Abstract
Background
A number of studies have examined human epidermal growth factor receptor 2 (HER2) status in primary gastric cancer (GC) and associated metastasis, some showed their great concordance in HER2 expression, but others demonstrated notable discordance. There is still little consensus on HER2 discordance, therefore, a systematic review and meta-analysis was conducted to assess the status on HER2 discordance between primary GC and its paired metastasis.
Methods
PubMed, EMBASE, ASCO and The Cochrane Library were searched for studies that explored the concordance between primary tumor and metastasis in patients with GC up to 10 March, 2014. Data of discordance of HER2 between primary GC and corresponding metastasis were extracted from the publications and random-effects models were used to estimate pooled discordance proportions.
Results
Eighteen articles including 1,867 patients were included for the meta-analysis in accordance with the selection criteria. Pooled discordance proportions were 7% [95% confidence interval (CI): 5-10%] for HER2 status. Pooled proportions of tumors shifting from positive to negative and from negative to positive were 17% (95% CI: 7-29%) and 4% (95% CI: 2-6%) respectively. No publication bias was found in the meta-analysis.
Conclusions
The discordance of HER2 status is not rare in primary and metastatic GC through our meta-analysis. Prospective studies are needed to testify the clinical significance of the discordance of HER2 status.
Keywords: Gastric cancer (GC), human epidermal growth factor receptor 2 (HER2), discordance, meta-analysis
Introduction
Gastric cancer (GC) keeps being one of the leading types of cancers worldwide. Based on the statistic estimate, there are still about one million new cases and over 700,000 deaths each year (1). Human epidermal growth factor receptor 2 (erbB2, or HER2/neu, or HER2), a proto-oncogene, is an important prognostic biomarker and key driver of tumorigenesis in GC (2). According to a great number of studies, HER2 positivity is detected in 7% to 34% of patients in GC (3-6). The results of the phase III ToGA (trastuzumab for gastric cancer) trials (7), indicated that trastuzumab plus chemotherapy became the standard regimen for patients with HER2 positive advanced GC. Therefore, it is now recommended that HER2 status should be evaluated in every GC patient eligible for trastuzumab treatment for most effective therapy. HER2 status was recommended to be tested by immunohistochemistry (IHC) first, followed by fluorescence in situ hybridization (FISH) for IHC 2+ patients, who was considered as cases with equivocal expression (8).
Probably since the tissues in metastatic sites are rarely removed or biopsied before treatment, HER2 status is generally evaluated in the primary lesions only, although trastuzumab-based therapy is used to treat metastatic disease. Several studies reported HER2 status in primary GC and associated metastasis, the discordance rate marked among these studies (9-12). Based on these disparate results and different opinions, arguments for or against HER2 testing of metastasis have therefore been aroused. Does the HER2 discordance come from the tumor-related behavior or testing error? As yet, little consensus has been reached on this controversy. Therefore, we performed a systematic review and meta-analysis to evaluate the discordance rate of HER2 status between primary tumor and corresponding metastasis and its clinical significance of treatment.
Methods
Search strategy and selection criteria
A systematic review of published work was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines. Electronic searches was performed to search the literatures reporting HER2 status in primary GC and its paired metastasis in PubMed, EMBASE, ASCO and The Cochrane Library using the combined text words ‘gastric’ or ‘stomach’ or ‘gastroesophageal’ and ‘cancer’ or ‘tumor’ or ‘neoplasm’ or ‘carcinoma’ and ‘HER2’ or ‘HER-2’ or ‘ERBB2’ or ‘ERBB-2’ or ‘c-erbb-2’ or ‘cerbb2’ or ‘neu’. The latest search was undertaken in 10 March, 2014. We also manually screened the reference lists of the retrieved articles to identify other relevant publications.
