Abstract
Objective
Extensively drug-resistant tuberculosis (XDR-TB)/HIV co-infection is difficult to treat with frequent adverse drug reactions, and high mortality. Adherence to antiretroviral therapy (ARV) and second-line TB medications may reduce mortality, prevent amplification of drug-resistance, and improve outcomes.
Methods
Prospective cohort study of XDR-TB patients on treatment in KwaZulu-Natal, South Africa. Adherence to ARV and TB medications was assessed separately at baseline and monthly. Knowledge, attitudes, and beliefs (KAB) were assessed at baseline. Optimal adherence was defined as self-report of taking all pills in the previous 7 days; missing any pills was defined as suboptimal adherence. Primary outcome was optimal adherence 6 months after initiation of XDR-TB treatment to TB medications, ARV, and both (‘dual-adherence’).
Results
104 XDR-TB patients (79.8% HIV co-infected, 84.3% on ARV at enrollment) were enrolled and followed monthly (median 8 visits; IQR 4–12). Six-month optimal adherence was higher for ARV (88.2%) than TB medications (67.7%) (p<0.001). Low educational attainment, male gender, and year of enrollment were independently associated with dual suboptimal adherence. At baseline participants indicated that XDR-TB was curable (76.0%), HIV and TB were linked (81.7%), and ARV improves TB outcomes (72.1%). Baseline KAB did not predict subsequent adherence.
Conclusions
Medication adherence was significantly higher for ARV than for TB medications in this cohort. Short course treatment regimens for drug-resistant TB with lower pill burden may increase adherence and improve outcomes in XDR-TB/HIV. Programmatic support for dual-adherence is critical in the treatment of drug-resistant TB and HIV.
Keywords: Extensively Drug-resistant Tuberculosis, HIV/AIDS, Adherence, Knowledge, Attitudes and Beliefs
Introduction
Extensively drug resistant tuberculosis (XDR-TB), the most resistant form of tuberculosis (TB),1 is difficult to treat,2 associated with substantial mortality,3,4 and poor treatment outcomes.5,6 Globally, the majority of reported cases of XDR-TB are from South Africa.7,8 XDR-TB in South Africa is characterized by a high percentage of HIV co-infection, early mortality, and poor 24-month treatment outcomes.9–11 XDR-TB-HIV treatment involves complex medication regimens with potential drug-interactions and adverse drug reactions.12 A recent prospective study of XDR-TB treatment in South Africa described ongoing community spread of drug resistant TB strains, and low rates of TB culture conversion with frequent reversion.13 Medication adherence was not measured in this study.
Medication adherence is critical for both HIV and TB outcomes, and suboptimal adherence mediates the development of antimycobacterial and antiretroviral drug resistance on treatment.14–16 Early studies have shown that approximately 95% adherence to antiretroviral therapy (ARV) is needed to ensure HIV viral suppression.17,18 Later studies using more potent and durable regimens have demonstrated viral suppression with lower adherence.19,20 Clinical trials of drug-susceptible TB treatment have shown that 95% of patients are capable of successful outcome with direct observation and support by study personnel.21 Under operational conditions many patients default their TB treatment and successful outcomes range from 55–95%.22,23 Medication adherence in patients with drug-resistant TB and HIV is understudied; to our knowledge there are no published reports in this group.
Patient adherence in HIV and TB treatment have been recently reviewed.24,25 A 'gold-standard' for measuring medication adherence in either field is controversial and each method has strengths and weaknesses.26 Patient-reported recall is widely used in measuring HIV medication adherence and has been shown to correlate with ARV pill count and HIV viral load suppression.27 There are no validated instruments to measure medication adherence in the treatment of drug-resistant TB.
Adherence to both TB medications and ARV may be affected by patient’s knowledge, attitudes, and beliefs (KAB).28,29 Factors associated with KAB include poverty, gender, education, perceived stigma around HIV or TB or both, and other social, structural, and cultural factors.24,30–32 In order to understand factors associated with treatment outcomes and survival in XDR-TB-HIV, we initiated a prospective study of XDR-TB treatment (PROX Study) in KZN, South Africa. Our primary aim was to measure adherence to ARV and TB medication, and understand factors associated with suboptimal adherence. A secondary aim was to understand the effect of baseline KAB on early self-reported adherence to TB treatment and ARV. Our hypothesis was that baseline knowledge of the connection between HIV and TB would be associated with improved adherence to ARV and second-line TB treatment among XDR-TB-HIV co-infected patients.33
Methods
We prospectively enrolled consecutive patients with culture confirmed XDR-TB admitted for initiation of XDR-TB treatment at a public TB specialist hospital in KwaZulu-Natal, South Africa from August 2009 through July 2011. Patients were eligible for enrollment if they were ≥18 years of age, diagnosed with active TB disease according to national TB program guidelines, had culture proven XDR-TB according to a standard case-definition, had not been previously treated for XDR-TB, agreed to start treatment for XDR-TB, and had capacity to give informed consent in either English or isiZulu. Prisoners, and patients previously treated for XDR-TB were excluded. Patients received usual care for XDR-TB (i.e. individualized therapy based on drug susceptibility pattern), HIV, and other diseases.
