102761471 | A/G | 0.046 | 0.92 | (0.85,1.00) | 0.046 |
| rs61125097 | 102895637 | G/A | 0.271 | 1.04 | (1.00,1.10) | 0.047 |
| rs56060310 | 102835566 | G/A | 0.009 | 0.82 | (0.67,1.00) | 0.047 |
| rs11111285 | 102895256 | G/A | 0.271 | 1.04 | (1.00,1.10) | 0.049 |
Chromosomal location based on NCBI build 37/hg19
Major/Minor allele
Minor allele frequency among controls
Discussion
In this fine-mapping study of prostate cancer risk among African Americans, we examined 334 IGF1 variants—8 of which were novel—and observed no statistically significant association between IGF1 variants and prostate cancer risk after correcting for multiple testing. The top association, a less common SNP, rs148371593, did not replicate in non-African American samples, for which it was found to be rare among Latinos and Native Hawaiians and non-existent among Japanese and European Americans.
Numerous studies have indicated a possible involvement of IGF1 in prostate development and associated higher circulating levels of IGF-I with an increased risk of developing prostate cancer (2–5). In our previous haplotype-based study (2, 3, 6), we identified two upstream IGF1 variants associated with prostate cancer risk in the Multiethnic Cohort Study (2). These two variants, rs7978742 and rs7965399, were originally reported to be associated with overall prostate cancer risk (p-values = 0.003 for both) (2). In this study, with a larger sample size of African Americans, we observed nominal positive associations for these SNPs in African Americans (rs7978742; p=0.03 and rs7965399; p=0.05), which was in line with our previously reported pattern of associations.
Subsequent studies confirmed an association at the IGF1 locus and prostate cancer risk in populations of European ancestry, in particular with variants rs7136446 and rs2033178 (13), and rs4764695 (14, 15). We observed a marginal nominal association with rs7136446 (p=0.06) and could not confirm an association with the other two reported variants (p>0.05) in African Americans.
Of the two variants (rs6220 and rs7136446) reported significantly associated with higher levels of circulating IGF-I by Johansson et al. (6) rs6220 was not examined in this study, though one correlated SNP, rs5009837, (r2=0.91 among African Americans) was tested in our study and was not associated with prostate cancer risk (p=0.47). The second variant, rs7136446, showed a nominal borderline association with prostate cancer risk in our study (p-value = 0.06). Two additional SNPs, rs1520220 and rs10735380, previously associated with high circulating levels of IGF-I (16, 17) were not associated with prostate cancer risk in our study (p values > 0.05).
Several genome-wide association studies (GWAS) of prostate cancer have failed to observe an association with IGF1 and prostate cancer (18). The absence of an IGF1 association in GWAS of prostate cancer coupled with our findings here would suggest common IGF1 variants do not play a role in prostate cancer susceptibility.
Sufficient statistical power is a major challenge of fine-mapping studies that aim to identify less common and rare risk alleles. While our study was able to observe nominal associations with variants that have a MAF ranging from <0.01 to 0.27, reduced study power is a limitation of our study. For less common variants with a MAF = 0.03, we had 80% to detect odds ratios of 1.60 among our African American sample, while for more common variants with a MAF = 0.20, we had 80% power to detect smaller odds ratios of 1.24.
Despite these limitations, the identification of novel and less common variants may be useful in further investigating the difference in prevalence and risk of prostate cancer between African Americans and other racial/ethnic groups. Large study populations of diverse ancestral populations are needed to confirm associations for less common variants.
Supplementary Material
Acknowledgments
This work was supported by the V Scholar Award from the Jim Valvano Foundation for Cancer Research. E.E. Giorgi is supported by the Center for Nonlinear Studies, LANL, LDRD 201110434DR.
Footnotes
The authors declare no conflict of interest.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission
- 2.Cheng I, Stram DO, Penney KL, Pike M, Le Marchand L, Kolonel LN, et al. Common genetic variation in IGF1 and prostate cancer risk in the Multiethnic Cohort. J Natl Cancer Inst. 2006;98:123–34. doi: 10.1093/jnci/djj013. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher FR, Cheng I, Freedman ML, Mucci L, Allen NE, Pollak MN, et al. A comprehensive analysis of common IGF1, IGFBP1 and IGFBP3 genetic variation with prospective IGF-I and IGFBP-3 blood levels and prostate cancer risk among Caucasians. Hum Mol Genet. 2010;19:3089–101. doi: 10.1093/hmg/ddq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price AJ, Allen NE, Appleby PN, Crowe FL, Travis RC, Tipper SJ, et al. Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2012;21:1531–41. doi: 10.1158/1055-9965.EPI-12-0481-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149:461–71. W83–8. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson M, McKay JD, Wiklund F, Rinaldi S, Verheus M, van Gils CH, et al. Implications for prostate cancer of insulin-like growth factor-I (IGF-I) genetic variation and circulating IGF-I levels. J Clin Endocrinol Metab. 2007;92:4820–6. doi: 10.1210/jc.2007-0887. [DOI] [PubMed] [Google Scholar]
- 7.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–57. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43:570–3. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30:69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng I, Kocarnik JM, Dumitrescu L, Lindor NM, Chang-Claude J, Avery CL, et al. Pleiotropic effects of genetic risk variants for other cancers on colorectal cancer risk: PAGE, GECCO and CCFR consortia. Gut. 2013 doi: 10.1136/gutjnl-2013-305189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 12.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson M, McKay JD, Stattin P, Canzian F, Boillot C, Wiklund F, et al. Comprehensive evaluation of genetic variation in the IGF1 gene and risk of prostate cancer. Int J Cancer. 2007;120:539–42. doi: 10.1002/ijc.22344. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher F, Cheng I, Freedman ML, Mucci L, Allen N, Pollak MN, Hayes RB, Ma J. IGF Genetic Marker Associations with Prostate Cancer Risk and Plasma Levels. Approaches to Complex Pathways in Molecular Epidemiology. 2007 [Google Scholar]
- 16.Al-Zahrani A, Sandhu MS, Luben RN, Thompson D, Baynes C, Pooley KA, et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006;15:1–10. doi: 10.1093/hmg/ddi398. [DOI] [PubMed] [Google Scholar]
- 17.Gu F, Schumacher FR, Canzian F, Allen NE, Albanes D, Berg CD, et al. Eighteen insulin-like growth factor pathway genes, circulating levels of IGF-I and its binding protein, and risk of prostate and breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2877–87. doi: 10.1158/1055-9965.EPI-10-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.