Studies which met the following criteria were eligible for this meta-analysis: (I) report HER2 status in primary GC and its paired metastasis with IHC or FISH or chromogenic in situ hybridization (CISH) or silver in situ hybridization (SISH) and etc.; (II) provide data of discordance rate of HER2 status (primary vs. metastasis), or the data can be derived from reported results. When there was more than one article using the overlapped populations, the latest publication was selected. Review articles, case reports, circulating tumor cells studies and studies that did not report needed data were excluded.
Data extraction
Data was extracted by two investigators (Zhi Peng and Jianling Zou) independently using a standard protocol. Any discrepancies were resolved by discussion and consensus was reached finally. The following data elements were extracted from each study: first author, year of publication, time of collection, race or country, number of patients (male/female), tumor stage, type and site of relapse, technique to detect HER2 status, corresponding scoring criteria, HER2 discordance rate, the prevalence shifting from positive to negative and vice versa.
Statistical analysis
The discordance proportion of HER2 between primary GC and metastasis with 95% confidence intervals (CIs) was calculated for each study. The Freeman-Tukey double arcsine transformation was used for the calculation of pooled estimates and corresponding 95% CIs (13). The chi-square test of homogeneity was performed to detect statistical heterogeneity (14). The Mantel-Haenszel fixed effect model was adopted if no significant difference was found in the homogeneity test. Otherwise, the DerSimonian-Laird random-effect model (D-Lmethod) was adopted for calculations.
In our study, subgroup analyses were conducted according to race, technique of detecting HER2 status, type and time of metastasis, type of sampling respectively. The quality of each study included in the meta-analysis was independently assessed by two reviewers (Zhi Peng and Jianling Zou) according to the Newcastle-Ottawa scale (NOS) (15). Studies that received more than six stars were considered as high quality, analogous to other reviews. We specifically classified studies at elevated risk of bias (1-3 stars), intermediate risk of bias (4-5 stars), or low risk of bias (6-9 stars). Any disagreements in studies’ quality assessment were resolved by a consensus discussion between the two reviewers. Furthermore, Publication bias was evaluated using funnel plots and the asymmetry test developed by Egger and colleagues (16). All analyses were carried out with the R software (http://cran.r-project.org/) with package ‘meta’. All the reported P values were two sided.
Results
Study characteristics
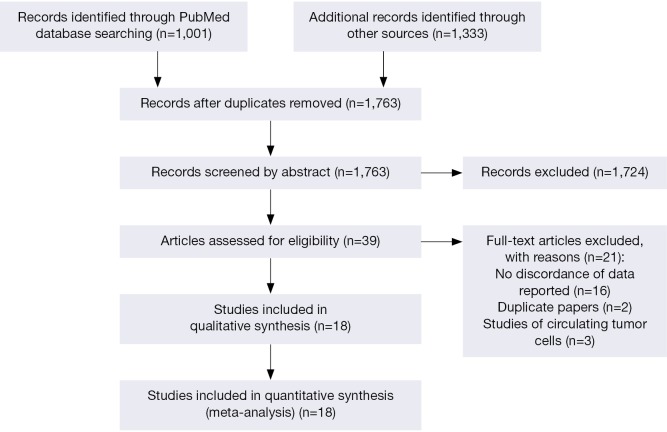
Totally 1,763 articles were identified from electronic databases, of which 39 studies were potentially related to our issue. Finally, eighteen articles (9-12,17-30) were included for the meta-analysis in accordance with the selection criteria (Figure 1), which provided paired HER2 data for primary and metastasis tumor in 1,867 subjects, ranging from 34 to 250 patients per study. Among the 18 studies, 16 studies offered the full texts and the 2 studies (11,21) offered the abstracts with discordance rate between primary GC and the metastasis disease. Among the 14 studies with full texts, 2 were in Chinese with English abstracts (23,28).
Figure 1.
Flow chart of the eligible studies. Flow chart of the eligible studies for the meta-analysis of HER2 status in paired primary gastric cancer and metastasis.