All participants gave written informed consent and the study protocol was approved by the ethical review committees of the University of KwaZulu-Natal, and the Albert Einstein College of Medicine.
Adherence was measured by separate seven-day recall for ARV and second-line TB medications (‘TB medications’) in a questionnaire administered by study staff fluent in both English and isiZulu at study intake and monthly, as previously described.34 For the purposes of analysis a cumulative six-month adherence variable, for TB and separately for ARV, was used which included responses given at baseline and at the monthly visits.18 Participants were considered ‘optimally adherent’ at each monthly visit only if they stated that they had taken all of their doses and missed none. If participants stated that they had not taken all or missed some of the medications they were considered ‘sub-optimally adherent’ for that class of medications (either ARV or TB medication).14 For the purposes of analysis a cumulative six-month adherence variable, for TB and separately for ARV, was used which included responses given at baseline and at the 1–6 month time points. The participants were considered optimally adherent for the cumulative six-month adherence for ARV, XDR-TB medications or both ARV and XDR-TB medications (‘dual adherence’) only if they were optimally adherent for all time points.
Knowledge, attitudes, and beliefs (KAB) around TB and HIV were assessed through a questionnaire administered at baseline prior to XDR-TB treatment initiation. A study staff person fluent in English and isiZulu administered the KAB questionnaire, adherence questions, and recorded clinical and demographic data.
Hospitalized patients were cared for in open wards by nursing staff with approximately 20 patients per health care worker. While all medications are given to patients, the ward nurse does not directly observe them taking their medications. In addition, patients may refuse a specific pill or injection. As outpatients, patients are enrolled in the public, provincial DOTS program which relies on a community or household DOTS supporter to provide patient support and attest through signature that the medications are being taken correctly and on schedule. Physicians check DOTS support cards at monthly visits.
During the second and third years of the study a patient support and education initiative was started to enhance adherence and improve patient care. This consisted of two staff members meeting with small groups of XDR-TB patients on a weekly basis. The purpose was to develop patient peer support and to encourage adherence as well as provide patient-oriented information around topics associated with drug-resistant TB and HIV.
Descriptive statistics were calculated using standard methods. Associations were tested with Fisher’s exact test; paired values were tested using McNemar’s test. To identify factors associated with incomplete adherence to ARV or TB medications we first identified factors in bivariate analysis and then constructed multiple logistic regression models including variables which were statistically significant and/or associated with >10% change in effect measure. Interaction between terms was assessed for significant change of the risk estimate. Test for trend was performed using Cochran-Armitage test. Statistical analysis was performed using SAS Version 9.3 (SAS Institute, Cary).
Results
Participant characteristics
During the study period, 110 consecutive patients age ≥18 with culture confirmed XDR-TB and without prior treatment for XDR-TB presented to the study site. Of these 5 patients either refused to participate, or lacked capacity to consent. One XDR-TB patient was a prisoner and therefore ineligible to enroll by study protocol. The remaining 104 XDR-TB patients were eligible, gave written informed consent and were prospectively enrolled in the study within two weeks of initiation of XDR-TB treatment. The majority of participants were female (52%), young (median age 35 years, range 18 to 60 years), and HIV co-infected (79.8%). Among HIV co-infected patients 84.3% were on ARV and median CD4 count at baseline was 267 (IQR135–452 cells/mm3). Of these patients 70/82 (85%) were on ARV at time of admission and an additional 9%(7/82) were started subsequently (median time to ARV initiation 136 days). These patients reported relatively high levels of educational attainment (32% completed secondary) and monthly income (median $296; IQR $185–588). The majority of patients had been previously treated for drug-sensitive TB (92.3%) and for multi-drug resistant TB (57.7%) (Table 1).
Table 1.
Demographic characteristics of extensively drug resistant tuberculosis (XDR-TB) patients initiated on XDR-TB therapy during study period
| Baseline Characteristic | XDR-TB patients N (%) |
|
|---|---|---|
| Sex | Male Female |
50 (48.1) 54 (51.9) |
| Age (years) | 18–25 26–35 36–50 >50 Median Age (IQR) |
21 (20.2) 32 (30.8) 42 (40.4) 9 (8.9) 35 (27–43) |
| Completed primary school | Yes No Missing |
81 (79.4) 21 (20.6) 2 |
| Household Income (US$/month)* |
Median (IQR) | $296 ($185–588) |
| HIV Status^ | Positive Negative |
83 (79.8) 21 (20.2) |
| CD4 T-cell Count#(cells/mm3) | Known Unknown Median CD4 Count (IQR) |
74 (89) 9 (11) 267 (135–452) |
| HIV positive on ARV**, # | Yes No |
77 (92.7) 6 (7.3) |
| TB Medications | Number (range) | 7 (4–9) |
| ARV Medications | Number (range) | 3 (2–5) |
| Previous TB history |
Yes No |
96 (92.3) 8 (7.7) |
| Previous MDR-TB** history |
Yes No |
60 (57.7) 44 (42.3) |
| History of being a Health Care Worker |
Yes No |
5 (4.8) 99 (95.2) |
Calculated from South African Rand at exchange rates from 7/2011. Income data missing for 8 patients.
