Abstract
Background
The purpose of this study is to evaluate the clinical efficacy and safety of abraxane-based chemotherapy with/without nedaplatin in elderly patients with non-small-cell lung cancer (NSCLC).
Materials and methods
From October 2009 to January 2013, 48 elderly patients (≥65 years) with NSCLC were investigated in this clinical trial. The patients were randomized and equally allocated into arms A and AP: (A) abraxane (130 mg/m2, days 1, 8); (B) abraxane + nedaplatin (20 mg/m2 days 1-3, q3w). The parameters of objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS) and side effects were evaluated between two arms.
Results
Over 80% of the patients completed four cycles of chemotherapy. The total ORR was 21.3%, DCR was 55.3%, PFS 4.5 months and OS 12.6 months. No significant difference was found between arms A and AP in terms of ORR (16.7% vs. 26.1%, P=0.665) or DCR (55.3% vs. 56.5%, P=0.871). The median PFS in arm A was 3.3 months [25-75% confidence interval (CI): 3.1-7.2] and 5.5 months (25-75% CI: 3.2-7.0) in arm AP with no statistical significance (P=0.640). The median OS in arm A was 12.6 months (25-75% CI: 5.7-26.2) and 15.1 months (25-75% CI: 6.4-35.3) in arm AP with no statistical significance (P=0.770). The side effects were mainly grade 1-2. The incidence of grade 3-4 toxicities was 29.1% in arm A and 62.5% in arm AP with a statistical significance (P=0.020).
Conclusions
Compared with combined therapy, abraxane alone chemotherapy was beneficial for elderly NSCLC patients with better tolerability and less adverse events, whereas did not significantly differ in terms of ORR, DCR, PFS or OS.
Keywords: Nab-paclitaxel, advanced non-small-cell lung cancer (NSCLC), elderly, pretreated, efficacy
Introduction
Lung cancer is one of the major diseases threatening the lives and health of the elderly population. In China, the highest incidence of pulmonary cancer occurred in the population aged between 75 and 79 years. In 2009, nearly 60 million patients died of lung cancer, especially the elderly population (1). It is predicted that an aged population of over 300,000 will be newly diagnosed with lung cancer by 2020, making the elderly patients vulnerable to this disease (1).
Non-small-cell lung cancer (NSCLC) is the most common lung cancer in elderly patients. Upon diagnoses, most patients were already at advanced stage with a median survival of only 6 to 10 months (2). Currently, the main treatment options for elderly patients with advanced NSCLC are chemotherapy and targeted therapy. According to clinical guidelines (3), lung cancer patients with genetic mutation could be treated with first-line targeted therapy, besides the conventional second-line targeted therapy. The elderly patients are more suitable for monotherapy or platinum-based combination therapy (3). However, for the patients resistant to the epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy, or benefitting little from first-, second- or even third-line chemotherapy, a variety of chronic diseases may occur during combined chemotherapy.
Currently, few studies have been dedicated to the treatments for elderly patients with advanced NSCLC, and the development of new drugs for the treatment is of the great interest to the community. Abraxane is a novel, albumin-stabilized, 130-nanometer particle form of paclitaxel. It does not need synthetic solvents as a carrier, nor pretreatment of corticosteroids or antihistamines. The infusion time is brief (30 min). Experimental studies also showed that, compared to solvent-based paclitaxel, albumin-bound paclitaxel significantly enhanced anti-tumor activity with superior safety (4). Hence, in the present study, we conducted abraxane-based chemotherapy (with or without nedaplatin) to treat elderly patients with advanced NSCLC, aiming to evaluate its efficacy and safety.