Their main characteristics including year of publication, race or country, number of subjects, type of metastasis and sampling, time of metastasis, corresponding techniques are reported in Table 1. All studies were retrospective. Nine (9,11,20-22,25-27,30) of the 18 studies (1,186 patients, 63.5%) were performed in Asian populations, and the remaining 9 (10,12,17-19,23,24,28,29) studies (681 patients, 36.5%) were in Western populations. The techniques used in the studies included IHC, ISH, southern blot hybridization technique and (or) the combination of the two or three. The tissues were mainly collected from surgery, while there were still some with the mixed methods of surgery and biopsy, and Pigni et al. (30) reported the study used the both methods for all the patients. Most of the metastatic tumors are synchronous loco-reginal lymph nodes, whereas there were still several studies reporting the cases with synchronous or metachronous distant metastasis (22,25,30), and they mainly located in liver, abdominal wall, intestine, uterus and so on.
Table 1. Main characteristics of studies included in meta-analysis.
| First author | Year of publication | Time frame | Race or country | No. of subjects (male/female) | Median age [range age] | Regional or distant metastasis | Synchronous or metachronous metastasis | Type of sampling | Techniques to test HER2 status |
|---|---|---|---|---|---|---|---|---|---|
| Chariyalertsak S | 1994 | 1990-1991 | Thailand | 97 (NR) | NR [NR] | Regional | Synchronous | Surgery or biopsy | IHC |
| Kim JS | 1997 | 1992-1993 | Korea | 45 (NR) | NR [NR] | Regional | Synchronous | Surgery | Southern blot |
| Kim JH | 2009 | 1995-2003 | Korea | 222 (NR) | 55.4 [NR] | Regional | Synchronous | Surgery | IHC |
| Marx AH | 2009 | NR | Germany | 51 (NR) | NR | Regional | Synchronous | Surgery | IHC and FISH |
| Kim JY | 2010 | NR | Caucasian | 193 (NR) | NR [NR] | Regional | Synchronous | Surgery | IHC and FISH |
| Kim MA | 2011 | 1990-2006 | Korea | 250 (NR) | NR | Regional and distant | Synchronous or metachronous | Surgery or biopsy | IHC and FISH |
| Geng YT | 2011 | 2003-2005 | China | 110 (79/31) | 63.5 [28-85] | Regional | Synchronous | Surgery | IHC |
| Bozzetti C | 2011 | NR | Italy | 39 (NR) | NR [40-88] | Regional or distant | Synchronous and metachronous | Surgery or biopsy | IHC and FISH |
| Zhang YL | 2012 | 2010-2011 | China | 112 (NR) | 59 [30-79] | Regional | Synchronous | Surgery | IHC and FISH |
| Tsapralis D | 2012 | 2004-2007 | Athens | 45 (NR) | NR [NR] | Regional | Synchronous | Surgery | IHC and CISH |
| Fassan M | 2012 | 2000-2003 | Italy | 47 (38/9) | 67.9 [49-87] | Regional | Synchronous | Surgery | IHC and CISH |
| Asioli S | 2012 | NR | Italy | 60 (NR) | NR [NR] | Regional or distant | Synchronous | Surgery | IHC |
| Shinozaki E | 2013 | NR | Caucasian | 58 (45/13) | NR [NR] | Distant | Synchronous and metachronous | Surgery | IHC |
| Pagni F | 2013 | 2008-2011 | Italy | 34 (16/18) | 68.5 [NR] | Regional or distant | Synchronous | Surgery and biopsy | IHC and FISH |
| Kochi M | 2013 | 1990-2010 | Japan | 102 (82/20) | 68 [29-86] | Regional | Synchronous | Surgery | IHC and FISH |
| Fusco N | 2013 | 1996-2008 | Italy | 154 (NR) | NR [NR] | Regional | Synchronous | Surgery | IHC |
| Cho EY | 2013 | 2000-2004 | Korea | 138 (NR) | NR [NR] | Distant | Synchronous and metachronous | Surgery | IHC and SISH |
| Geng Y | 2014 | 2003-2005 | China | 110 (79/31) | 63.5 [28-85] | Regional | Synchronous | Surgery | IHC |
HER2, human epidermal growth factor receptor 2; NR, not reported; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; CISH, chromogenic in situ hybridization; SISH, silver in situ hybridization.