HIV-infected includes known HIV infected on admission (82) and diagnosed as HIV-infected subsequently (1).
Among HIV infected patients; patients with known CD4 T cell counts who had at least 1 count during the study period
ARV – antiretroviral therapy
On ARV includes ARV on admission (70) and ARV initiated subsequently (7)
XDR-TB treatment was based on drug susceptibility testing to six antimycobacterial drugs (isoniazid, rifampicin, ofloxacin, streptomycin, kanamycin, and ethambutol) and consisted of on average 7 (range 4–9) antimycobacterial drugs. The majority of patients were on an initial TB treatment regimen including capreomycin, moxifloxacin, para-amino salicylic acid (PAS), ethionamide (98.1%), terizidone, and pyrazinamide (PZA) (96.2%). XDR-TB patients were started on a median 7 (range 4–9) TB medications and 3 ARV (range 3–5). Patients were treated with ARV regimens that included non-nucleotide reverse transcriptase inhibitors (efavirenz N=68; neviripine N=3; Not recorded N=6) and nucleotide reverse transcriptase inhibitors. During the study period two patients were changed to protease inhibitor-containing regimens after clinically failing treatment. Overall median time of follow-up for all XDR-TB patients on treatment was 9 months (IQR 2–19). Median inpatient time was 144 days (IQR 77–189).
Adherence data
We obtained self-reported TB medication adherence data for all 104 XDR-TB patients and adherence data for 68/77 patients (88%) on ARV. On average each patient had 8 monthly visits (IQR 3–14) during which adherence to ARV and TB medications were assessed by self-reported seven-day recall. Among all XDR-TB patients on treatment, 67.3% reported optimal six-month adherence to TB medications while among XDR-TB-HIV co-infected patients on ARV 88.2% reported optimal adherence to ARV. (Table 2a) Among XDR-TB-HIV co-infected patients on both XDR-TB medications and ARV, optimal six-month adherence to ARV was 88.2% and optimal six-month adherence to TB medications was 67.7% (P<0.001). 64.3% reported optimal six-month adherence to both TB medications and ARV (‘dual-adherence’). Optimal adherence to ARV was significantly associated with optimal adherence to TB medications (OR 21.0 (95% CI 2.38–184.89), p=0.006). The largest subset (15/25) of patients non-adherent to either ARV or second-line TB medications reported optimal adherence to ARV but not to TB medications. (Table 2b)
Table 2.
| a. Cumulative six-month optimal adherence to ARV and TB medications in all XDR-TB patients (N=104) | ||
|---|---|---|
| TB Medication Adherence | ARV Adherence* | Dual Adherent** |
| 70/104 (67.3) | 60/68 (88.2) | 45/68 (66.2) |
| b. Cumulative six-month optimal adherence in XDR-TB-HIV Co-infected Patients on both ARV and TB medications (N=68) | ||
|---|---|---|
| XDR-TB Medication Adherence | ARV Adherence | |
| Optimal Adherence | Sub-optimal Adherence | |
| Optimal Adherence | 45 | 1 |
| Sub-optimal Adherence Sometimes Non-Adherent (%) Often Non-Adherent (%) Always Non-Adherent (%) |
15 9 (60.0) 4 (26.7) 2 (13.3) |
7 4 (57.1) 3 (42.9) 0 |
| XDR-TB Medication Adherence | 46/68 (67.7%) | |
| ARV Adherence | 60/68 (88.2%) | p<0.001 |
75 patients reported being on ARV at six months; 68 provided adherence data
Calculated among HIV infected individuals on ARV with adherence data. Count includes individuals that are dual adherent to both ARVs and TB medications. One patient provided ARV adherence data, but not TB adherence data.