Materials and methods
Patient selection
This study was conducted in strict accordance with Good Clinical Practice (GCP) requirements (Figure 1). Eligible patients with stage IIIB (with or without pleural effusion) or IV NSCLC were included (5). Inclusion criteria included aged ≥65 years; with measurable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) (6); Eastern Cooperative Oncology Group (ECOG) performance status (PS) score from 0 to 1; estimated lifetime of >3 months; resistant to EGFR-TKI therapy or first-, second- or even third-line chemotherapy; meeting the indicators for inspectional chemotherapy indications; no history of radiotherapy and chemotherapy in the previous 1 month or above. Patients were excluded from the study if having severe cardiovascular diseases; failing to tolerate chemotherapy; having mental disorders and psychiatric diseases; having symptomatic brain metastases; severe side effects or poor compliance.
Figure 1.
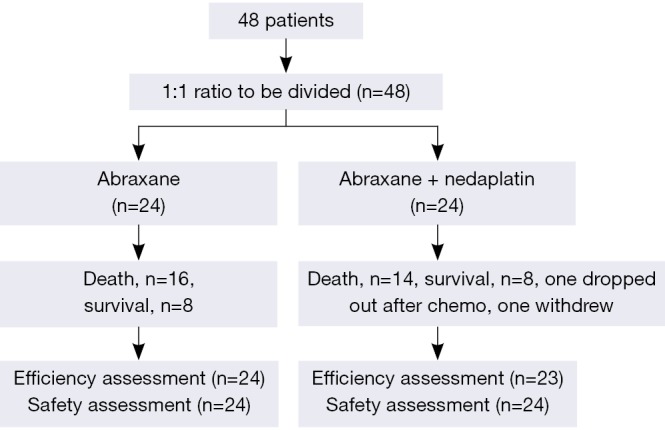
Criteria of patients’ selection.
Informed consents were obtained from each participant in this clinical trial. This study was approved by the ethnic committee of the first affiliated hospital of Guangzhou University of Chinese Medicine.
Treatment plan
The rationale of nedaplatin and nab-paclitaxel dosage chosen for this study referred to the treatment regime proposed by the clinical trials conducted by Tang and Li et al. (7,8). Arm A: abraxane 130 mg/m2 was administrated through intravenous infusion of 30 minutes on days 1 and 8. Arm AP: abraxane 130 mg/m2 was administrated through intravenous infusion of 30 minutes on days 1 and 8; nedaplatin 20 mg/m2 was administrated between days 1 and 3. Treatment cycles were repeated every 3 weeks. All participants were randomly divided into these two arms. Each patient received at least two cycles and followed every two cycles until death.
Measurements
The primary endpoint was objective response rate (ORR). The secondary endpoints of the study included disease control rate (DCR), progression-free survival (PFS), overall survival (OS) and safety. Tumors evaluated using RECIST (6), safety evaluation using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI CTCAE 3.0).
Statistical analysis
SPSS 19.0 statistical software was utilized to complete data analysis. The ORR and DCR within arms were compared by chi-square test and PFS and OS were analyzed by the Kaplan-Meier method. Statistical comparison between the two arms was conducted by log-rank test. The incidence of adverse reactions between the two arms was compared using Wilcoxon test. P<0.05 was considered as statistical significance.
Results
Patient characteristics
From October 2009 to January 2013, 48 patients diagnosed with NSCLC were enrolled and one patient in the AP group withdrew from this study after the first chemotherapy due to intolerable adverse events. Thus, the patient was merely included for adverse event analysis rather than intent to treat (ITT) analysis. The patients’ clinical data, such as age, gender, tumor node metastasis (TNM) stage, ECOG, EGFR, smoking status, etc. were illustrated in Table 1.