Meta-analysis of HER2 discordance between primary tumor and metastasis
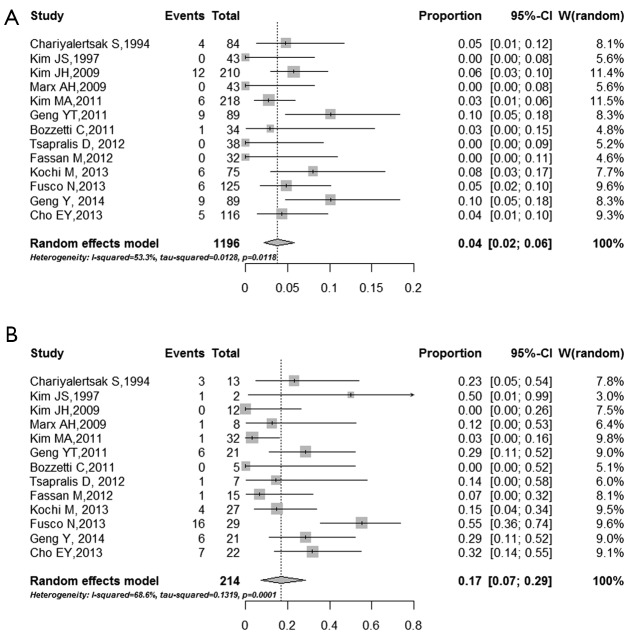
Among all the studies, the discordance rates between primary and metastasis tumor ranged from 2% to 24% (I2 =72.2%, τ2 =0.03, P<0.0001), and the pooled estimate of the proportion of the total studies was 7% (95% CI: 5-10%) (Figure 2). The proportions of patients whose HER2 status changed from positive to negative and from negative to positive were also conducted. The pooled proportion of negative conversion was 17% (95% CI: 7-29%) (Figure 3A), and the positive conversion was 4% (95% CI: 2-6%) (Figure 3B). The results reached significantly statistical difference (P<0.001).
Figure 2.
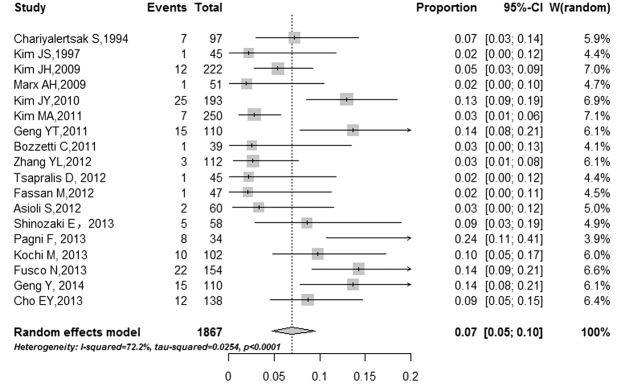
Forest plot for proportion of discordance HER2 status. Pooled estimate for HER2 discordant in studies reporting HER2 status in primary gastric cancer and paired metastasis. Weights are from random-effects analysis. Squares indicate the point estimates of the effect of study (proportion of discordance) and diamonds, the summary estimate from the pooled studies; 95% confidence intervals are indicated by horizontal bars and shown in square brackets.
Figure 3.
Forest plot for proportion of negative and positive conversion of HER2 status. The pooled proportions of patients whose HER2 status changed from positive to negative (A) and from negative to positive (B). Weights are from random-effects analysis. Squares indicate the point estimates of the effect of study (proportion of discordance) and diamonds, the summary estimate from the pooled studies; 95% confidence intervals are indicated by horizontal bars and shown in brackets.