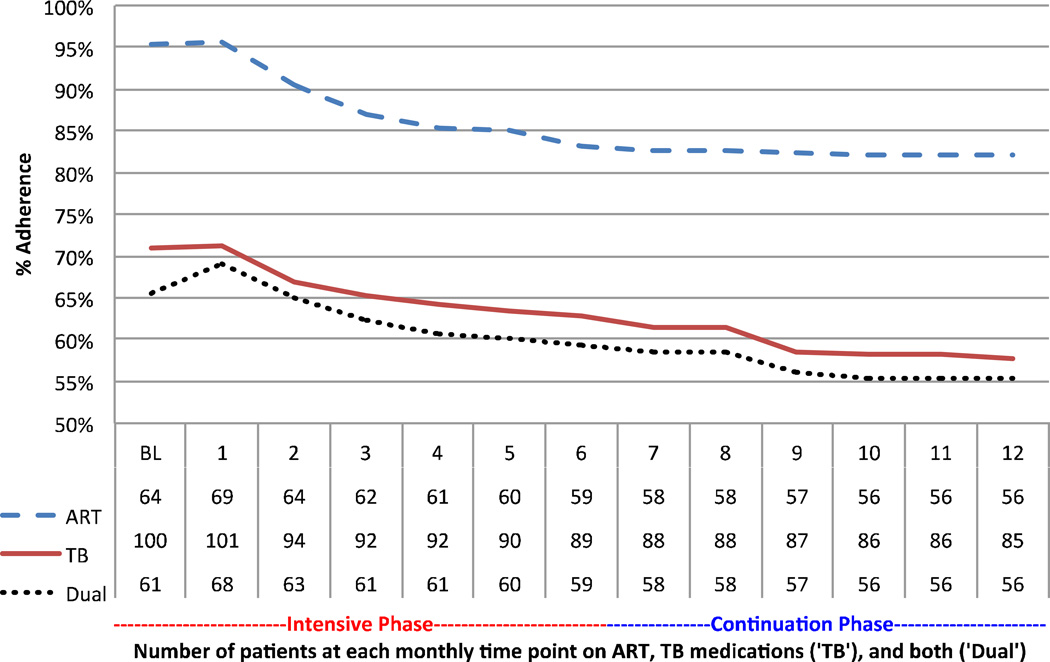
On longitudinal analysis initially 95% of patients reported optimal adherence to ARV, 71% reported optimal adherence to TB medications and among patients on ARV and TB medications 67% reported optimal adherence to both. At month 4 cumulative optimal adherence to ART (87%) TB medications (67%) and both (64%) were lower and continued to slowly decline through the first twelve months of treatment. However, there was not a significant decline in adherence after the end of the intensive phase, which included nurse-administered injectable agents for the majority of patients (92%). (Figure 1)
Figure 1.
Percentage of patients with baseline (BL) and monthly cumulative optimal treatment adherence to ARV, TB medications. Numbers reporting adherence data for antiretroviral therapy (ARV), TB medications (TB), or both (Dual) at each monthly visit in the table below. Intensive treatment phase includes injectable agents. Data censored at time of death. (N=19/104).
Among the patients who reported suboptimal adherence to TB medications 13/22 (59%) reported they took ‘most’ or ‘missed few’ of their medications; 7/22 (32%) reported taking few or often missing their TB medications; 2/22 (9%) reported always missing or never taking their TB medications. Among patients on ARV who reported suboptimal adherence 7/8 (88%) reported they took ‘most’ or ‘missed few’ of their ARV; 1/8 (12%) reported always missing or never taking his ARV. XDR-TB-HIV patients with at least six consecutive monthly visits 57.6% report six visits with optimal ARV adherence as compared to 37.5% reporting optimal TB medication adherence on six consecutive visits. Number of monthly study visits with self-reported optimal adherence to TB medications was correlated with TB culture conversion over the course of XDR-TB treatment. TB culture conversion was defined as two consecutive negative cultures taken ≥30 days apart. (Supplemental Figure 1)
XDR-TB-HIV co-infected patients were less adherent to TB medications (65.1%) than HIV negative XDR-TB patients (76.2%) (p=0.33). For XDR-TB patients not on ARV at baseline, optimal TB medication adherence was 63.6%, not significantly different compared to TB medication adherence in XDR-TB patients on ARV (65.7%) (p=1.00). Patients appeared to be less adherent to their overall XDR-TB medication regimens if they included cycloserine (33.3%) but this difference was not statistically significant (p=0.09). (Supplemental Figure 2)
On multivariate analysis higher educational attainment was associated with optimal dual adherence at six months (OR 5.39; 95% CI 1.03 – 28.25). Women were more likely to report being optimally adherent (OR 4.68; 95% CI 1.11 – 19.68) as were younger patients though this association was not statistically significant (OR 2.95; 95% CI 0.65 – 13.42). In addition, there was significantly lower dual optimal adherence among patients who enrolled earlier, in 2009 compared to patients who enrolled later, in 2011, (test for trend, p<0.001). The variable ‘adverse drug reaction’ was not included in the multivariate analysis since including the variable did not change estimates of effect and many participants had missing data (N=20). (Table 3)
Table 3.