Table 1. Patient demographics and other baseline characteristics (n=48).
| Characteristics | All patients, n=48 | Arm A, n=24 | Arm AP, n=24 | P |
|---|---|---|---|---|
| Age, years | 0.510 | |||
| Mean [range] | 70.8 [65-80] | 71.0 [65-80] | 70.5 [66-79] | |
| Gender, n [%] | 0.917 | |||
| Male | 31 [64.6] | 16 [66.7] | 15 [62.5] | |
| Female | 17 [35.4] | 8 [33.3] | 9 [37.5] | |
| ECOG PS, n [%] | 0.401 | |||
| 0 | 17 [35.4] | 10 [41.7] | 7 [29.2] | |
| 1 | 31 [64.6] | 14 [58.3] | 17 [70.8] | |
| TNM stage, n [%] | 0.374 | |||
| IIIB | 13 [27.1] | 8 [33.3] | 5 [20.8] | |
| IV | 35 [72.9] | 16 [66.7] | 19 [79.2] | |
| Histology, n [%] | 0.471 | |||
| Adenocarcinoma | 32 [66.7] | 17 [70.8] | 15 [62.5] | |
| Squamous | 16 [33.3] | 7 [29.2] | 9 [37.5] | |
| Smoking status, n [%] | 0.474 | |||
| Yes | 27 [56.3] | 15 [62.5] | 12 [50.0] | |
| No | 21 [43.8] | 9 [37.5] | 12 [50.0] | |
| EGFR mutation, n [%] | 0.000 | |||
| EGFR (–) | 38 [79.2] | 18 [75.0] | 20 [83.3] | |
| EGFR (+) | 10 [20.8] | 6 [25.0] | 4 [16.7] | |
| Pretreated with nab-paclitaxel | 0.932 | |||
| Yes | 13 [27.1] | 6 [25.0] | 7 [29.2] | |
| No | 35 [72.9] | 18 [75.0] | 17 [70.8] | |
| Prior treatment | 1.000 | |||
| Radiotherapy | 7 [14.6] | 4 [16.7] | 3 [12.5] | |
| Targeted therapy | 18 [37.5] | 10 [41.7] | 8 [33.3] | |
| Chemotherapy | 42 [87.5] | 20 [83.3] | 22 [91.7] | |
| Median treatment cycle | 4 [1-8] | 4 [2-8] | 4 [1-6] | 1.000 |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; TNM, tumor node metastasis; EGFR, epidermal growth factor receptor.
ORR and DCR
Based on the assessment of clinical efficacy, partial response (PR) was obtained from a total of 10 subjects (21.3%) and no complete response (CR) was found. The results showed that ORR was 21.3% [95% confidence interval (CI): 9.58-32.98%] and DCR was 55.3% (95% CI: 41.11-69.53%). In arm A, PR was assessed from four subjects (16.7%; 95% CI: 1.76-31.58%). Nine had stable disease (SD) with a DCR of 54.2% (95% CI: 34.23-74.10%). In arm AP, PR was obtained from six patients (26.1%; 95% CI: 8.14-44.03%). Seven had SD with a DCR of 56.5% (95% CI: 36.26-76.78%). No significant difference was observed between the two arms in terms of PR (Tables 2,3).
Table 2. Best overall response (n=47).
| Arm, n (%) | PR, n (%) | SD, n (%) | PD, n (%) | ORR (CR+PR), n (%) | DCR (CR+PR+SD), n (%) |
|---|---|---|---|---|---|
| Total, n=47 | 10 (21.3) | 16 (34.0) | 21 (44.7) | 10 (21.3) | 26 (55.3) |
| Arm A, n=24 | 4 (16.7) | 9 (37.5) | 11 (45.8) | 4 (16.7) | 13 (54.2) |
| Arm AP, n=23 | 6 (26.1) | 7 (30.4) | 10 (43.5) | 6 (26.1)* | 13 (56.5)§ |
*, ORR between arms A and AP by χ2 test was χ2=0.187, P=0.665, no significant difference between the two arms; §, DCR between arms A and AP by χ2 test was χ2=0.026, P=0.871, no significant difference between the two arms. PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; CR, complete response; DCR, disease control rate.