Stratified analysis was conducted according to the technique used to define HER2 status, site of relapse, the type to sampling and the time of metastasis. As it is shown in the Table 2, the pooled discordance proportion was 8% (95% CI: 6-11%) in studies using IHC alone, and 2% (95% CI: 1-5%) with studies using ISH alone, and 15% (95% CI: 4-31%) with studies using IHC and ISH. When it is about the site of relapse, the pooled discordance proportion was 7% (95% CI: 5-10%) for loco-regional lymph nodes only, and 3% (95% CI: 0-9%) for distant relapse only, and 7% (95% CI: 0-21%) for loco-regional lymph nodes or distant relapse. Different pooled discordance proportion was found when the time to metastasize was taken into account. When the relapse was synchronous to the primary cancer, the pooled discordance proportion was 7% (95% CI: 5-10%), and when asynchronous, the pooled discordance proportion was 0% (95% CI: 0-3%), and when mixed with synchronous and asynchronous, the pooled discordance proportion was 7% (95% CI: 4-11%). While the different pooled discordance of type of sampling was also analyzed, the pooled discordance proportion was 7% (95% CI: 5-10%) when the sampling was through surgery only, and 26% (95% CI: 13-43%) when the sampling was through biopsy only, and 4% (95% CI: 1-7%) when the studies was mixed with surgery and biopsy sampling. Influence analysis showed that removal any research would not cause a significant change in the results.
Table 2. Subgroup analysis about HER2 discordant proportion.
| Type of subgroups (No. of studies providing data for analysis, No. of subjects) | HER2 discordant% (95% CI) |
|---|---|
| Different race | |
| Asian population (9 studies, 1,186 subjects) | 0.07 (0.03-0.12) |
| Western population (9 studies, 681 subjects) | 0.07 (0.04-0.10) |
| Test used to determine HER2 status | |
| Immunohistochemistry (IHC) (16 studies, 1,788 subjects) | 0.08 (0.06-0.11) |
| In situ hybridization (ISH) (8 studies, 902 subjects) | 0.02 (0.01-0.05) |
| Immunohistochemistry (IHC)/in situ hybridization (IHC/ISH) (2 studies, 136 subjects) | 0.15 (0.04-0.31) |
| Type of metastasis included in paired testing of primary cancer and metastasis | |
| Studies of loco-regional lymph nodes only (15 studies, 1,548 subjects) | 0.07 (0.05-0.1) |
| Studies of distant metastasis only (3 studies, 225 subjects) | 0.03 (0.00-0.09) |
| Studies of loco-regional lymph nodes and distant metastasis (3 studies, 162 subjects) | 0.07 (0.00-0.21) |
| Metastasis synchronous or asynchronous to primary cancer | |
| Studies of synchronous metastasis (15 studies, 1,571 subjects) | 0.07 (0.05-0.1) |
| Studies of asynchronous metastasis (1 study, 61 subjects) | 0.00 (0.00-0.03) |
| Studies of synchronous or asynchronous metastasis (3 studies, 235 subjects) | 0.07 (0.04-0.11) |
| Type of sampling | |
| Surgery only (15 studies, 1,481 subjects) | 0.07 (0.05-0.1) |
| Biopsy only (1 study, 34 subjects) | 0.26 (0.13-0.43) |
| Surgery or biopsy (3 studies, 386 subjects) | 0.04 (0.01-0.07) |
HER2, human epidermal growth factor receptor 2.
Risk of bias
In this review, we assessed the studies’ quality according to the NOS guideline. Overall, 12 studies were assessed to be high-quality and 6 medium-quality; no studies was deemed to be low-quality (Table S1). For publication bias estimating, no visually and statistically significant asymmetry was found according to the inverted funnel plot (Figure 4) and Egger’s test in all analyses (P=0.835), which means no publication bias was found between studies. We also tested the publication bias among the subgroups and no significant publication bias was observed either.