Univariate and multivariate analysis of factors associated with optimal adherence to TB Medications, ARV, and both (Dual) at 6 months
| TB Medication adherence N=104 (%)* |
ARV adherence N=70 (%)** |
Dual adherence N=68 (%)*** |
Dual Adherence Univariate OR (95% CI) |
Dual Adherence Multivariate OR (95%CI) |
||
|---|---|---|---|---|---|---|
| Gender | Male Female |
29/50 (58.0) 41/54 (75.9) |
25/31 (80.7) 35/39 (89.7) |
16/29 (55.2) 29/39 (74.4) |
1.00 (ref) 2.72 (0.99 – 7.44) |
1.00 (ref) 4.68 (1.11–19.68) |
| Age | <35 >=35 |
35/49 (71.4) 35/55 (63.6) |
24/28 (85.7) 36/42 (85.7) |
18/28 (64.3) 27/40 (67.5) |
1.00 (ref) 1.00 (0.37–2.71) |
2.95 (0.65 – 13.42) 1.00 (ref) |
| Completed Primary school | No Yes |
8/21 (38.1) 60/81 (74.1) |
10/16 (62.5) 48/52 (92.3) |
5/16 (31.3) 38/52 (73.1) |
1.00 (ref) 5.97 (1.76–20.26) |
1.00 (ref) 5.39 (1.03–28.25) |
| Previous MDR-TB Hx | No Yes |
24/44 (54.6) 46/60 (76.7) |
24/29 (82.8) 36/41 (87.8) |
16/29 (55.2) 29/41 (70.7) |
1.00 (ref) 1.96 (0.73–5.31) |
1.00 (ref) 0.78 (0.19–3.15) |
| Income(ZAR) | <R2010 >=R2010 |
32/48 (66.7) 32/48 (66.7) |
31/38 (81.6) 27/30 (90.0) |
23/38 (60.5) 20/30 (66.7) |
1.00 (ref) 1.30 (0.48–3.54) |
1.00 (ref) 1.96 (0.44–8.66) |
| Year Enrolled | 2011 2010 2009 |
38/42 (90.5) 28/42 (66.7) 4/20 (20.0) |
27/30 (90.0) 26/29 (89.7) 7/11 (63.6) |
26/30 (86.7) 18/29 (62.1) 1/11 (9.1) |
1.00 (ref) 0.25 (0.07–0.92) 0.02 (0.00–0.15) |
1.00 (ref) 0.41 (0.09–1.78) 0.01 (0.00–0.13) |
| Adverse Events on Treatment† | No Yes |
13/24 (54.2) 30/51 (58.8) |
11/15 (73.3) 28/33 (84.9) |
7/15 (46.7) 17/33 (51.5) |
1.00 (ref) 1.21 (0.36–4.12) |
Calculation based on individuals with adherence data (N=104) within the first 6 months follow-up
Calculation based on individuals with ARV adherence data (N=70) within the first 6 months follow-up
Analysis includes patients with ARV and TB adherence data (N=68)
Within the first 6 months of follow-up
In the baseline KAB questionnaire, participants were optimistic regarding adhering to XDR-TB treatment for >2 years (80.8%); being cured of XDR-TB (76.0%), and successfully completing treatment (96.2%). Participants identified linkages between TB and HIV (81.7%); ARV and XDR-TB treatment (72.1%); and modes of TB transmission (97.1%). About half of all patients (48.1%) incorrectly identified sharing a cup as a possible mode of transmission. Answers to these KAB questions were not significantly associated with increased percentages of optimal adherence to TB medications or to both TB medications and ARV at six months. (Table 4)
Table 4.
Baseline knowledge, attitudes and beliefs questions (KAB) with overall stratified by percent adherent to TB medications in all XDR-TB patients (n=104) and both TB medications and ARV adherent (‘dual’) in XDR-TB-HIV patients (N=68)
| KAB Question |
Overall N (%) |
TB medication Adherent n/N (%) |
Dual Adherent n/N (%) |
|
|---|---|---|---|---|
| Is XDR-TB curable? | No | 7 (6.7) | 4/7 (57.1) | 2/5 (40.0) |
| Yes | 79 (76.0) | 55/79 (69.6) | 38/56 (67.9) | |
| Don't Know | 18 (17.3) | 11/18 (61.1) | 5/7 (71.4) | |
| p-value | 0.66 | 0.43 | ||
| Is XDR-TB Treatment >2 years? | No | 4 (3.8) | 1/4 (25.0) | 1/1 (100.0) |
| Yes | 84 (80.8) | 59/84 (70.2) | 40/60 (66.7) | |
| Don't Know | 16 (15.4) | 10/16 (62.5) | 4/7 (57.1) | |
| p-value | 0.15 | 0.79 | ||
| Not taking medications every day will likely make my XDR-TB worse? | No | 1 (1.0) | 0/1 (0.0) | 0/1 (0.0) |
| Yes | 95 (91.3) | 64/95 (67.4) | 44/65 (6.7) | |
| Don't Know | 8 (7.7) | 6/8 (75.0) | 1/2 (50.0) | |
| p-value | 0.40 | 0.41 | ||
| I think I will complete XDR-TB treatment successfully? | No | 0 (0.0) | - | - |
| Yes | 100 (96.2) | 67/100 (67.0) | 44/66 (66.7) | |