Table 3. Sub-arm analysis (n=47).
| Arm, n (%) | PR, n (%) | SD, n (%) | PD, n (%) | ORR (CR+PR), n (%) | DCR (CR+PR+SD), n (%) |
|---|---|---|---|---|---|
| Arm A | |||||
| Pathological examination | |||||
| Adenocarcinoma (n=17) | 2 (11.8) | 7 (41.2) | 8 (47.1) | 2 (11.8) | 9 (52.9) |
| Squamous carcinoma (n=7) | 2 (28.6) | 2 (28.6) | 3 (42.9) | 2 (28.6)△ | 4 (57.1)▽ |
| Previous injection of paclitaxel | |||||
| Yes (n=6) | 2 (33.3) | 1 (16.7) | 3 (50.0) | 2 (33.3) | 3 (50.0) |
| No (n=18) | 2 (11.1) | 8 (44.4) | 8 (44.4) | 2 (11.1)# | 10 (55.6)※ |
| Arm AP | |||||
| Pathological examination | |||||
| Adenocarcinoma (n=14) | 5 (35.7) | 3 (21.4) | 6 (42.9) | 5 (35.7) | 8 (57.1) |
| Squamous carcinoma (n=9) | 1 (11.1) | 4 (44.4) | 4 (44.4) | 1 (11.1)□ | 5 (55.6)◆ |
| Previous injection of paclitaxel | |||||
| Yes (n=6) | 1 (16.7) | 2 (33.3) | 3 (50.0) | 1 (16.7) | 3 (50.0) |
| No (n=17) | 5 (29.4) | 5 (29.4) | 7 (41.2) | 5 (29.4)◻ | 10 (58.8)■ |
A sub-arm: △, ORR in squamous carcinoma and non-squamous carcinoma by Fisher’s exact test, P=0.552, no significant difference between the two arms; ▽, DCR in squamous carcinoma and non-squamous carcinoma by Fisher’s exact test was P=1.000, no significant difference between the two arms; #, ORR with or without the application of paclitaxel by Fisher’s exact test was P=0.251, no significant difference between the two arms; ※, DCR with or without the application of paclitaxel by Fisher’s exact test was P=1.000, no significant difference between the two arms; AP sub-arm: □, ORR in squamous carcinoma and non-squamous carcinoma by Fisher’s exact test, P=0.34, no significant difference between the two arms; ◆, DCR in squamous carcinoma and non-squamous carcinoma by Fisher’s exact test was P=1.000, no significant difference between the two arms; ◻, ORR with or without the application of paclitaxel by Fisher’s exact test was P=1.000, no significant difference between the two arms; ■, DCR with or without the application of paclitaxel by Fisher’s exact test was P=1.000, no significant difference between the two arms. PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; CR, complete response; DCR, disease control rate.
Progression-free survival (PFS)
In all 48 patients, the median PFS was 4.5 months (95% CI: 2.29-6.71). In details, the median PFS in arm A was 3.3 months (25-75% CI: 3.1-7.2) and 5.5 months (25-75% CI: 3.2-7.0) in arm AP with no significant difference between two arms (P=0.738), as illustrated in Figure 2.
Figure 2.
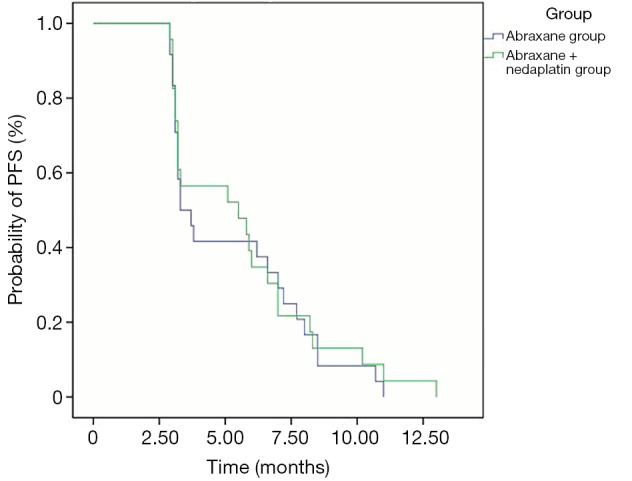
Progression-free survival (PFS) curves between two arms.