Figure 4.
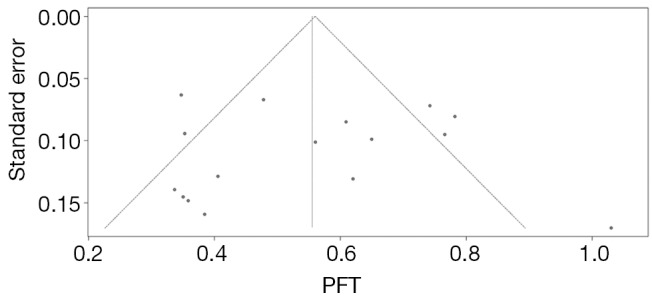
Funnel plots for the publication bias estimating. The dashed line represents 95% confidence intervals. Circles represent individual studies.
Discussion
The discordance of HER2 status between primary GC and metastasis and its clinical implications are still in debate. Accurate detection of HER2 will provide important information for determining whether trastuzumab therapy is needed. In this study, we synthesized the published data on HER2 status discordance between paired primary GC and corresponding metastasis. Our results showed that the rates of HER2 discordance were 7% (95% CI: 5-10%).
The choice of systemic therapy in advanced GC is dependent on the appropriate targeting of HER2 receptor. The HER2 status of metastatic disease is usually assumed to be the same as that of the primary tumor, but there is evidence for discordance in receptor status between primary and metastatic tumor. In Japan, HER2 testing should be routinely performed in patients with metastatic or recurrent GC, which recommended by the Japanese Society of Pathology. Our data showed that the discordance of negative conversion is higher than that of positive conversion (0.17 vs. 0.04). The data imply 4% of patients may loss the chance of anti-HER2 therapy if they only detect primary GC site. Therefore, the HER2 testing should be performed in both primary and metastatic sites whenever possible for anti-HER2 therapy.
However, results of the meta-analysis should be interpreted with caution. The ‘true’ discordance rates may be lower than those reported in the literature. Firstly, debates still exist related to the intratumoral heterogeneity. Marx’s study showed that HER2 amplification is highly homogenous in GC (20). However, Bilous et al. (31) and Grabsch et al. (32) indicated that there was heterogeneity among HER2 protein expression and amplification. It is suggested that HER2 testing in endoscopic biopsies before treatment is prone to false-negative results. Secondly, the limited discordant HER2 status found between the primary and the paired metastatic GC specimens might be partially due to the method of detection. HER2 overexpression in gastric tumor is heterogeneous and comprises a minority of tumor cells, which represent an important drawback for HER2 testing. It will produce low-reproducible results for HER2. In our meta-analysis, the pooled discordance is 8% using IHC, only 2% using ISH. It is consistent with paired HER2 status in primary breast cancer and its paired metastasis, which generally suggested that the potentially greater accuracy of ISH relative to IHC (33). Previous studies have reported that variability in IHC testing, including variable types of fixation, different antigen retrieval methods, and subjectivities in observer analysis, might affect results (34). Other factors such as relatively small sample size, retrospective studies and without centralized evaluation are also need to be addressed.
In addition to intratumoral heterogeneity and technique issues, the true discordance of HER2 may be due to other factors, such as clonal selection, tumor progression and other unknown reasons. HER2 status may change during the metastatic process and tumorigenesis. It is suggested that biopsy of metastasis appears to be beneficial in breast cancer in a prospective study (35). Correspondingly, the inconsistent chemotherapy response usually exists in clinical. Our results underlie the therapeutic importance of HER2 testing in both primary and metastatic site in GC. In breast cancer, patients with receptor discordance between primary and recurrent tumor are associated with poor survival, which might lead to inappropriate targeted therapy (36). For breast cancer, guidelines recommend HER2 testing of primary breast cancer or at the time of metastatic relapse, and some recommend testing metastasis if HER2 status of the primary is unknown (37). However, to the best of our knowledge, there are no relevant data to elucidate the clinical significance for treatment change of HER2 status discordance between primary and metastasis in GC. Whether a finding of discordance altered management of metastatic disease and whether altered management improved patient outcomes need to be investigated in the future.