| Don't Know | 4 (3.8) | 3/4 (75.0) | 1/2 (50.0) | |
| p-value | 1.00 | 1.00 | ||
| Is there a link between TB and HIV? | No | 7 (6.7) | 4/7 (57.1) | 0/1 (0.0) |
| Yes | 85 (81.7) | 58/85 (68.2) | 40/58 (69.0) | |
| Don't Know | 12 (11.5) | 8/12 (66.7) | 5/9 (55.6) | |
| p-value | 0.85 | 0.32 | ||
| ARV for my HIV helps to fight XDR-TB? | No | 5 (4.8) | 3/5 (60.0) | 2/4 (50.0) |
| Yes | 75 (72.1) | 55/75 (73.3) | 38/53 (71.7) | |
| Don't Know | 24 (23.1) | 12/24 (50.0) | 5/11 (45.5) | |
| p-value | 0.10 | 0.17 | ||
| XDR-TB transmitted by air when XDR-TB patient coughs? | No | 1 (1.0) | 1/1 (100.0) | 1/1 (100.0) |
| Yes | 101 (97.1) | 68/101 (67.3) | 44/66 (66.7) | |
| Don't Know | 2 (1.9) | 1/2 (50.0) | 0/1 (0.0) | |
| p-value | 1.00 | 0.57 | ||
| XDR-TB transmitted by sharing a cup? | No | 41 (39.4) | 27/41 (65.9) | 17/27 (63.0) |
| Yes | 50 (48.1) | 37/50 (74.0) | 25/35 (71.4) | |
| Don't Know | 13 (12.5) | 6/13 (46.2) | 3/6 (50.0) | |
| p-value | 0.17 | 0.51 | ||
| Enough information about XDR-TB? | No | 63 (60.6) | 44/63 (69.8) | 27/39 (69.2) |
| Yes | 34 (32.7) | 20/34 (58.8) | 15/25 (60.0) | |
| Don't Know | 7 (6.7) | 6/7 (85.7) | 3/4 (75.0) | |
| p-value | 0.37 | 0.77 | ||
| XDR-TB patients treated respectfully? | No | 7 (7.1) | 6/7 (85.7) | 3/3 (100.0) |
| Yes | 86 (86.9) | 56/86 (65.1) | 36/56 (64.3) | |
| Don't Know | 6 (6.1) | 4/6 (66.7) | 3/5 (60.0) | |
| Missing | 5 | |||
| p-value | 0.58 | 0.78 | ||
| Told family and friends about XDR-TB? | No | 10 (9.6) | 6/10 (60.0) | 3/6 (50.0) |
| Yes | 93 (89.4) | 63/93 (67.7) | 41/61 (67.2) | |
| Don't Know | 1 (1.0) | 1/1 (100.0) | 1/1 (100.0) | |
| p-value | 0.82 | 0.61 | ||
| Family/friends treat differently with XDR-TB? | No | 87 (85.3) | 60/87 (69.0) | 41/59 (69.5) |
| Yes | 9 (8.8) | 4/9 (44.4) | 1/5 (20.0) | |
| Don't Know | 6 (5.9) | 5/6 (83.3) | 2/3 (66.7) | |
| Missing | 2 | |||
| p-value | 0.26 | 0.11 | ||
| Should treat XDR-TB at patient’s community level? | No | 1 (1.0) | 0/1 (0.0) | 0/1 (0.0) |
| Yes | 99 (95.2) | 67/99 (67.7) | 44/65 (67.7) | |
| Don't Know | 4 (3.8) | 3/4 (75.0) | 1/2 (50.0) | |
| p-value | 0.53 | 0.41 |
Discussion
To our knowledge this is the first prospective study of adherence in the integrated treatment of drug-resistant TB and HIV. Our main finding is that patients being treated for XDR-TB and HIV co-infection are significantly more likely to report complete adherence to ARV compared to TB medications through the first six months of their XDR-TB treatment. It is probable that these self-reported adherence figures are an over-estimate since patients may be hesitant to report the extent of their non-adherence to study staff.35,36 In our cohort, knowledge, attitudes, and beliefs including knowledge of the relationship between TB and HIV; attitudes around XDR-TB and HIV stigma; and beliefs regarding adherence did not predict subsequent self-reported adherence at six months.
Adherence in the treatment of drug-resistant TB-HIV is critically important since adherence mediates treatment outcomes, the development of drug-resistance on treatment, the infectivity of the patients on treatment and is therefore associated with transmission of TB infection in the community. Improved adherence to ARV is associated with improved survival in XDR-TB-HIV patients 10. However, ARV does not appear to be associated with improved rates of TB culture conversion in drug-susceptible or drug-resistant TB.5,10,37 Therefore if XDR-TB patients are adherent to ARV but poorly adherent to TB medications the concern is that they will survive to spread drug-resistant TB strains in the hospital and in the community, and be more likely to develop amplification of drug-resistance on treatment.