Overall survival (OS)
During subsequent follow-up, a total of 30 patients (65.2%) died, including 16 cases in arm A and 14 in arm AP. The overall median OS was 12.6 months (25-75% CI: 5.9-26.2). In arm A, the median OS was 12.6 months (25-75% CI: 5.7-26.2) and 15.1 months (25-75% CI: 6.4-35.3) in arm AP with no statistical significance (P=0.770) (Figure 3).
Figure 3.
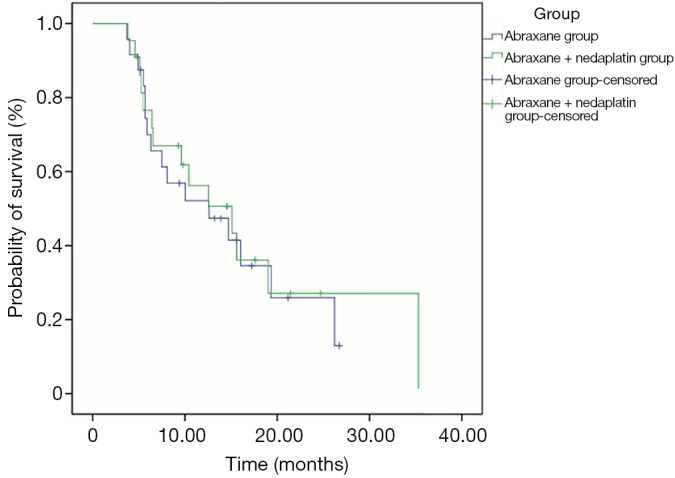
Overall survival (OS) curves between two arms.
Safety evaluation
Throughout this study, 80% of patients completed four cycles of chemotherapy (range: 1-8 cycles). One patient presented with grade IV hemoglobin (HGB) decline and required blood transfusion. The patient eventually decided to withdraw from this clinical trial. No treatment-related death was reported yet.
Adverse reactions were assessed based on hematologic toxicity. The following adverse reactions had an incidence of >10%, including anemia, neutropenia and thrombocytopenia syndrome (Table 4). Most of the adverse reactions were graded as 1-2 toxicities. Grade 3-4 toxicities included leucocyte, neutropenia, anemia, fatigue and joint pain. The incidence of neutropenia in arm AP was significantly higher than that in arm A (62.5% vs. 29.1%, P=0.020). For those non-hematologic reactions, the signs of alopecia, peripheral nerve numbness, joint pain, fatigue, myalgia, nausea and vomiting, etc., were equally observed. The incidence of neurotoxicity in arm A was 8.3% (n=3) and 25.0% (n=6) in arm AP. The incidence of fatigue in arm A was 4.2% (1) and 16.7% (Greene et al., 2002) in arm AP. The incidence of nausea and vomiting in arm A was 4.2% (1) and 16.7% (5) in arm AP. Rank sum test revealed no statistical significance in terms of the adverse reactions between the two arms.
Table 4. Percentage of patients with treatment-related toxicities/adverse events (reported in ≥10% of patients) (n=48).
| Toxicities/adverse event | A arm, n=24 |
AP arm, n=24 |
P# | |||
|---|---|---|---|---|---|---|
| Grade 3*, n (%) | Grade 4*, n (%) | Grade 3*, n (%) | Grade 4*, n (%) | |||
| Hematologic | ||||||
| Decrease in hemoglobin | 2 (8.3) | 0 | 5 (20.8) | 0 | 0.225 | |
| Neutropenia | 5 (20.8) | 2 (8.3) | 9 (37.5) | 6 (25.0) | 0.020 | |
| Thrombocytopenia | 2 (8.3) | 1 (4.2) | 7 (29.2) | 1 (4.2) | 0.105 | |
| Non-hematologic | ||||||
| Peripheral neurotoxicity | 3 (12.5) | 0 | 6 (25.0) | 0 | 0.589 | |
| Muscle pain | 0 | 0 | 0 | 0 | – | |
| Joint pain | 0 | 0 | 0 | 0 | – | |
| Fatigue | 1 (4.2) | 0 | 4 (16.7) | 0 | 0.161 | |
| Nausea and vomiting | 1 (4.2) | 0 | 4 (16.7) | 0 | 0.145 | |
| Alopecia | 0 | 0 | 0 | 0 | – | |
*, If a patient reported the same toxicity more than one incidence, the patient was counted only for that toxicity, but with the highest grade; #, exact P values were obtained by Wilcoxon rank-sum test.