In clinical practice, there are also several factors need to be noted. Delaying systemic therapy to await biopsy of a metastatic lesion and its characterization is a concern. Biopsy of metastatic disease cannot be easy to be carried out due to potential complications and due to the need of surgical approaches. Patient’s preference to undertake an invasive procedure is also concerned (35,38).
Conclusions
In conclusion, the discordance of HER2 status is not rare in primary and metastatic GC through our meta-analysis. Wherever possible, HER2 status testing is recommended to perform in both primary and metastatic sites. Prospective studies are needed to testify the clinical significance of the discordance of HER2 status.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81172110), National High Technology Research and Development Program (No. 2012AA 02A 504), Beijing Municipal Science & Technology Commission Program (No. Z11110706730000), Beijing Natural Science Foundation (7142034), and China Postdoctoral Science Foundation (2013M530494).
Disclosure: The authors declare no conflict of interest.
Table S1. Assessment of study quality with Newcastle-Ottawa scale.
| Study ID | Year | Selection |
Comparability |
Exposure |
No. of star | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | C2 | E1 | E2 | E3 | |||||
| Chariyalertsak S | 1994 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||||
| Kim JS | 1997 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||||
| Kim JH | 2009 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | |||
| Marx AH | 2009 | ★ | ★ | ★ | ★ | ★ | 5 | ||||||
| Kim JY | 2010 | ★ | ★ | ★ | ★ | ★ | 5 | ||||||
| Kim MA | 2011 | ★ | ★ | ★ | ★ | ★ | 5 | ||||||
| Geng YT | 2011 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||
| Bozzetti C | 2011 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||||
| Zhang YL | 2012 | ★ | ★ | ★ | ★ | ★ | 5 | ||||||
| Tsapralis D | 2012 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||||
| Fassan M | 2012 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||
| Asioli S | 2012 | ★ | ★ | ★ | ★ | ★ | 5 | ||||||
| Shinozaki E | 2013 | ★ | ★ | ★ | ★ | 4 | |||||||
| Pagni F | 2013 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||||
| Kochi M | 2013 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||
| Fusco N | 2013 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | |||
| Cho EY | 2013 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||
| Geng Y | 2014 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||||
Selection contains four criteria: S1, is the case definition adequate? S2, representativeness of the cases; S3, selection of controls; S4, definition of controls. Comparability means: C1, comparability of cases; C2, controls on the basis of the design or analysis. Exposure contains three criteria: E1, ascertainment of exposure; E2, same method of ascertainment for cases and controls; E3, non-response rate.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.Gravalos C, Jimeno A.HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [DOI] [PubMed] [Google Scholar]
- 3.Barros-Silva JD, Leitão D, Afonso L, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer 2009;100:487-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jørgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology 2010;78:26-33. [DOI] [PubMed] [Google Scholar]
- 5.Shan L, Ying J, Lu N.HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population. Diagn Pathol 2013;8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol 2009;135:1331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [DOI] [PubMed] [Google Scholar]
- 8.Boku N.HER2-positive gastric cancer. Gastric Cancer 2014;17:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusco N, Rocco EG, Del Conte C, et al. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol 2013;26:816-24. [DOI] [PubMed] [Google Scholar]
- 10.Cho EY, Park K, Do I, et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol 2013;26:677-84. [DOI] [PubMed] [Google Scholar]
- 11.Shinozaki E, Yamamoto N, Saiura A, et al. Concordance of HER2 and its related molecules between primary and paired liver metastatic sites in gastric cancer. J Clin Oncol 2013;31:abstr 4108.