Risk factors for incomplete adherence at six months included male gender and low educational attainment, which is consistent with some, but not all previous studies.30,35,38,39 Year of admission was also associated with complete adherence, which may be due to improved patient education after our patient support intervention, differential reporting bias, or other factors.33,40 We have identified risk groups (i.e. men and lower educated patients) who may be amenable to specific interventions to improve adherence. In addition the finding that the majority of TB suboptimal adherent patients do report optimal ARV adherence is helpful. It suggests that these patients are not intrinsically non-adherent and that with enhanced patient support, education, and most importantly more tolerable TB drug regimens it may be possible to improve adherence for these patients.
The six-month adherence data predominantly represent in-hospital adherence since patients were hospitalized for a median of 4.7 months. Given high patient-to-nurse ratios therapy in hospital is seldom directly observed. Self-reported seven-day adherence to ARV, XDR-TB medications decreased significantly during the first twelve months on treatment and may represent in part the transition from inpatient to outpatient care. On discharge from hospital to outpatient care, patients are enrolled in the local directly observed therapy (DOTS) program. This program is under-resourced and relies on family members and friends to provide patient support. It is likely that the reported limitations of DOTS for drug-susceptible TB lead to even less effective adherence in the treatment of drug-resistant TB-HIV.
A major limitation of our study is the reliance on self-reported seven-day recall for adherence. Self-reported seven-day recall was the only means of measuring adherence in this study. Seven-day recall has been used extensively in ARV adherence studies where it has been shown to correlate with percentage of patients with undetectable HIV RNA viral load 31,36. Seven-day recall has not been validated as a measure of adherence in the treatment of drug-resistant TB.14,34 Self-reported adherence based on recall may lead to overestimation or less likely underestimation of actual adherence due to reporting or recall bias. It is unlikely that there was differential error with respect to ARV and TB medications and therefore it is likely that XDR-TB patients in this cohort do have lower rates of adherence to TB medications than ARV. Other limitations include relatively short duration of follow up, which limits our ability to comment on implications for treatment outcome or mortality. Another limitation is that we lack precise data on how changes in medications or adverse drug reactions during treatment affect adherence.
The potential implication of our main finding is that XDR-TB-HIV patients in KwaZulu-Natal, South Africa may have reduced mortality with higher adherence to ARV and yet have decreased TB culture conversion due to poor adherence to XDR-TB medications. Incomplete TB adherence with prolonged survival may also result prolonged transmission of infectious strains in the community and in amplification of TB drug-resistance on treatment, which is consistent with the epidemiology of drug-resistant TB in KwaZulu-Natal province.41 To address these findings, shorter duration regimens for treatment of drug-resistant TB are needed. As such TB regimens are introduced it will be important to introduce programs for patient and adherence support including ongoing monitoring and evaluation. Additional resources need to be devoted to enhancing adherence for drug-resistant TB-HIV within the context of TB-HIV control programs.
Supplementary Material
Acknowledgements
We acknowledge Ms. Nokwanda Depargo for her efforts in recruiting and enrolling patients. Our sincere appreciation goes to the physicians and nurses of King Dinuzulu Hospital.
Source of Funding: M.O. was supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases [5K23AI098479], Albert Einstein Center for Global Health & Clinical and Translational Research Institute, and Stony Wold-Herbert Fund. M.O. and N.P. were supported by the Centre for AIDS Programme of Research in South Africa (CAPRISA).
Footnotes
Conflicts of Interest: No conflicts of interest to declare.
REFERENCES
- 1.Benesova Y, Vasku A, Novotna H, et al. Matrix metalloproteinase-9 and matrix metalloproteinase-2 as biomarkers of various courses in multiple sclerosis. Mult Scler. 2009;15(3):316–322. doi: 10.1177/1352458508099482. [DOI] [PubMed] [Google Scholar]
- 2.Kliiman K, Altraja A. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;33(5):1085–1094. doi: 10.1183/09031936.00155708. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 4.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375(9728):1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13(7):855–861. [PMC free article] [PubMed] [Google Scholar]
- 6.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283(19):2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 7.Wolf C, Mohr H, Schneider-Axmann T, et al. CACNA1C genotype explains interindividual differences in amygdala volume among patients with schizophrenia. European archives of psychiatry and clinical neuroscience. 2013 doi: 10.1007/s00406-013-0427-y. [DOI] [PubMed] [Google Scholar]