Discussion
It is an extremely challenging task to treat advanced NSCLC in elderly patients. Few clinical studies had been dedicated to providing better care for elderly patients with advanced NSCLC due to the deteriorated physical functions and various clinical complications.
Previous studies have demonstrated in the feasibility and efficacy of nedaplatin in treatment of pulmonary cancer. Tang et al. statistically compared the clinical efficacy of S-1 plus nedaplatin and the standard second-line chemotherapy in EGFR-negative lung adenocarcinoma after failure of first-line chemotherapy in 179 patients and proved that the combination of S-1 and nedaplatin was well tolerated and making this technique as a potentially strong candidate for the treatment of advanced non-small-cell lung adenocarcinoma (7). Li et al. conducted a retrospective, randomized, control study including 619 NSCLC patients and observe the short-term efficacy, long-term survival and adverse responses with nedaplatin or cisplatin concomitant with other chemotherapy in treating NSCLC. The results revealed that nedaplatin concomitant with other chemotherapy is effective for treating NSCLC with higher clinical efficacy than cisplatin combined with chemotherapy (8).
Paclitaxel is the third-generation of cancer chemotherapy drug, recommended for first- or second-line treatment. In the first-line treatment for elderly patients, weekly injection of paclitaxel can achieve an efficiency rate between 23% and 37.5% (9-11). The combined therapy of paclitaxel and carboplatin can yield an efficiency rate of 19% to 55% (12-15). The results in our study demonstrated that, in combination with platinum (carboplatin or cisplatin), the treatment of paclitaxel for elderly patients with advanced NSCLC had better efficacy rate in overall performances of the patients. However, the excipient of polyoxyethylene castor oil can cause allergic reactions, needing steroid pretreatment, thus limiting the usage of paclitaxel (16,17). Abraxane is a new formulation of paclitaxel, which causes mild allergic reactions, requires short infusion time, and may benefit the patients with NSCLC. Previous study showed that abraxane monotherapy per week achieved the efficiency rate of 30%, PFS of 5 months and OS of 11 months in patients with stage IV NSCLC (18). In addition, abraxane monotherapy for 3 weeks yielded an ORR of 16% and a median survival of 11 months (4). In combination with carboplatin as the first-line treatment for phase III lung cancer patients, weekly abraxane regimen achieved the ORR of 33% and PFS of 6.3 months, significantly better than the outcomes of monotherapy of paclitaxel injection (19).
In the current study, 48 patients, who previously received first- or second- or even third-line treatments, were administrated with abraxane-based chemotherapy and achieved an ORR of 21.3%, DCR of 55.3%, PFS of 4.5 months and OS of 12.6 months. The adverse reactions were mainly hematologic toxicities including grade 3/4 neutropenia syndrome (45.8%), thrombocytopenia (22.9%), anemia (14.6%) and peripheral nerve toxicity (18.8%). No allergic reactions were observed and the tolerance rate was acceptable. The ORR or DCR did not significantly differ between arms A and AP, suggesting that no cross-resistance was observed between abraxane and paclitaxel. It may be explained by the structural characteristics of abraxane, as it utilizes the natural biological properties of albumin through the gp-60-mediated endothelial cell membrane transport (20), and its interaction between albumin binding protein SPARC (an acidic cysteine-rich secretory protein) to increase uptake and accumulation (21).