- 12.Kochi M, Fujii M, Masuda S, et al. Differing deregulation of HER2 in primary gastric cancer and synchronous related metastatic lymph nodes. Diagn Pathol 2013;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JJ. The Inverse of the Freeman-Tukey Double Arcsine Transformation. Am Stat 1978;32:138. [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Well GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chariyalertsak S, Sugano K, Ohkura H, et al. Comparison of c-erbB-2 oncoprotein expression in tissue and serum of patients with stomach cancer. Tumour Biol 1994;15:294-303. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Choi CW, Kim BS, et al. Amplification of c-erbB-2 proto-oncogene in cancer foci, adjacent normal, metastatic and normal tissues of human primary gastric adenocarcinomas. J Korean Med Sci 1997;12:311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Kim MA, Lee HS, et al. Comparative analysis of protein expressions in primary and metastatic gastric carcinomas. Hum Pathol 2009;40:314-22. [DOI] [PubMed] [Google Scholar]
- 20.Marx AH, Tharun L, Muth J, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol 2009;40:769-77. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Park KM, Cho EY, et al. HER2 overexpression in primary and metastatic carcinomas in pT2b gastric adenocarcinoma: Comparison of antibodies A4085 and CB11 with correlation to results of fluorescent in situ hybridization. Laboratory Investigation 2010;90:134A-73A. [Google Scholar]
- 22.Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 2011;104:1372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng YT, Qiu JR, Wang R, et al. Expression of HER-2 and leptin in gastric cancer and their clinical significance. Zhonghua Zhong Liu Za Zhi 2011;33:764-9. [PubMed] [Google Scholar]
- 24.Kim MA, Lee HJ, Yang HK, et al. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 2011;59:822-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asioli S, Maletta F, Verdun di Cantogno L, et al. Approaching heterogeneity of human epidermal growth factor receptor 2 in surgical specimens of gastric cancer. Hum Pathol 2012;43:2070-9. [DOI] [PubMed] [Google Scholar]
- 26.Fassan M, Ludwig K, Pizzi M, et al. Human epithelial growth factor receptor 2 (HER2) status in primary and metastatic esophagogastric junction adenocarcinomas. Hum Pathol 2012;43:1206-12. [DOI] [PubMed] [Google Scholar]
- 27.Tsapralis D, Panayiotides I, Peros G, et al. Human epidermal growth factor receptor-2 gene amplification in gastric cancer using tissue microarray technology. World J Gastroenterol 2012;18:150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YL, Hua YW, Zhang ZD, et al. HER-2 amplification and over expression in primary tumors and paired lymph node metastases of gastric cancer. World Chinese Journal of Digestology 2012;20:2485-90. [Google Scholar]
- 29.Geng Y, Chen X, Qiu J, et al. Human epidermal growth factor receptor-2 expression in primary and metastatic gastric cancer. Int J Clin Oncol 2014;19:303-11. [DOI] [PubMed] [Google Scholar]
- 30.Pagni F, Zannella S, Ronchi S, et al. HER2 status of gastric carcinoma and corresponding lymph node metastasis. Pathol Oncol Res 2013;19:103-9. [DOI] [PubMed] [Google Scholar]
- 31.Bilous M, Osamura RY, Rüschoff J, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol 2010;41:304-5; author reply 305-6. [DOI] [PubMed] [Google Scholar]
- 32.Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol 2010;32:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer 2014;50:277-89. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita-Kashima Y, Shu S, Yorozu K, et al. Importance of formalin fixing conditions for HER2 testing in gastric cancer: immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer 2014;17:638-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 2012;30:587-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat 2011;125:553-61. [DOI] [PubMed] [Google Scholar]
- 37.Perez EA, Cortés J, Gonzalez-Angulo AM, et al. HER2 testing: current status and future directions. Cancer Treat Rev 2014;40:276-84. [DOI] [PubMed] [Google Scholar]
- 38.Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol 2011;22:2227-33. [DOI] [PubMed] [Google Scholar]