- 8.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS medicine. 2012;9(8):e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdool-Karim Q, Abdool-Karim SS. The evolving HIV epidemic in South Africa. Int J Epidemiol. 2002;31(1):37–40. doi: 10.1093/ije/31.1.37. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR., Jr Treatment Outcomes for Extensively Drug-Resistant Tuberculosis and HIV Co-infection. Emerging infectious diseases. 2013;19(3) doi: 10.3201/eid1903.120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. American journal of respiratory and critical care medicine. 2010;181(1):80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 12.Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8(5):e63057. doi: 10.1371/journal.pone.0063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014 doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor JL, Gardner EM, Mannheimer SB, et al. Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. The Journal of infectious diseases. 2013;208(1):40–49. doi: 10.1093/infdis/jis731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner EM, Sharma S, Peng G, et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. Aids. 2008;22(1):75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni P, Akarte S, Mankeshwar R, Bhawalkar J, Banerjee A, Kulkarni A. Non-adherence of new pulmonary tuberculosis patients to anti-tuberculosis treatment. Annals of medical and health sciences research. 2013;3(1):67–74. doi: 10.4103/2141-9248.109507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of internal medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson J. AIDS researchers target poor adherence. JAMA : the journal of the American Medical Association. 1999;281(12):1069. doi: 10.1001/jama.281.12.1069. [DOI] [PubMed] [Google Scholar]
- 19.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 20.Glass TR, Rotger M, Telenti A, et al. Determinants of sustained viral suppression in HIV-infected patients with self-reported poor adherence to antiretroviral therapy. PloS one. 2012;7(1):e29186. doi: 10.1371/journal.pone.0029186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elzinga G, Raviglione MC, Maher D. Scale up: meeting targets in global tuberculosis control. Lancet. 2004;363(9411):814–819. doi: 10.1016/S0140-6736(04)15698-5. [DOI] [PubMed] [Google Scholar]
- 22.Nunn AJ, Phillips PP, Gillespie SH. Design issues in pivotal drug trials for drug sensitive tuberculosis (TB) Tuberculosis. 2008;88(Suppl 1):S85–S92. doi: 10.1016/S1472-9792(08)70039-8. [DOI] [PubMed] [Google Scholar]
- 23.Cox HS, Morrow M, Deutschmann PW. Long term efficacy of DOTS regimens for tuberculosis: systematic review. Bmj. 2008;336(7642):484–487. doi: 10.1136/bmj.39463.640787.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS medicine. 2007;4(7):e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS and behavior. 2011;15(7):1381–1396. doi: 10.1007/s10461-011-9942-x. [DOI] [PubMed] [Google Scholar]
- 26.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS and behavior. 2013;17(1):284–297. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and behavior. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potchoo Y, Tchamdja K, Balogou A, Pitche VP, Guissou IP, Kassang EK. Knowledge and adherence to antiretroviral therapy among adult people living with HIV/AIDS treated in the health care centers of the association"Espoir Vie Togo" in Togo, West Africa. BMC clinical pharmacology. 2010;10:11. doi: 10.1186/1472-6904-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, Lu W, Zhou Y, Zhu L, Shen H, Wang J. Adherence to anti-tuberculosis treatment among pulmonary tuberculosis patients: a qualitative and quantitative study. BMC health services research. 2009;9:169. doi: 10.1186/1472-6963-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naidoo P, Peltzer K, Louw J, Matseke G, McHunu G, Tutshana B. Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC public health. 2013;13:396. doi: 10.1186/1471-2458-13-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finlay A, Lancaster J, Holtz TH, Weyer K, Miranda A, van der Walt M. Patient- and provider-level risk factors associated with default from tuberculosis treatment, South Africa, 2002: a case-control study. BMC public health. 2012;12:56. doi: 10.1186/1471-2458-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowe KA, Makhubele B, Hargreaves JR, Porter JD, Hausler HP, Pronyk PM. Adherence to TB preventive therapy for HIV-positive patients in rural South Africa: implications for antiretroviral delivery in resource-poor settings? The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2005;9(3):263–269. [PubMed] [Google Scholar]
- 33.Gebremariam MK, Bjune GA, Frich JC. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC public health. 2010;10:651. doi: 10.1186/1471-2458-10-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34(8):1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 35.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 36.Balikuddembe R, Kayiwa J, Musoke D, et al. Plasma drug level validates self-reported adherence but predicts limited specificity for nonadherence to antiretroviral therapy. ISRN pharmacology. 2012;2012:274978. doi: 10.5402/2012/274978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brust JC, Lygizos M, Chaiyachati K, et al. Culture conversion among HIV co-infected multidrug-resistant tuberculosis patients in Tugela Ferry, South Africa. PLoS One. 2011;6(1):e15841. doi: 10.1371/journal.pone.0015841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyser M, Buchacz K, Bush TJ, et al. Factors associated with non-adherence to antiretroviral therapy in the SUN study. AIDS Care. 2011;23(5):601–611. doi: 10.1080/09540121.2010.525603. [DOI] [PubMed] [Google Scholar]
- 39.Kliiman K, Altraja A. Predictors and mortality associated with treatment default in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14(4):454–463. [PubMed] [Google Scholar]
- 40.Mindachew M, Deribew A, Tessema F, Biadgilign S. Predictors of adherence to isoniazid preventive therapy among HIV positive adults in Addis Ababa, Ethiopia. BMC public health. 2011;11:916. doi: 10.1186/1471-2458-11-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klopper M, Warren RM, Hayes C, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerging infectious diseases. 2013;19(3):449–455. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.