To sum up, the two-drug combination therapy did not significantly improve efficacy for elderly patients with advanced NSCLC, as compared to mono-chemotherapy. However, due to the incidence of serious adverse reactions and various chronic diseases after receiving combined therapy, mono-chemotherapy may be a better option for elderly patients with advanced NSCLC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [DOI] [PubMed] [Google Scholar]
- 2.Vamvakas L, Saloustros E, Karampeazis A, et al. Advanced non-small-cell lung cancer in the elderly. Clin Lung Cancer 2009;10:158-67. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology. Lung Cancer Screening version 1, 2013. Available online: http://www.respiratory-thessaly.gr/assets/lung_screening%201.2013.pdf
- 4.Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol 2006;17:1263-8. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, et al. eds. AJCC Cancer Staging Manual 7th Edition. New York: Springer, 2010:253-70. [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y, Wang W, Teng XZ, et al. Efficacy of S-1 plus nedaplatin compared to standard second-line chemotherapy in EGFR-negative lung adenocarcinoma after failure of first-line chemotherapy. Tumour Biol 2014;35:8945-51. [DOI] [PubMed] [Google Scholar]
- 8.Li CH, Liu MY, Liu W, et al. Randomized control study of nedaplatin or cisplatin concomitant with other chemotherapy in the treatment of advanced non-small cell lung cancer. Asian Pac J Cancer Prev 2014;15:731-6. [DOI] [PubMed] [Google Scholar]
- 9.Fidias P, Supko JG, Martins R, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res 2001;7:3942-9. [PubMed] [Google Scholar]
- 10.Rossi D, Dennetta D, Ugolini M, et al. Weekly paclitaxel in elderly patients (aged > or = 70 years) with advanced non-small-cell lung cancer: an alternative choice? Results of a phase II study. Clin Lung Cancer 2008;9:280-4. [DOI] [PubMed] [Google Scholar]
- 11.Bauman JE, Eaton KD, Wallace SG, et al. A Phase II study of pulse dose imatinib mesylate and weekly paclitaxel in patients aged 70 and over with advanced non-small cell lung cancer. BMC Cancer 2012;12:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto I, Moriyama E, Fujii S, et al. Phase II study of carboplatin-paclitaxel combination chemotherapy in elderly patients with advanced non-small cell lung cancer. Jpn J Clin Oncol 2005;35:188-94. [DOI] [PubMed] [Google Scholar]
- 13.Giorgio CG, Pappalardo A, Russo A, et al. A phase II study of carboplatin and paclitaxel as first line chemotherapy in elderly patients with advanced non-small cell lung cancer (NSCLC). Lung Cancer 2006;51:357-62. [DOI] [PubMed] [Google Scholar]
- 14.Ramalingam S, Perry MC, La Rocca RV, et al. Comparison of outcomes for elderly patients treated with weekly paclitaxel in combination with carboplatin versus the standard 3-weekly paclitaxel and carboplatin for advanced nonsmall cell lung cancer. Cancer 2008;113:542-6. [DOI] [PubMed] [Google Scholar]
- 15.Sakakibara T, Inoue A, Sugawara S, et al. Randomized phase II trial of weekly paclitaxel combined with carboplatin versus standard paclitaxel combined with carboplatin for elderly patients with advanced non-small-cell lung cancer. Ann Oncol 2010;21:795-9. [DOI] [PubMed] [Google Scholar]
- 16.Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001;37:1590-8. [DOI] [PubMed] [Google Scholar]
- 17.Uziely B, Jeffers S, Muggia F.Low doses of dexamethasone protect against paclitaxel (Taxol)-related hypersensitivity reactions following cycle 1. Ann Oncol 1994;5:474. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi NA, Riely GJ, Azzoli CG, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol 2008;26:639-43. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [DOI] [PubMed] [Google Scholar]
- 20.John TA, Vogel SM, Tiruppathi C, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol 2003;284:L187-96. [DOI] [PubMed] [Google Scholar]
- 21.Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol Biol Cell 2003;14:3977-88. [DOI] [PMC free article] [PubMed] [Google Scholar]


